New Insight on Insulinoma Treatment in a Pet Rat—A Case Report
Abstract
Simple Summary
Abstract
1. Introduction
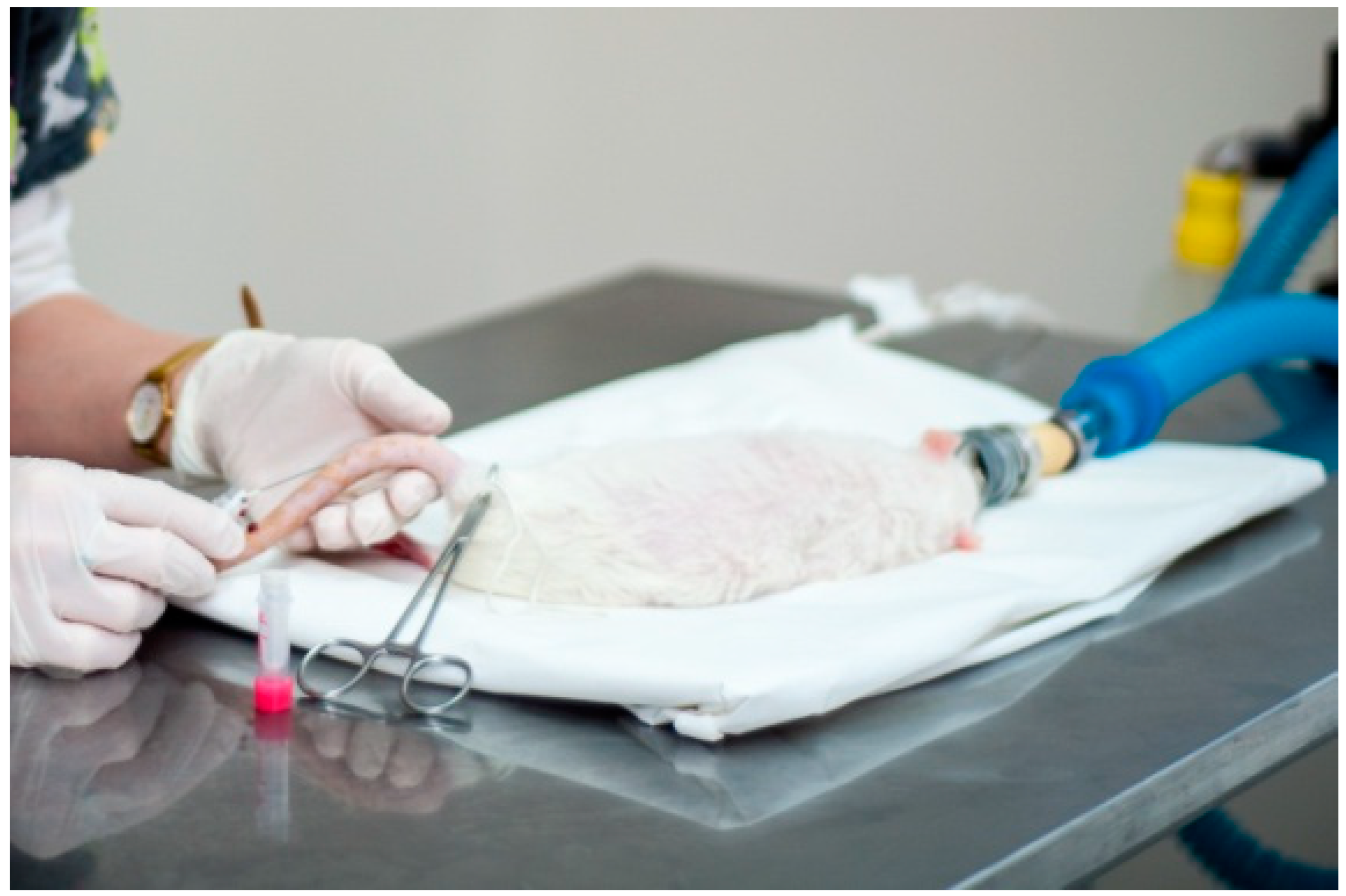
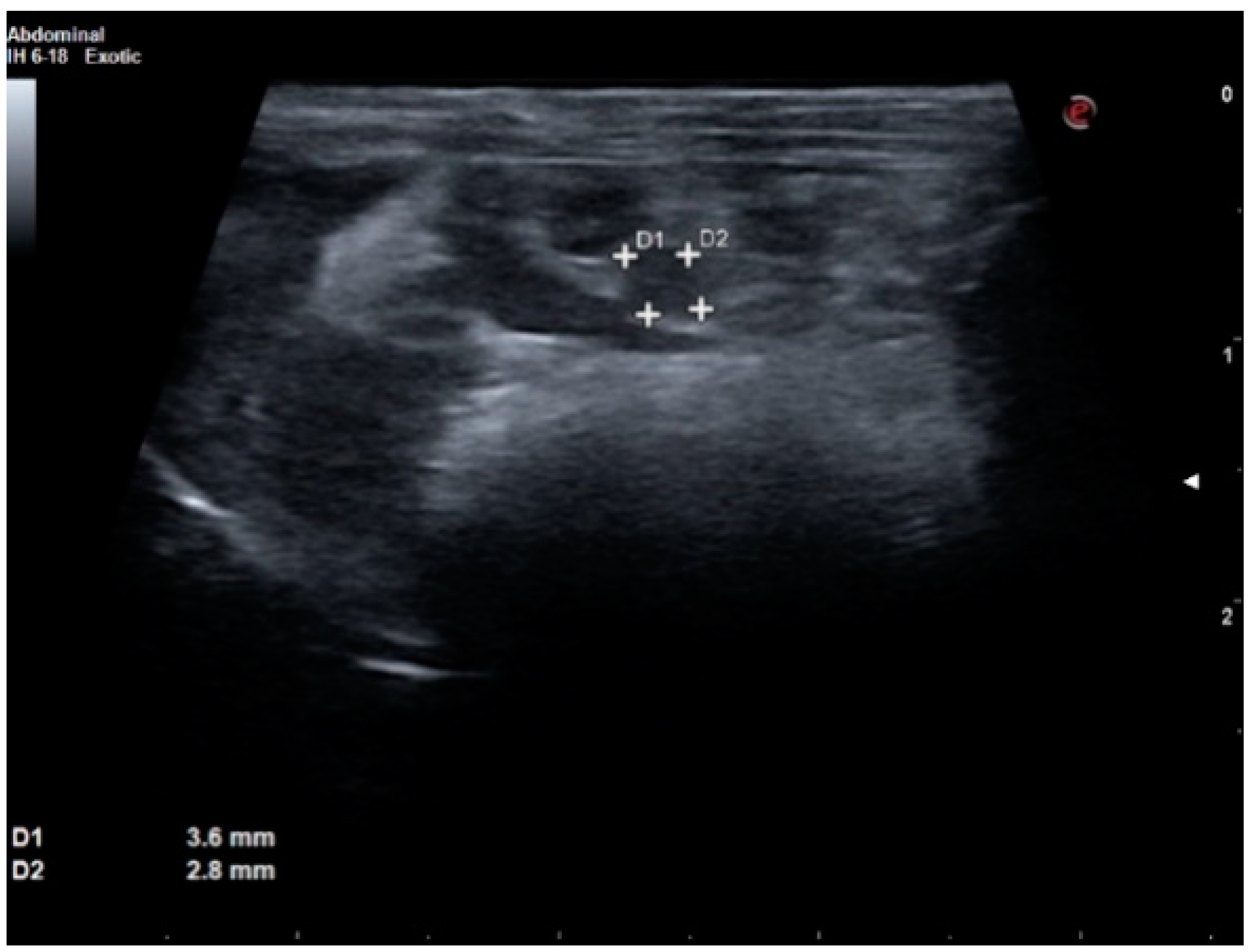
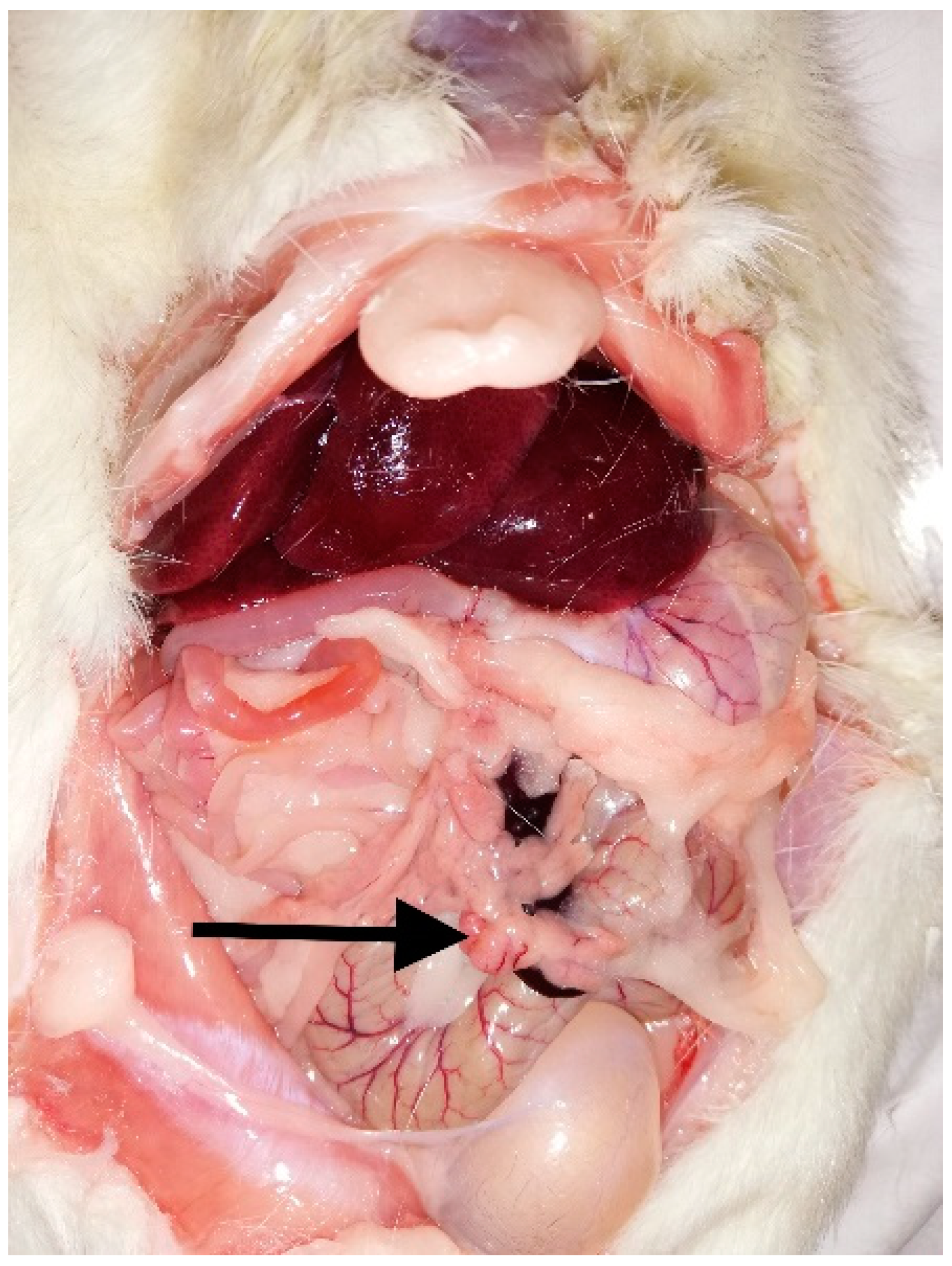
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S. Advanced diagnostic approaches and current medical management of insulinomas and adrenocortical disease in ferrets (Mustela putorius furo). Vet. Clin. North Am. Exot. Anim. Pract. 2010, 13, 439–452. [Google Scholar] [CrossRef]
- Oglesbee, B.L. Blackwell’s Five-Minute Veterinary Consult: Small Mammal, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 126–127, 132–134. [Google Scholar]
- Quesenberry, K.E.; Orcutt, C.J.; Mans, C.; Carpenter, J.W. Ferrets, Rabbits, and Rodents, 4th ed.; Elsevier: St. Louis, MI, USA, 2021; pp. 77–91. [Google Scholar]
- Mitchell, M.; Tully, T. Current Therapy in Exotic Pet Practice; Elsevier: London, UK, 2016; pp. 294–296. [Google Scholar]
- Agúndez, M.G.; Velasco, C.I. Case report of a guinea pig (Cavia porcellus) with a surgically treated insulinoma. J. Exot. Pet. Med. 2020, 33, 50–53. [Google Scholar] [CrossRef]
- Hess, L.R.; Ravich, M.L.; Reavill, D.R. Diagnosis and treatment of an insulinoma in a guinea pig (Cavia porcellus). J. Am. Vet. Med. Assoc. 2013, 242, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Vannevel, J.Y.; Wilcock, B. Insulinoma in 2 guinea pigs (Cavia porcellus). Can. Vet. J. 2005, 46, 339–341. [Google Scholar] [PubMed]
- Adissu, H.A.; Turner, P.V. Insulinoma and squamous cell carcinoma with peripheral polyneuropathy in an aged Sprague-Dawley rat. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 856–859. [Google Scholar] [PubMed]
- Robertson, J.; Brand, J.; Blas-Machado, U.; Cohen, E.; Mayer, J. Spontaneous pancreatic islet cell adenoma with peripheral neuropathy in a pet rat (Rattus norvegicus). J. Exot. Pet. Med. 2019, 28, 166–172. [Google Scholar] [CrossRef]
- Carpenter, J.W.; Marion, C.J. Exotic Animal Formulary, 5th ed.; Elsevier: Oxford, UK, 2017; pp. 459–493, 533–557. [Google Scholar]
- He, Q.; Su, G.; Liu, K.; Zhang, F.; Jiang, Y.; Gao, J.; Liu, L.; Jiang, Z.; Jin, M.; Xie, H. Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method. PLoS ONE 2017, 12, e0189837. [Google Scholar] [CrossRef] [PubMed]
- Chick, W.L.; Warren, S.; Chute, R.N.; Like, A.A.; Lauris, V.; Kitchen, K.C. Transplantable insulinoma in the rat. Proc. Natl. Acad. Sci. USA 1977, 74, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R.; Tan, K.S.; Bailey, C.J.; Powell, C.J.; Swanston-Flatt, S.K.; Marks, V. Effects of transplantation and resection of a radiation-induced rat insulinoma on glucose homeostasis and the endocrine pancreas. Br. J. Cancer 1987, 54, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.H.; Mølck, A.-M.; Heydenreich, A.; Jensen, K.J.; Bertelsen, L.O.; Alifrangis, L.; Andersen, L.; Søeborg, H.; Chapman, M.; Lykkesfeldt, J.; et al. Histopathological nerve and skeletal muscle changes in rats subjected to persistent insulin-induced hypoglycemia. J. Toxicol. Pathol. 2016, 29, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Yagihashi, S. Peripheral nerve pathology in rats with streptozotocin-induced insulinoma. Acta Neuropathol. 1996, 91, 616–623. [Google Scholar] [CrossRef]
- Sugimoto, K.; Baba, M.; Suda, T. Peripheral neuropathy and microangiopathy in rats with insulinoma: Association with chronic hyperinsulinemia. Diabetes Metab. Res. Rev. 2003, 19, 392–400. [Google Scholar] [CrossRef]
- Stromberg, P.C.; Wilson, F.; Capen, C.C. Immunocytochemical demonstration of insulin in spontaneous pancreatic islet cell tumors of Fisher rats. Vet. Pathol. 1983, 20, 291–297. [Google Scholar] [CrossRef]
- Bomhard, E. Frequency of spontaneous tumors in Wistar rats in 30-months studies. Exp. Toxicol. Pathol. 1992, 44, 381–392. [Google Scholar] [CrossRef]
- Walsh, K.M.; Poteracki, J. Spontaneous neoplasms in control Wistar rats. Fundam Appl. Toxicol. 1994, 22, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; Andreu, M.; Greaves, P. Neoplasia and hyperplasia of pancreatic endocrine tissue in the rat: An immunocytochemical study. Vet. Pathol. 1986, 23, 11–15. [Google Scholar] [CrossRef]
- Son, W.-C.h.; Bell, D.; Taylor, I.; Mowat, V. Profile of Early Occurring Spontaneous Tumors in Han Wistar Rats. Toxicol. Pathol. 2010, 38, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, A.; Herr, F.; Rona, G. The functional significance of a spontaneous pancreatic islet change in aged rats. Diabetologia 1968, 4, 44–47. [Google Scholar] [CrossRef][Green Version]
- Turnbull, G.J.; Lee, P.N.; Roe, F.J. Relationship of body-weight gain to longevity and to risk of development of nephropathy and neoplasia in Sprague-Dawley rats. Food Chem. Toxicol. 1985, 23, 355–361. [Google Scholar] [CrossRef]
- Keenan, K.P.; Soper, K.A.; Smith, P.F.; Ballam, G.C.; Clark, R.L. Diet, over-feeding and moderate dietary restriction in control Sprague–Dawley rats: I. Effects on spontaneous neoplasms. Toxicol. Pathol. 1995, 23, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Molon-Noblot, S.; Keenan, K.P.; Coleman, J.B.; Hoe, C.M.; Laroque, P. The effects of ad libitum overfeeding and moderate and marked dietary restriction on age-related spontaneous pancreatic islet pathology in Sprague–Dawley rats. Toxicol. Pathol. 2001, 29, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.H.; Mølck, A.-M.; Chapman, M.; Alifrangis, L.; Andersen, L.; Lykkesfeldt, J.; Bøgh, I.B. Chronic Hyperinsulinaemic Hypoglycaemia in Rats Is Accompanied by Increased Body Weight, Hyperleptinaemia, and Decreased Neuronal Glucose Transporter Levels in the Brain. Int. J. Endocrinol. 2017, 2017, 7861236. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, J.; Nukada, H. Hypoglycaemic neuropathy: Microvascular changes due to recurrent hypoglycaemic episodes in rat sciatic nerve. Brain Res. 2002, 947, 84–89. [Google Scholar] [CrossRef]
- Sidenius, P.; Jakobsen, J. Peripheral neuropathy in rats induced by insulin treatment. Diabetes 1983, 32, 383–386. [Google Scholar] [CrossRef][Green Version]
- Bradley, A.E.; Bolon, B.; Butt, M.T.; Cramer, S.D.; Czasch, S.; Garman, R.H.; George, C.; Gröters, S.; Kaufmann, W.; Kovi, R.C.; et al. Proliferative and Nonproliferative Lesions of the Rat and Mouse Central and Peripheral Nervous Systems: New and Revised INHAND Terms. Toxicol. Path. 2020, 48, 827–844. [Google Scholar] [CrossRef]
- Ettinger, S.J.; Feldman, E.C.; Cote, E. Textbook of Veterinary Internal Medicine Expert Consult, 8th ed.; Elsevier: St. Louis, MI, USA, 2010; pp. 1762–1767. [Google Scholar]
- Alan, I.S.; Alan, B. Side effects of glucocorticoids. In Pharmacokinetics and Adverse Effects of Drugs-Mechanisms and Risk Factors; Intechopen: London, UK, 2018; pp. 93–124. [Google Scholar] [CrossRef]
- Christensson, C.; Thoren, A.; Lindberg, B. Safety of inhaled budesonide: Clinical manifestations of systemic corticosteroid-related adverse effects. Drug Saf. 2008, 31, 965–988. [Google Scholar] [CrossRef]
- Schmid, H.A.; Brueggen, J. Effects of somatostatin analogs on glucose homeostasis in rats. J. Endocrinol. 2012, 212, 49–60. [Google Scholar] [CrossRef]
| Parameter | Blood Results before Starting Treatment with Dexamethasone | Blood Results after 5 Weeks of Taking Dexamethasone | Reference Range According to Carpenter [10] and He et al. [11] * |
|---|---|---|---|
| RBC | 8.35 T/l | 8.42 T/l | 7–8 T/l |
| Ht | 43.2% | 48.1% | 35–45% |
| Hb | 15.4 g/dL | 16.5 g/dL | 12–18 g/dL |
| MCV | 51.7 fL | 57.1 fL | 55.21–64.8 fL * |
| MCH | 18.4 pg | 19.6 pg | 18.7–21.2 pg * |
| MCHC | 35.6 g/dL | 34.3 g/dL | 31.8–34.7 g/dL * |
| WBC | 3.11 G/L | 2.71 G/L | 5–23 G/L |
| neutrophils | 1.12 G/L | 1.98 G/L | 10–50% |
| lymphocytes | 1.58 G/L | 0.5 G/L | 50–70% |
| monocytes | 0.357 G/L | 0.220 G/L | 0–10% |
| eosinophils | 0.03 G/L | 0.003 G/L | 0–5% |
| basophils | 0.01 G/L | 0.003 G/L | 0–1% |
| PLT | 647 G/L | 465 G/L | 840–1240 G/L |
| ALT (GPT) | 32.0 U/L | 77.4 U/L | 20–92 U/L |
| AST (GOT) | 135.6 U/L | 186.6 U/L | |
| alpha-Amylase | 534.2 U/L | 488.9 U/L | |
| TP | 65.1 g/L | 73.7 g/L | 56–76 g/L |
| CK | 549 U/L | 382.0 U/L | |
| GLDH | 14.4 U/L | 98.6 U/L | |
| creatinine | 0.64 mg/dL | 0.81 mg/dL | 0.2–0.8 mg/dL |
| BUN | 27.38 mg/dL | 58.37 mg/dL | 15–21 mg/dL |
| glucose | 20.88 mg/dL | 94.5 mg/dL | 50–135 mg/dL |
| K+ | 5.57 mmol/L | 5.9 mmol/L | |
| Na+ | 146.5 mmol/L | 135–155 mmol/L | |
| Cl- | 104.7 mmol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewska, A.; Barszcz, K.; Orzechowska, A.; Małek-Sanigórska, A. New Insight on Insulinoma Treatment in a Pet Rat—A Case Report. Animals 2022, 12, 2783. https://doi.org/10.3390/ani12202783
Godlewska A, Barszcz K, Orzechowska A, Małek-Sanigórska A. New Insight on Insulinoma Treatment in a Pet Rat—A Case Report. Animals. 2022; 12(20):2783. https://doi.org/10.3390/ani12202783
Chicago/Turabian StyleGodlewska, Agata, Karolina Barszcz, Aleksandra Orzechowska, and Aleksandra Małek-Sanigórska. 2022. "New Insight on Insulinoma Treatment in a Pet Rat—A Case Report" Animals 12, no. 20: 2783. https://doi.org/10.3390/ani12202783
APA StyleGodlewska, A., Barszcz, K., Orzechowska, A., & Małek-Sanigórska, A. (2022). New Insight on Insulinoma Treatment in a Pet Rat—A Case Report. Animals, 12(20), 2783. https://doi.org/10.3390/ani12202783





