Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database of Vespa velutina Nests Detected in Galicia
2.2. Nests Handling Procedures
2.3. Nest’s Structure Analysis
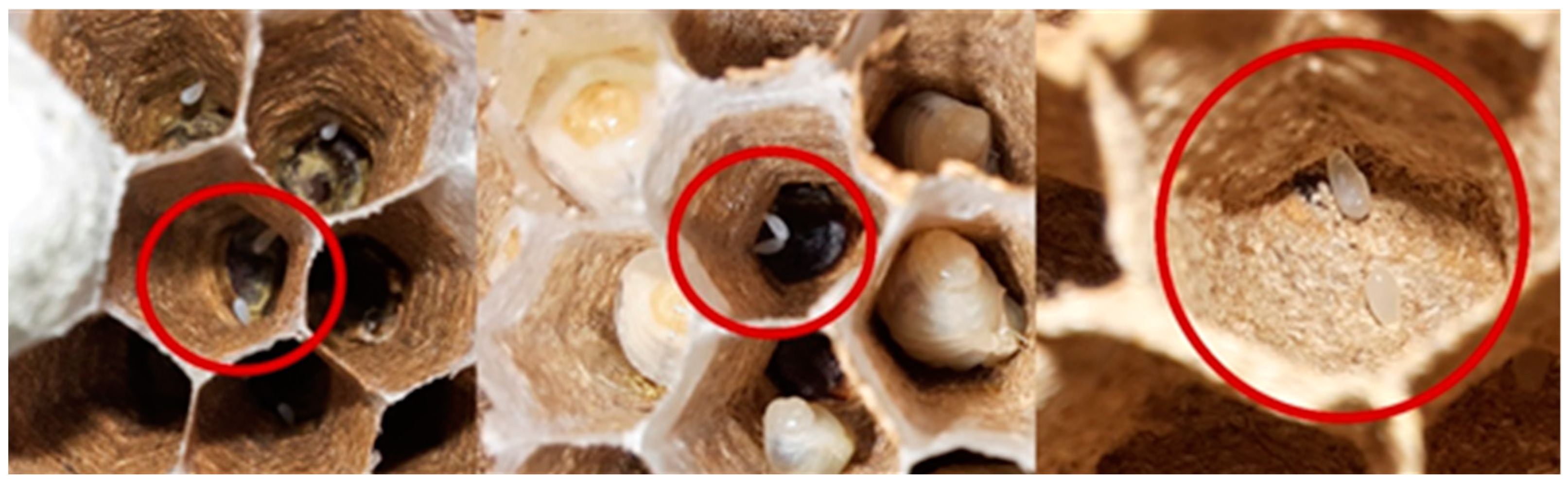
2.4. Nest Population
2.5. Statistical Analyses
3. Results
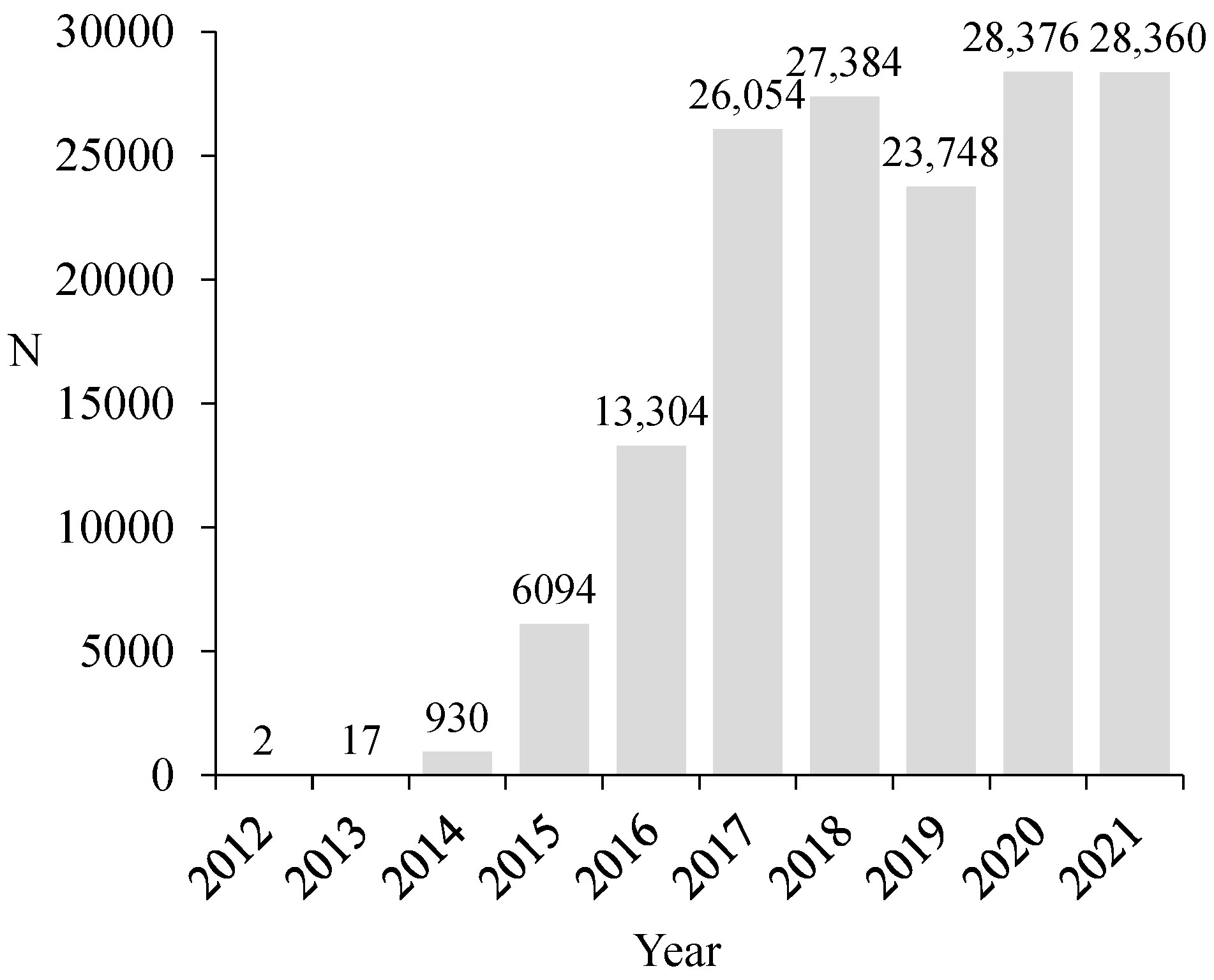
3.1. Vespa velutina Nest Locations in Galicia
3.2. Nesting Structure: Characteristics of Embryo Nests
3.3. Nesting Structure: Characteristics of Relocation Nests
3.4. Nesting Structure: Characteristics of Secondary Nests
3.5. Nesting Population Characteristics of the Different Types of Nests
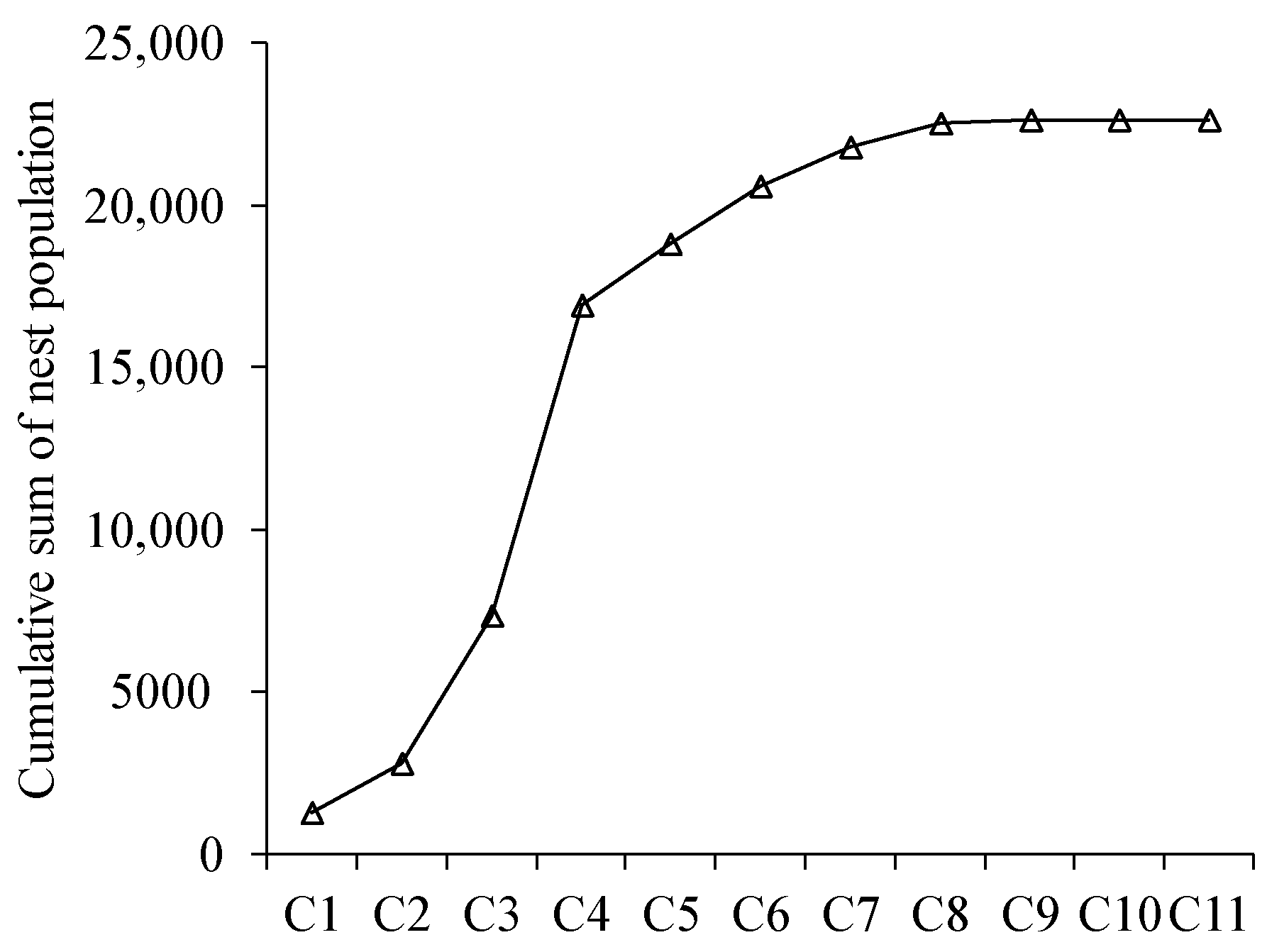
3.6. Estimation of the Population by Meconium Content
4. Discussion
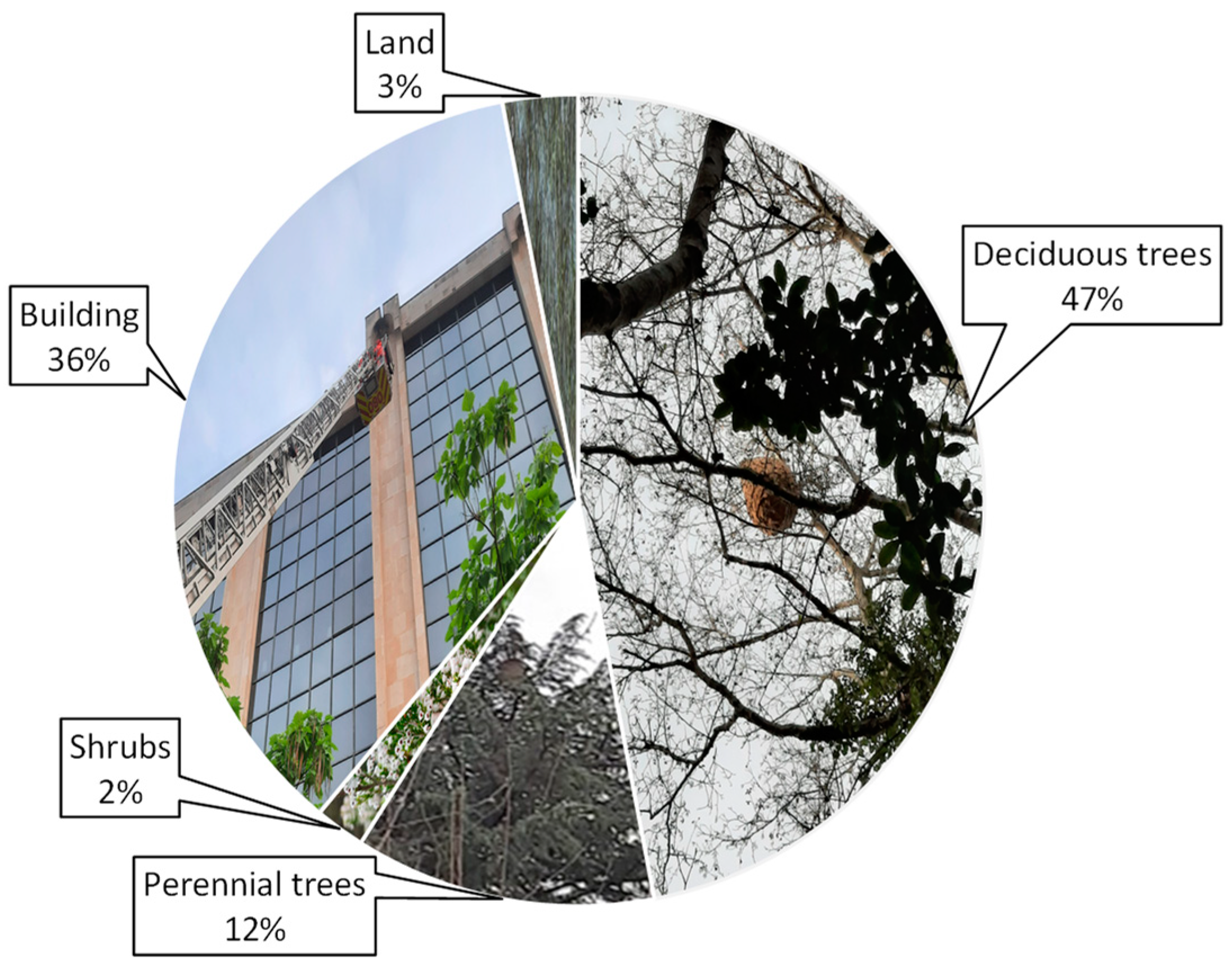
4.1. Nesting Habitat
4.2. Life Cycle Dynamics and Nest Type
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Arca, M. Caractérisation génétique et étude comportementale d’une espèce envahissante en France: Vespa velutina Lepeletier (Hymenoptera, Vespidae). Ph.D. Dissertation, Université Pierre et Marie Curie, Paris, France, 2012. [Google Scholar]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Seijo-Rodríguez, A.; Escuredo, O.; Seijo-Coello, M.d.C. Spreading of Vespa velutina in northwestern Spain: Influence of elevation and meteorological factors and effect of bait trapping on target and non-target living organisms. J. Pest Sci. 2019, 92, 557–565. [Google Scholar] [CrossRef]
- Rojas-Nossa, S.V.; Gil, N.; Mato, S.; Garrido, J. Vespa velutina: Características e impactos de una exitosa especie exótica invasora. ECOS 2021, 30, 1–10. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, L. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 8–11. [Google Scholar] [CrossRef]
- Arca, M.; Mougel, F.; Guillemaud, T.; Dupas, S.; Rome, Q.; Perrard, A.; Muller, F.; Fossoud, A.; Capdevielle-Dulac, C.; Torres-Leguizamon, M.; et al. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol. Invasions 2015, 17, 2357–2371. [Google Scholar] [CrossRef]
- Poidatz, J.; Bressac, C.; Bonnard, O.; Thiéry, D. Delayed sexual maturity in males of Vespa velutina. Insect Sci. 2018, 25, 679–689. [Google Scholar] [CrossRef]
- Prezoto, F.; Nascimento, F.S.; Barbosa, B.C.; Somavilla, A. Neotropical Social Wasps; Springer: Cham, Brazil, 2021; Volume 10, pp. 320–326. [Google Scholar]
- Castro, L.C.; Pagola-Carte, S. Vespa velutina Lepeletier, 1836 (Hymenoptera: Vespidae), recolectada en la Península Ibérica. Heteropterus Rev. Entomol. 2010, 10, 193–196. [Google Scholar]
- Goldarazena, A.; Gonzalez, M.; Heredia, G. De Spread of the yellow-legged hornet Vespa velutina nigrithorax du Buysson (Hymenoptera: Vespidae) across Northern. Bulletin OEPP/EPPO 2015, 45, 133–138. [Google Scholar] [CrossRef]
- Beggs, J.R.; Brockerhoff, E.G.; Corley, J.C.; Kenis, M.; Masciocchi, M.; Muller, F.; Rome, Q.; Villemant, C. Ecological effects and management of invasive alien Vespidae. BioControl 2011, 56, 505–526. [Google Scholar] [CrossRef]
- Villemant, C.; Barbet-Massin, M.; Perrard, A.; Muller, F.; Gargominy, O.; Jiguet, F.; Rome, Q. Predicting the invasion risk by the alien bee-hawking Yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biol. Conserv. 2011, 144, 2142–2150. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Muller, F.; Perrard, A.; Villemant, C.; Jiguet, F. Climate change increases the risk of invasion by the Yellow-legged hornet. Biol. Conserv. 2013, 157, 4–10. [Google Scholar] [CrossRef]
- Diéguez-Antón, A.; Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C. Monitoring study in honey bee colonies stressed by the invasive hornet Vespa velutina. Vet. Sci. 2022, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Salles, J.M.; Courchamp, F. The economic cost of control of the invasive yellow-legged Asian hornet. NeoBiota 2020, 55, 11–25. [Google Scholar] [CrossRef]
- Choi, M.B.; Martin, S.J.; Lee, J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia Pac. Entomol. 2012, 15, 473–477. [Google Scholar] [CrossRef]
- Ikegami, M.; Tsujii, K.; Ishizuka, A.; Nakagawa, N.; Kishi, S.; Sakamoto, Y.; Sakamoto, H.; Goka, K. Environments, spatial structures, and species competitions: Determining the impact of yellow-legged hornets, Vespa velutina, on native wasps and bees on Tsushima Island, Japan. Biol. Invasions 2020, 22, 3131–3143. [Google Scholar] [CrossRef]
- Rojas-Nossa, S.V.; Calviño-Cancela, M. The invasive hornet Vespa velutina affects pollination of a wild plant through changes in abundance and behaviour of floral visitors. Biol. Invasions 2020, 22, 2609–2618. [Google Scholar] [CrossRef]
- Rome, Q.; Perrard, A.; Muller, F.; Fontaine, C.; Quilès, A.; Zuccon, D.; Villemant, C. Not just honeybees: Predatory habits of Vespa velutina (Hymenoptera: Vespidae) in France. Ann. De La Société Entomol. De Fr. 2021, 57, 1–11. [Google Scholar] [CrossRef]
- Choi, M.B. Foraging behavior of an invasive alien hornet (Vespa velutina) at Apis mellifera hives in Korea: Foraging duration and success rate. Entomol. Res. 2021, 51, 143–148. [Google Scholar] [CrossRef]
- Mazzei, M.; Forzan, M.; Cilia, G.; Sagona, S.; Bortolotti, L.; Felicioli, A. First detection of replicative deformed wing virus (DWV) in Vespa velutina nigrithorax. Bull. Insectology 2018, 71, 211–216. [Google Scholar]
- Marzoli, F.; Forzan, M.; Bortolotti, L.; Pacini, M.I.; Rodríguez-Flores, M.S.; Felicioli, A.; Mazzei, M. Next generation sequencing study on RNA viruses of Vespa velutina and Apis mellifera sharing the same foraging area. Transbound. Emerg. Dis. 2021, 68, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Fedele, E.; Gervasini, E.; Cardoso, A.C.; La Notte, A.; Vallecillo, S.; Tsiamis, K.; Maes, J. Invasive Alien Species Impact on Ecosystem Services. Asian Hornet (Vespa Velutina Nigrithorax Case Study); Technical Reports; Publications Office, European Union: Luxembourg, 2019.
- de Haro, L.; Labadie, M.; Chanseau, P.; Cabot, C.; Blanc-Brisset, I.; Penouil, F. Medical consequences of the Asian black hornet (Vespa velutina) invasion in Southwestern France. Toxicon 2010, 55, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Laborde-castérot, H.; Darrouzet, É.; Roux, G.L.; Labadie, M.; Delcourt, N.; Haro, L.D.; Vodovar, D.; Langrand, J.; Laborde-castérot, H.; Darrouzet, É.; et al. Ocular lesions other than stings following yellow-legged hornet (Vespa velutina nigrithorax) projections, as reported to French poison control centers. JAMA Ophthalmol. 2020, 139, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J. The Asian Hornet (Vespa velutina)-Threats, Biology & Expansion; International Bee Research Association (IBRA) & Northern Bee Books: Bristol, UK, 2017. [Google Scholar]
- Van Itterbeeck, J.; Feng, Y.; Zhao, M.; Wang, C.; Tan, K.; Saga, T.; Nonaka, K.; Jung, C. Rearing techniques for hornets with emphasis on Vespa velutina (Hymenoptera: Vespidae): A review. J. Asia. Pac. Entomol. 2021, 24, 103–117. [Google Scholar] [CrossRef]
- Rome, Q.; Muller, F.J.; Touret-Alby, A.; Darrouzet, E.; Perrard, A.; Villemant, C. Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. J. Appl. Entomol. 2015, 139, 771–782. [Google Scholar] [CrossRef]
- Feás Sánchez, X.; Charles, R.J. Notes on the Nest Architecture and Colony Composition in Winter of the Yellow-Legged Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae), in Its Introduced Habitat in Galicia (NW Spain). Insects 2019, 10, 237. [Google Scholar] [CrossRef]
- Haouzi, M.; Gévar, J.; Khalil, A.; Darrouzet, E. Nest structures display specific hydrocarbon profiles: Insights into the chemical ecology of the invasive yellow-legged hornet Vespa velutina nigrithorax. Chemoecology 2021, 31, 227–238. [Google Scholar] [CrossRef]
- Crespo, N.; Louzada, J.; Fernandes, L.S.; Tavares, P.B.; Aranha, J. Microscopic Identification of Anatomical Elements and Chemical Analysis of Secondary Nests of Vespa velutina nigrithorax du Buysson. Insects 2022, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.E. Taxonomy, distribution and nesting biology of species of the genera Provespa Ashmead and Vespa Linneaus (Hymenoptera, Vespidae). Entomol. Mon. Mag. 2008, 144, 69–101. [Google Scholar]
- Archer, M.E. The queen colony phase of vespine wasps (Hymenoptera, Vespidae). Insectes Soc. 2010, 57, 133–145. [Google Scholar] [CrossRef]
- Bessa, A.S.; Carvalho, J.; Gomes, A.; Santarém, F. Climate and land-use drivers of invasion: Predicting the expansion of Vespa velutina nigrithorax into the Iberian Peninsula. Insect Conserv. Divers. 2016, 9, 27–37. [Google Scholar] [CrossRef]
- Monceau, K.; Thiéry, D. Vespa velutina nest distribution at a local scale: An 8-year survey of the invasive honeybee predator. Insect Sci. 2017, 24, 663–674. [Google Scholar] [CrossRef]
- Robinet, C.; Suppo, C.; Darrouzet, E. Rapid spread of the invasive yellow-legged hornet in France: The role of human-mediated dispersal and the effects of control measures. J. Appl. Ecol. 2017, 54, 205–215. [Google Scholar] [CrossRef]
- Carvalho, J.; Hipólito, D.; Santarém, F.; Martins, R.; Gomes, A.; Carmo, P.; Rodrigues, R.; Grosso-Silva, J.; Fonseca, C. Patterns of Vespa velutina invasion in Portugal using crowdsourced data. Insect Conserv. Divers. 2020, 13, 501–507. [Google Scholar] [CrossRef]
- Yamane, S. On the collection technique of vespine nests, based chiefly on practices through a survey in Taiwan from 1972 to 1974 (Hymenoptera: Vespidae). Seibutsu Kyozai 1977, 12, 42–59. [Google Scholar]
- Nakamura, M.; Sonthichai, S. Nesting habits of some hornet species (Hymenoptera, Vespidae) in Northern Thailand. Kasetsart J. Nat. Sci. 2004, 38, 196. [Google Scholar]
- Nadolski, J. Structure of nests and colony sizes of the European Hornet (Vespa crabro) and Saxon wasp (Dolichovespula saxonica) (Hymenoptera: Vespinae) in urban conditions. Sociobiology 2012, 59, 1075–1120. [Google Scholar] [CrossRef]
- Ganor, E.; Barenholz–Paniry, V.; Ishay, J.S. Cell wall structure and composition in the comb built by hornets and wasps (Vespinae, Hymenoptera). J. Morphol. 1986, 189, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö. Determination of some structural features of the nest paper materials of Dolichovespula saxonica Fabricius, 1793 (Hymenoptera: Vespinae) in Turkey. Entomol. Res. 2017, 47, 286–294. [Google Scholar] [CrossRef]
- Barbosa, B.C.; Maciel, T.T.; Gonzaga, D.R.; Prezoto, F. Social wasps in an urban fragment: Seasonality and selection of nesting substrates. J. Nat. Hist. 2020, 54, 1581–1591. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Ford, S.M.; Poidatz, J.; Thiéry, D.; Osborne, J.L. Searching for nests of the invasive Asian hornet (Vespa velutina) using radio-telemetry. Commun. Biol. 2018, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lioy, S.; Bianchi, E.; Biglia, A.; Bessone, M.; Laurino, D.; Porporato, M. Viability of thermal imaging in detecting nests of the invasive hornet Vespa velutina. Insect Sci. 2021, 28, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Darrouzet, E.; Gévar, J.; Guignard, Q.; Aron, S. Production of early diploid males by European colonies of the invasive hornet Vespa velutina nigrithorax. PLoS ONE 2015, 10, e0136680. [Google Scholar] [CrossRef]
- Dazhi, D.; Yunzhen, W. A preliminary study on the biology of wasps Vespa velutina auraria Smith and Vespa tropica ducalis Smith. Zool. Res. 1989, 10, 162–163. [Google Scholar]
- Kumano, N.; Kasuya, E. An alternative strategy for maintenance of eusociality after nest destruction: New nest construction in a primitively eusocial wasp. Insectes Soc. 2006, 53, 149–155. [Google Scholar] [CrossRef]
- Quaresma, A.; Henriques, D.; Godinho, J.; Maside, X.; Bortolotti, L.; Pinto, M.A. Invasion genetics of the Asian hornet Vespa velutina nigrithorax in Southern Europe. Biol. Invasions 2022, 24, 1479–1494. [Google Scholar] [CrossRef]
- Van der Vecht, J. The Vespinae of the Indo-Malayan and Papuan areas (Hymenoptera: Vespidae). Zool. Verh. 1957, 34, 1–82. [Google Scholar]
- Herrera, C.; Maqués, A.; Colomar, V.; Leza, M.M. Analysis of the secondary nest of the yellow-legged hornet found in the Balearic Islands reveals its high adaptability to Mediterranean isolated ecosystems. Isl. Invasives: Scaling Up Meet Chall. 2019, 62, 375–380. [Google Scholar]
- Kuo, M.; Yeh, W. Ecological studies on Vespa basalis Smith, Vespa velutina flavitarsus Sonan and Tropica pseudosoror Vecht. (Study on Vespidae in Taiwan II). J. Natl. Chiayi Inst. Agric. 1985, 11, 95–106. [Google Scholar]
- Taylor, B.A.; Cini, A.; Cervo, R.; Reuter, M.; Sumner, S. Queen succession conflict in the paper wasp Polistes dominula is mitigated by age-based convention. Behav. Ecol. 2020, 31, 992–1002. [Google Scholar] [CrossRef]
- Cappa, F.; Cini, A.; Pepiciello, I.; Petrocelli, I.; Inghilesi, A.F.; Anfora, G.; Dani, F.R.; Bortolotti, L.; Wen, P.; Cervo, R. Female volatiles as sex attractants in the invasive population of Vespa velutina nigrithorax. J. Insect Physiol. 2019, 119, 103952. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Berlín, Germany, 1990. [Google Scholar]
- Ishay, J. Observations sur la biologie de la guêpe orientale Vespa orientalis F. Ins. Soc. 1964, 11, 193–206. [Google Scholar] [CrossRef]




| Nests Type | Season | N | Perimeter (cm) | Height (cm) | Combs | Nest Cells | Cells Area (cm2) |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Min-Max | Mean ± SD | Mean ± SD | |||
| Embryo | Spring | 62 | 21.8 ± 4.8 | 7.6 ± 1.4 | 1–2 | 45 ± 33 | 0.31 ± 0.05 |
| Secondary | Spring | 7 | 43 ± 11.6 a | 12.8 ± 3.2 a | 2–4 | 575 ± 376 a | 0.39 ± 0.06 a |
| Summer | 11 | 61.5 ± 26.8 a | 17.9 ± 7.1 a | 2–7 | 942 ± 1353 a | 0.47 ± 0.07 ab | |
| Autumn | 15 | 116.7 ± 27.9 b | 46.5 ± 16.5 b | 3–11 | 8043 ± 5920 b | 0.54 ± 0.06 bc | |
| Winter | 11 | 113.5 ± 11.1 b | 56.2 ± 26.6 b | 5–11 | 6593 ± 4005 b | 0.56 ± 0.03 c | |
| All | 44 | 94 ± 37 | 37.6 ± 23.5 | 2–11 | 4805 ± 5199 | 0.51 ± 0.08 |
| Nests type | Season | N | Larvae | Eggs | Hornets Emerged from Meconium | Nest Population |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Embryo | Spring | 62 | 14 ± 12 | 12 ± 11 | 17 ± 20 | 32 ± 28 |
| Secondary | Spring | 7 | 158 ± 129 a | 112 ± 88 a | 369 ± 193 a | 85 ± 165 a |
| Summer | 11 | 356 ± 505 a | 109 ± 125 a | 1347 ± 1135 ab | 1501± 1107 ab | |
| Autumn | 15 | 473 ± 416 a | 514 ± 10 b | 12,599 ± 10,479 c | 13,224 ± 10,561 b | |
| Winter | 11 | 298 ± 429 a | 101 ± 71 a | 12,145 ± 5984 bc | 12,255 ± 6040 ab | |
| All | 44 | 351 ± 407 | 162 ± 163 | 7643 ± 8746 | 8227 ± 8923 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diéguez-Antón, A.; Escuredo, O.; Seijo, M.C.; Rodríguez-Flores, M.S. Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain). Animals 2022, 12, 2781. https://doi.org/10.3390/ani12202781
Diéguez-Antón A, Escuredo O, Seijo MC, Rodríguez-Flores MS. Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain). Animals. 2022; 12(20):2781. https://doi.org/10.3390/ani12202781
Chicago/Turabian StyleDiéguez-Antón, Ana, Olga Escuredo, María Carmen Seijo, and María Shantal Rodríguez-Flores. 2022. "Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain)" Animals 12, no. 20: 2781. https://doi.org/10.3390/ani12202781
APA StyleDiéguez-Antón, A., Escuredo, O., Seijo, M. C., & Rodríguez-Flores, M. S. (2022). Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain). Animals, 12(20), 2781. https://doi.org/10.3390/ani12202781









