Modification of Morphology and Glycan Pattern of the Oviductal Epithelium of Baboon Papio hamadryas during the Menstrual Cycle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Evaluation of the Stage of the Menstrual Cycle
2.3. Hormonal Assays
2.4. Vaginal Cytology
2.5. Sampling and Tissue Preparation for Histological Analysis
2.6. Lectin Histochemistry
2.7. Sialidase Treatment
2.8. Morphometrical Analysis
2.9. Statistical Analysis
3. Results
3.1. Steroid Hormones
3.2. Vaginal Cytology
3.3. Histological Analysis
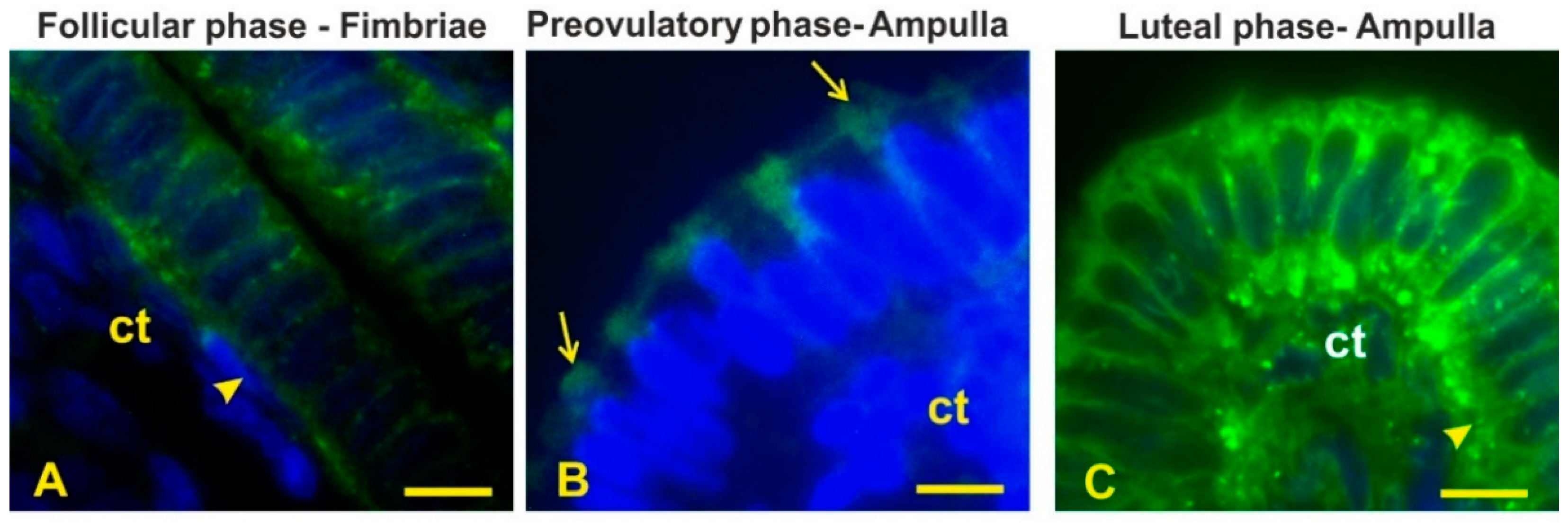
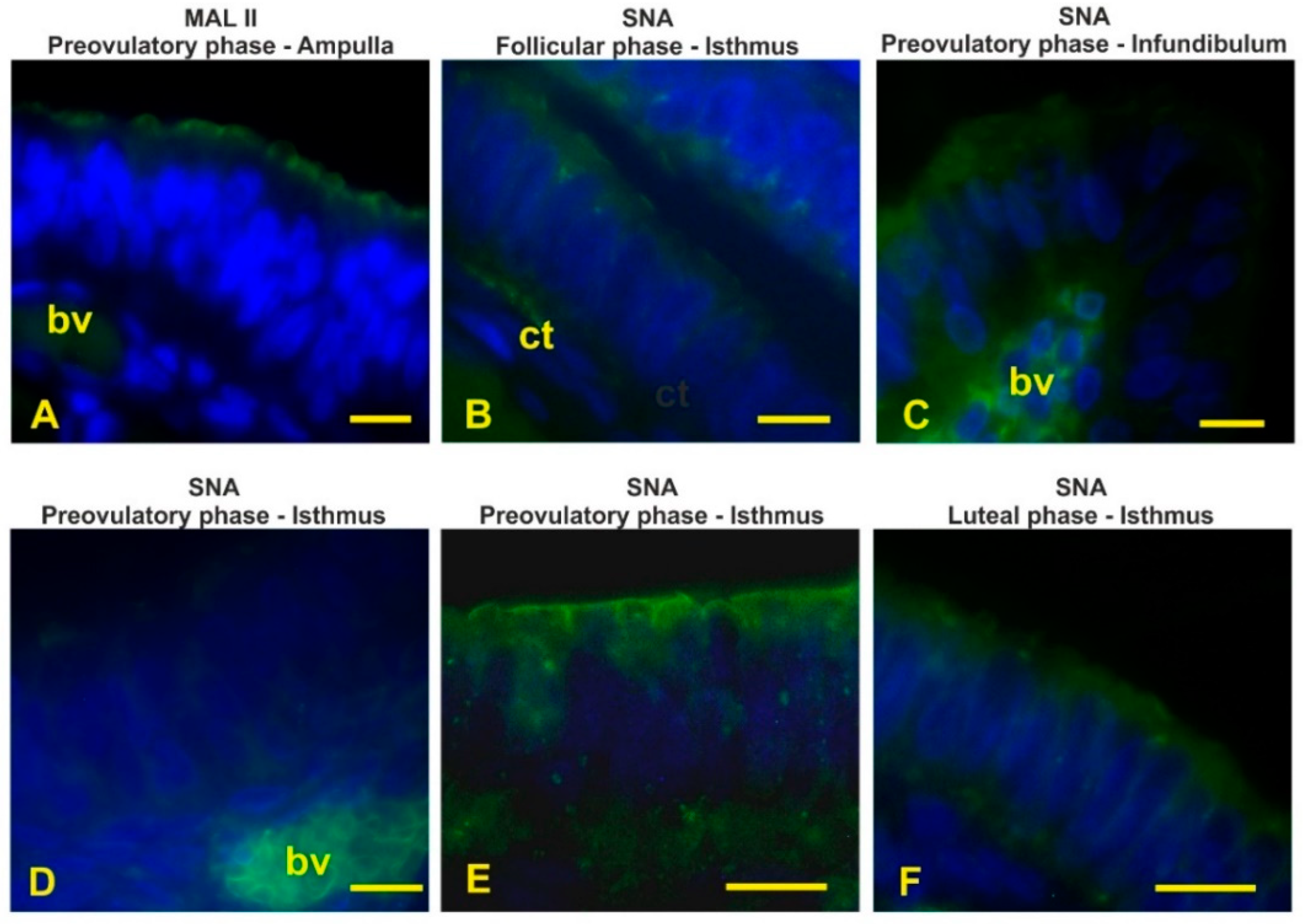
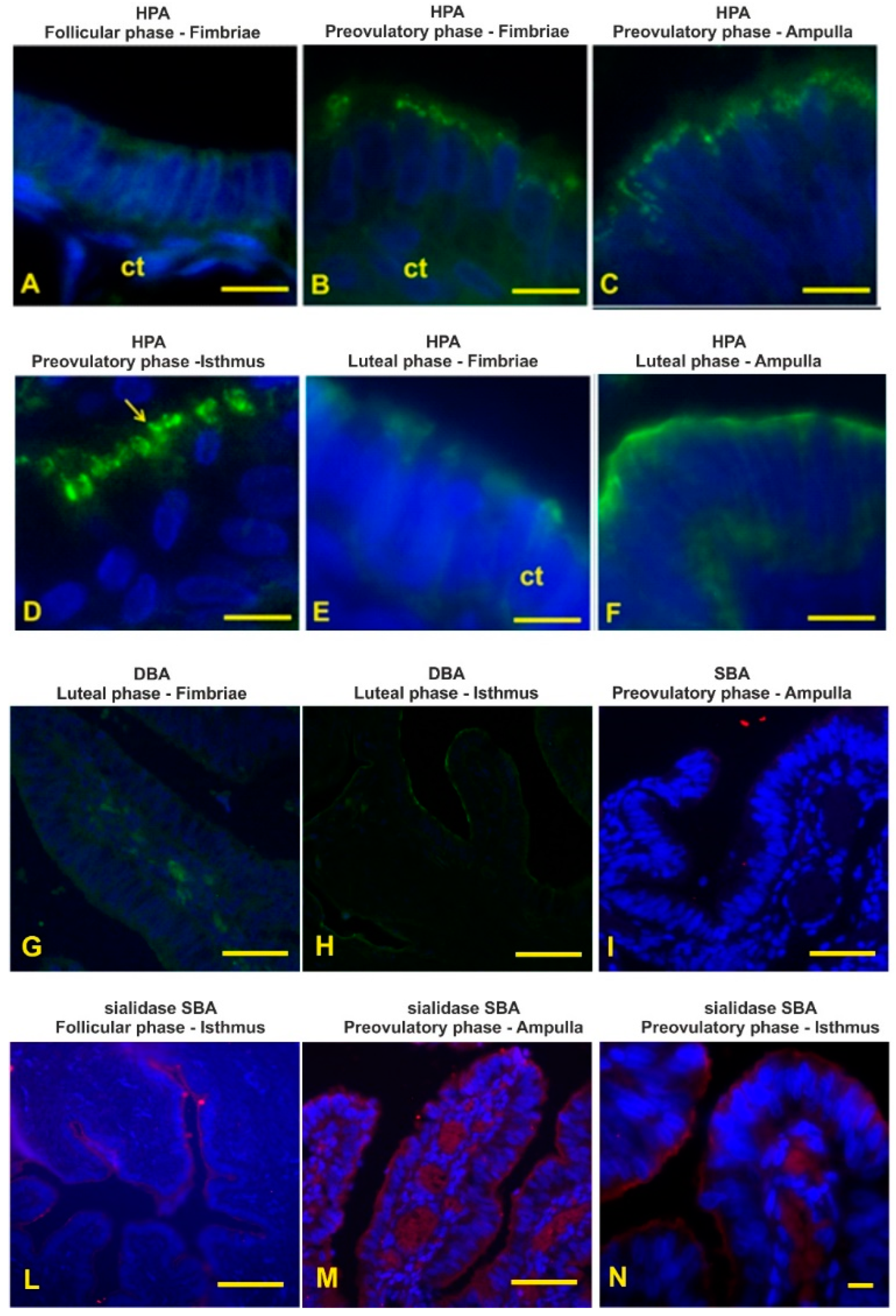
3.4. Lectin Histochemistry
3.5. High-Mannose Glycans
3.6. Sialoglycans
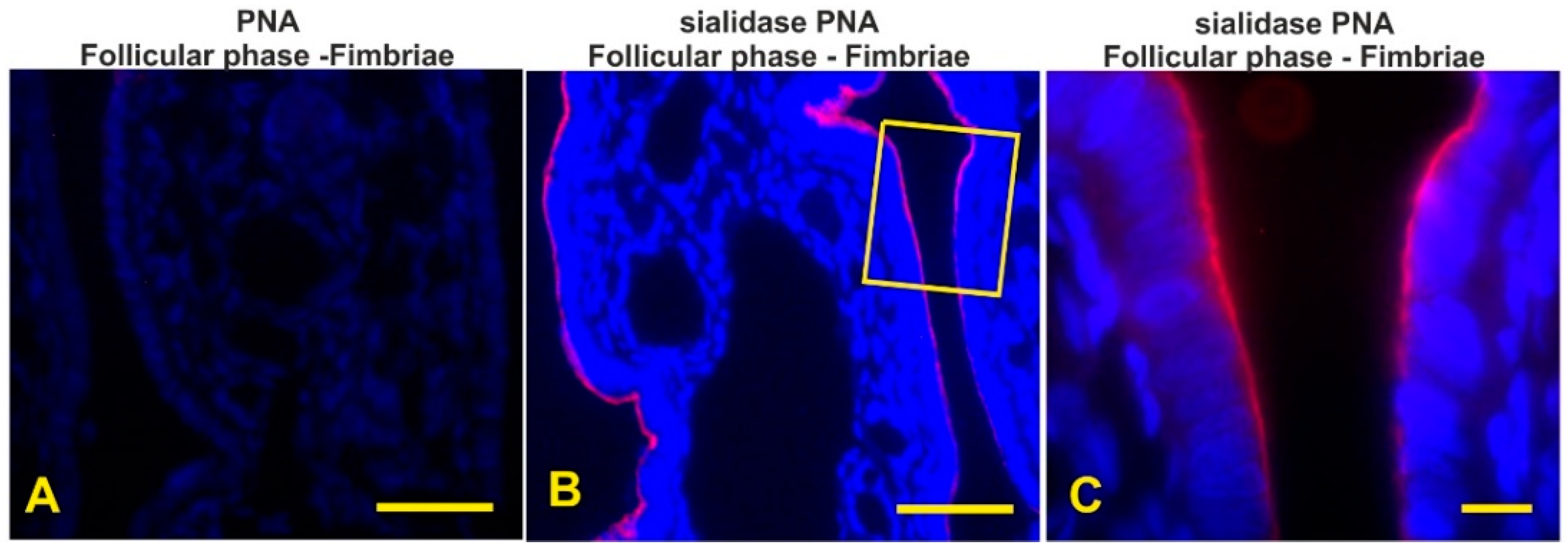
3.7. Detection of the Terminal and Sialic Acid Penultimate T-Antigen with PNA and KOH-Sialidase (s)PNA
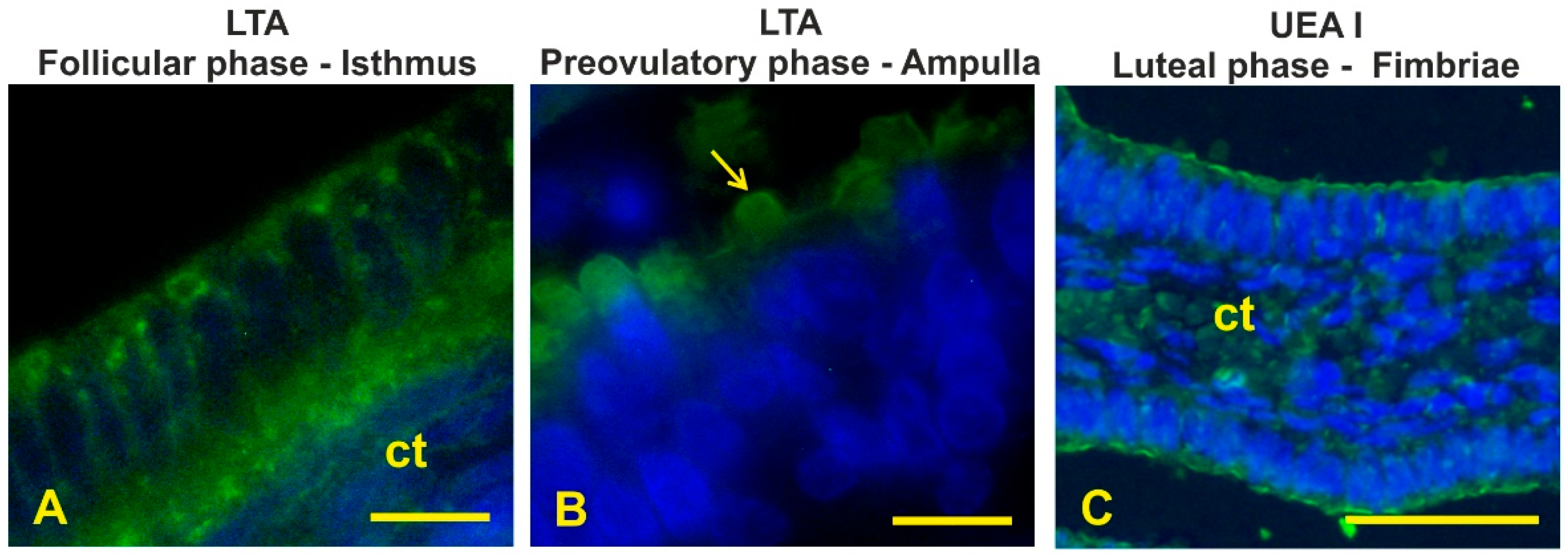
3.8. Fucosylated Glycans
3.9. GalNAc-Terminating Glycans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef]
- Miller, D.J. Sperm movement, storage, and release from the oviduct. J. Anim. Sci. 2020, 98, 188. [Google Scholar] [CrossRef]
- Verhage, H.G.; Bareither, M.L.; Jaffe, R.C.; Akbar, M. Cyclic changes in ciliation, secretion, and cell height of the oviduct epithelium in women. Am. J. Anat. 1979, 156, 505–522. [Google Scholar] [CrossRef]
- Brenner, R.M.; Carlisle, K.S.; Hess, D.L.; Sandow, B.A.; West, N.B. Morphology of the oviduct and endometria of cynomolgus macaques during the menstrual cycle. Biol. Reprod. 1983, 29, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.M.; Maslar, I.A. The primate oviduct and endometrium. In The Physiology of Reproduction; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1988; pp. 303–329. [Google Scholar]
- Aguilar, J.; Reyley, M. The uterine tubal fluid: Secretion, composition and biological effects. Anim. Reprod. 2005, 2, 91–105. [Google Scholar]
- Ashraf, H.; Siddiqui, A.M.; Rana, M.A. Fallopian tube analysis of the peristaltic-ciliary flow of third grade fluid in a finite narrow tube. Chin. J. Phys. 2018, 56, 605–621. [Google Scholar]
- Coy, P.; Yanagimachi, R. The common and species-specific roles of oviductal proteins in mammalian fertilization and embryo development. BioScience 2015, 65, 973–984. [Google Scholar] [CrossRef]
- Li, S.; Winuhayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [PubMed]
- Verhage, H.G.; Fazleabas, A.T. The in vitro synthesis of estrogen-dependent proteins by the baboon (Papio anubis) oviduct. Endocrinology 1988, 123, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Verhage, H.G.; Fazleabas, A.T.; Mavrogianis, P.A.; Day-Bowman, M.B.; Donnelly, K.M.; Arias, E.B.; Jaffe, R.C. The baboon oviduct: Characteristics of an oestradiol-dependent oviduct-specific glycoprotein. Hum. Reprod. Update 1997, 3, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E.; Herrera, G.G.; Anamthathmakula, P.; Rock, J.K.; Willie, A.M.; Harris, E.A.; Takemaru, K.-I.; Winuthayanon, W. Roles of steroid hormones in oviductal function. Reproduction 2020, 159, R125–R137. [Google Scholar] [CrossRef] [PubMed]
- Moros-Nicolás, C.; Fouchécourt, S.; Goudet, G.; Monget, P. Genes encoding mammalian oviductal proteins involved in fertilization are subjected to gene death and positive selection. J. Mol. Evol. 2018, 86, 655–667. [Google Scholar] [CrossRef]
- Williams-Blangero, S.; Vandeberg, J.S.; Blangero, J.; Konigsberg, L.; Dyke, B. Genetic differentiation between baboon subspecies: Relevance for biomedical research. Am. J. Primatol. 1990, 20, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kopp, G.H.; Roos, C.; Thomas, M.; Butynski, T.M.; Wildman, D.E.; Alagaili, A.N.; Groeneveld, L.F.; Zinner, D. Out of Africa, but how and when? The case of hamadryas baboons (Papio hamadryas). J. Hum. Evol. 2014, 76, 154–164. [Google Scholar] [CrossRef]
- Gippoliti, S. Papio hamadryas. The IUCN Red List of Threatened Species. 2019. Available online: https://ielc.libguides.com/sdzg/factsheets/hamadryasbaboon/bibliography (accessed on 1 December 2021).
- Goffe, A.S.; Zinner, D.; Fischer, J. Sex and friendship in a multilevel society: Behavioral patterns and associations between female and male Guinea baboons. Behav. Ecol. Sociobiol. 2016, 70, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.C.; Sparks, S.G.; Powell, J.E. Levels of estrogens, progestogens and luteinizing hormone during the menstrual cycle of the baboon. Endocrinology 1970, 87, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception 2015, 92, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R. Walker’s Mammals of the World, 6th ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 1166–1170. [Google Scholar]
- Verhage, H.G.; Mavrogianis, P.A.; Boice, M.A.; Li, W.; Fazleabas, A.T. Oviductal epithelium of the baboon: Hormonal control and the immuno-gold localization of oviduct-specific glycoproteins. Am. J. Anat. 1990, 187, 81–90. [Google Scholar] [CrossRef]
- Rapisarda, J.J.; Mavrogianis, P.A.; O’Day-Bowman, M.B.; Fazleabas, A.T.; Verhage, H.G. Immunological characterization and immunocytochemical localization of an oviduct-specific glycoprotein in the human. J. Clin. Endocrinol. Metab. 1993, 76, 1483–1488. [Google Scholar] [PubMed]
- Jaffe, R.C.; Arias, E.B.; O’Day-Bowman, M.B.; Donnelly, K.M.; Mavrogianis, P.A.; Verhage, H.G. Regional distribution and hormonal control of estrogen-dependent oviduct-specific glycoprotein messenger ribonucleic acid in the baboon (Papio anubis). Biol. Reprod. 1996, 55, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Verhage, H.G.; Fazleabas, A.T.; Donnelly, K. The in vitro synthesis and release of proteins by the human oviduct. Endocrinology 1988, 122, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Lok, I.H.; Briton-Jones, C.M.; Yuen, P.M.; Haines, C.J. Variable expression of oviductin mRNA at different stages of human reproductive cycle. J. Assist. Reprod. Genet. 2002, 19, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Buhi, W. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 2002, 123, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Aoki, K.; Doungkamchan, K.; Tiemeyer, M.; Bovin, N.; Miller, D.J. Sulfated Lewis A trisaccharide on oviduct membrane glycoproteins binds bovine sperm and lengthens sperm lifespan. J. Biol. Chem. 2019, 294, 13445–13463. [Google Scholar] [CrossRef] [PubMed]
- Desantis, S.; Accogli, G.; Silvestre, F.; Binetti, F.; Caira, M.; Lacalandra, G.M. Modifications of carbohydrate residues in the sheep oviductal ampulla after superovulation. Theriogenology 2015, 83, 943–952. [Google Scholar] [CrossRef]
- Knauf, S.; Batamuzi, E.K.; Mätz-Rensing, K.; Wehrend, A.; Leendertz, F.H. Exfoliative vaginal cytology: A diagnostic tool for sexual cycle stages in nonhuman primates. Tanz. Vet. J. 2009, 26, 1–6. [Google Scholar] [CrossRef]
- Moore, D. Hormonal cytology. J. Obst. Gynaecol. 1964, 7, 19–23. [Google Scholar]
- Desantis, S.; Acone, F.; Corriero, A.; Deflorio, M.; Zubani, D.; Ventriglia, G.; Palmieri, G.; De Metrio, G. Distribution of sialoglycoconjugates in the oviductal isthmus of the horse during anoestrus, oestrus and pregnancy: A lectin histochemistry study. Eur. J. Histochem. 2004, 48, 403–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jolly, C. Species, subspecies, and baboon systematics. In Species, Species Concepts, and Primate Evolution; Kimbel, W., Martin, L., Eds.; Plenum Publishing: New York, NY, USA, 1993; pp. 67–107. [Google Scholar]
- Goncharov, N.; Aso, T.; Cekan, Z.; Pachalia, N.; Diczfalusy, E. Hormonal changes during the menstrual cycle of the baboon (Papio hamadryas). Acta Endocrinol. 1976, 82, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Micha, J.P.; Quimby, F. Baboon cervical colposcopy, Histology and cytology. Gynecol. Oncol. 1984, 17, 308–313. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Matzuk, M.M. The menstrual cycle: Basic biology. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef]
- Accogli, G.; Monaco, D.; El Bahrawy, K.A.; El-Sayed, A.A.H.; Ciannarella, F.; Beneult, B.; Lacalandra, G.M.; Desantis, S. Morphological and glycan features of the camel oviduct epithelium. Ann. Anat. 2014, 196, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. The mammalian oviductal epithelium: Regional variations in cytological and functional aspects of the oviductal secretory cells. Histol. Histopathol. 1996, 11, 743–768. [Google Scholar]
- Desantis, S.; Zizza, S.; Accogli, G.; Acone, F.; Rossi, R.; Resta, L. Morphometric and ultrastructural features of the mare oviduct epithelium during oestrus. Theriogenology 2011, 75, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Slayden, O.D.; Luo, F.; Bishop, C.V. Physiological action of progesterone in the nonhuman primate oviduct. Cells 2022, 11, 1534. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Henning, G.W.; Zheng, H.; Yan, W. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef]
- Cummings, R.D.; Darvill, A.G.; Etzler, M.E.; Hahn, M.G. Glycan-recognizing probes as tools. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 611–625. [Google Scholar]
- Brockhausen, I.; Stanley, P. O-GalNAcglycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 113–123. [Google Scholar]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 99–111. [Google Scholar]
- Varki, A.; Schnaar, R.L.; Schauer, R. Sialic acids and other nonulosonic acids. In Essentials of Glycobiology, 3rd ed.; Varki, A., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 179–195. [Google Scholar]
- Geisler, C.; Jarvis, D.L. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 2011, 21, 988–993. [Google Scholar] [CrossRef]
- Schulte, B.A.; Rao, K.P.; Kreutner, A.; Thomopopoulos, G.N.; Spicer, S.S. Histochemical examination of glycoconjugates of epithelial cells in the human fallopian tube. Lab. Investig. 1985, 52, 207–219. [Google Scholar]
- Jones, C.J.P.; Ortíz, M.E.; Croxatto, H.B.; Manzur, A.; Slevin, G.; Aplin, J.D. Muc1 and glycan expression in the oviduct and endometrium of a new world monkey, Cebus Apella. Biol. Reprod. 2001, 64, 1535–1544. [Google Scholar] [CrossRef][Green Version]
- Lyng, R.; Shur, D. Mouse oviduct-specific glycoprotein is an egg-associated ZP3-independent sperm-adhesion ligand. J. Cell Sci. 2009, 122, 3894–3906. [Google Scholar] [CrossRef]
- Walter, I.; Bavdek, S. Lectin binding patterns of porcine oviduct mucosa and endometrium during the oestrous cycle. J. Anat. 1997, 190, 299–307. [Google Scholar] [CrossRef]
- Flamini, M.A.; Barbeito, C.G.; Portiansky, E.L. A morphological, morphometric and histochemical study of the oviduct in pregnant and non-pregnant females of the plains viscacha (Lagostomus maximus). Acta Zool. 2012, 95, 186–195. [Google Scholar] [CrossRef]
- Kiss, H.; Walter, I.; Lehner, R.; Egarter, C.; Breitenecker, G.; Böck, P. Lectin histochemistry of fallopian tube epithelial cells. J. Reprod. Med. 1998, 43, 535–538. [Google Scholar] [PubMed]
- Lefebvre, R.; Suarez, S. Bovine sperm binding to homologous oviductal epithelium involves fucose recognition. Biol. Reprod. 1996, 54, 185. [Google Scholar] [CrossRef]
- Kadirvel, G.; Machado, A.S.; Korneli, C.; Collins, E.; Miller, P.; Bess, K.N.; Aoki, K.; Tiemeyer, M.; Bovin, N.; Miller, D.J. Porcine sperm bind to specific 6-sialylated biantennary glycans to form the oviduct reservoir. Biol. Reprod. 2012, 87, 147. [Google Scholar] [CrossRef]
- Machado, S.A.; Sharif, M.; Kardivel, G.; Bovin, N.; Miller, D.J. Adhesion to oviduct glycans regulates porcine sperm Ca2+ influx and viability. PLoS ONE 2020, 15, e0237666. [Google Scholar] [CrossRef]
- Desantis, S.; Accogli, G.; Silvestre, F.; Binetti, F.; Cox, S.N.; Roscino, M.; Caira, M.; Lacalandra, G.M. Glycan profile of oviductal isthmus epithelium in normal and superovulated ewes. Theriogenology 2016, 85, 1192–1202. [Google Scholar] [CrossRef]
- Gagneux, P.; Cheriyan, M.; Hurtado-Ziola, N.; Brinkman van der Linden, E.C.M.; Anderson, D.; McClure, H.; Varki, A.; Varki, N.M. Human-specific regulation of α2–6-linked sialic acids. J. Biol. Chem. 2003, 278, 48245–48250. [Google Scholar] [CrossRef]
- Norwood, T.; Hein, C.E.; Halbert, S.A.; Anderson, R.G.W. Polycationic macromolecules inhibit cilia-mediated ovum transport in the rabbit oviduct. Proc. Natl. Acad. Sci. USA 1978, 75, 4413–4416. [Google Scholar] [CrossRef]
- Ito, T.; Newkirk, C.; Strum, J.M.; McDowell, E.M. Changes in glycoconjugates revealed by lectin staining in the developing airways of syrian golden hamsters. Anat. Rec. 1990, 228, 151–162. [Google Scholar] [CrossRef]
- Menghi, G.; Bondi, A.M.; Materazzi, G. Distribution of lectin binding sites in rabbit oviduct. Anat. Rec. 1985, 211, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Mijakovac, A.; Jurić, J.; Kohrt, W.M.; Krištić, J.; Kifer, D.; Gavin, K.M.; Miškec, K.; Azra Frkatović, A.; Vučković, F.; Pezer, M.; et al. Effects of estradiol on immunoglobulin g glycosylation: Mapping of the downstream signaling mechanism. Front. Immunol. 2021, 12, 680227. [Google Scholar] [CrossRef] [PubMed]
- O’Day-Bowman, M.B.; Mavrogianis, P.A.; Reuter, L.M.; Johnson, D.E.; Fazleabas, A.T.; Verhage, H.G. Association of oviduct-specific glycoproteins with human and baboon (Papio anubis) ovarian oocytes and enhancement of human sperm binding to human hemizonae following in vitro incubation. Biol. Reprod. 1996, 54, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Saccary, L.; She, Y.M.; Oko, R.; Kan, F.W. Hamster oviductin regulates tyrosine phosphorylation of sperm proteins during in vitro capacitation. Biol. Reprod. 2013, 89, 38. [Google Scholar] [CrossRef] [PubMed]
- Kouba, A.J.; Abeydeera, L.R.; Alvarez, I.M.; Day, B.N.; Buhi, W.C. Effects of the porcine oviduct-specific glycoprotein on fertilization, polyspermy, and embryonic development in vitro. Biol. Reprod. 2000, 63, 242–250. [Google Scholar] [CrossRef]
- Coy, P.; Canovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; Matás, C.; Avilés, M. Oviduct-specific glycoprotein and heparin modulate sperm–zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 5809–15814. [Google Scholar] [CrossRef]
- Pradeep, M.A.; Jagadeesh, J.; De, A.K.; Kaushik, J.K.; Malakar, D.; Kumar, S.; Dang, A.K.; Das, S.K.; Mohanty, A.K. Purification, sequence characterization and effect of goat oviduct specific glycoprotein on in vitro embryo development. Theriogenology 2011, 75, 1005–1015. [Google Scholar] [CrossRef]
- Desantis, S.; Accogli, G.; Albrizio, M.; Rossi, R.; Cremonesi, F.; Lange Consiglio, A. Glycan profiling analysis of equine amniotic progenitor mesenchymal cells and their derived extracellular microvesicles. Stem. Cells Dev. 2019, 28, 812–821. [Google Scholar] [CrossRef]
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150, 59–69. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.; Fernandez-Rufete, M.; Corbin, E.; Tsikis, G.; Uzbekov, R.; Garanina, A.S.; Coy, P.; Almiñana, C.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilizsation. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.Á.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.E.; Da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef]
- González-Brusi, L.; Algarra, B.; Moros-Nicolás, C.; Izquierdo-Rico, M.J.; Avilés, M.; Jiménez-Movilla, M. A comparative view on the oviductal environment during the periconception period. Biomolecules 2020, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Brañes, M.C.; Morales, B.; Ríos, M.; Villalón, M.J. Regulation of the immunoexpression of aquaporin 9 by ovarian hormones in the rat oviductal epithelium. Am. J. Physiol. Cell Physiol. 2005, 288, C1048–C1057. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Skowronska, A.; Nielsen, S.J. Fluctation of aquaporin 1, 5 and 9 expressionexpressions in the pig oviduct during the oestrous cycle and early pregnancy. J. Histochem. Cytochem. 2011, 59, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bjørkskov, F.B.; Krabbe, S.L.; Nurup, C.N.; Missel, J.W.; Spulber, M.; Bomholt, J.; Molbaek, K.; Helix-Nielsen, C.; Gotfryd, K.; Gourdon, P.; et al. Purification and functional comparison of nine human Aquaporins produced in Saccharomyces cerevisiae for the purpose of biophysical characterization. Sci. Rep. 2017, 7, 16899. [Google Scholar] [CrossRef]
- Tanski, D.; Skowronska, A.; Eliszewski, M.; Gromadzinski, L.; Kempisty, B.; Skowronski, M.T. Changes in aquaporin 1, 5 and 9 gene expression in the porcine oviduct according to estrous cycle and early pregnancy. Int. J. Mol. Sci. 2020, 21, 2777. [Google Scholar] [CrossRef] [PubMed]
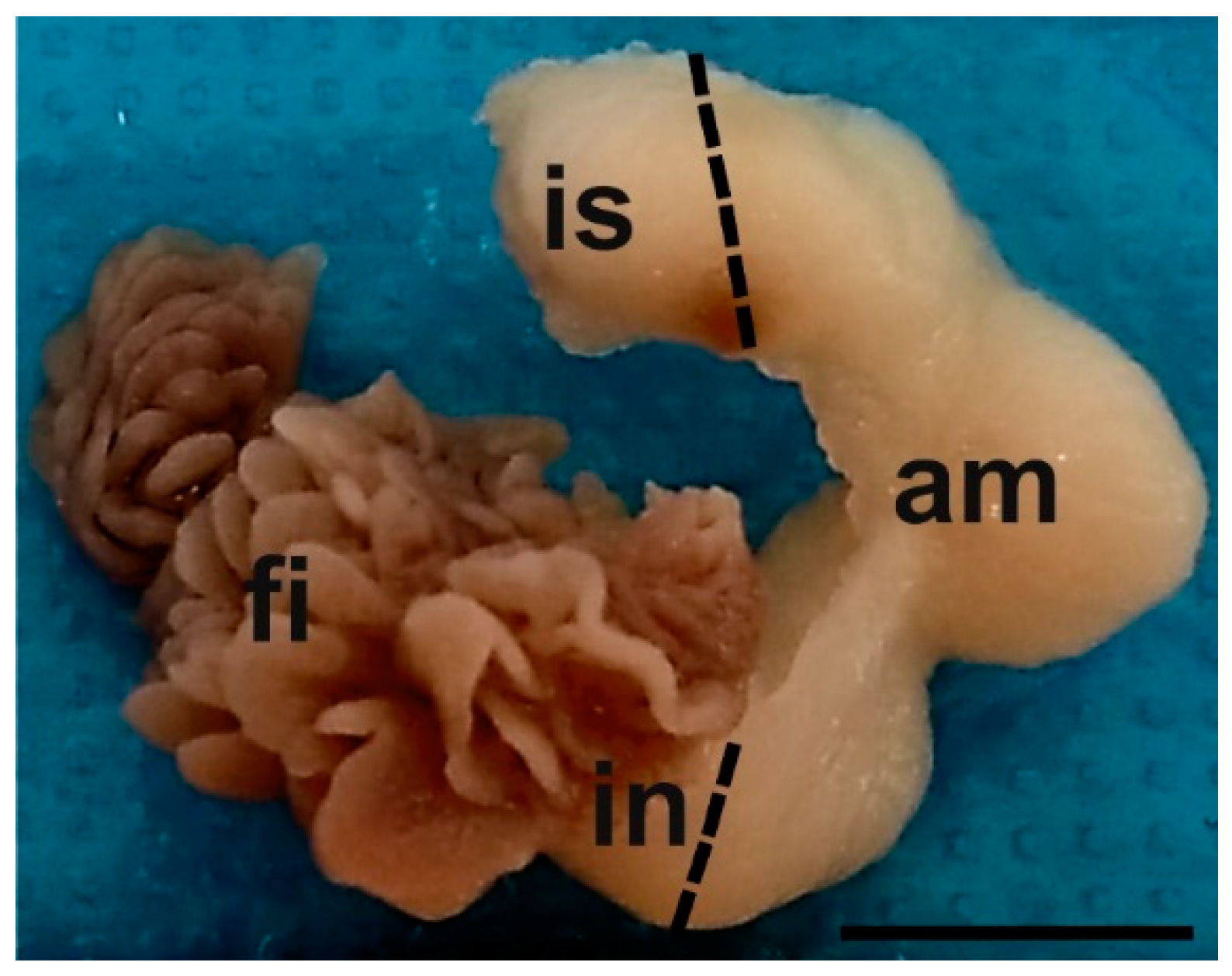

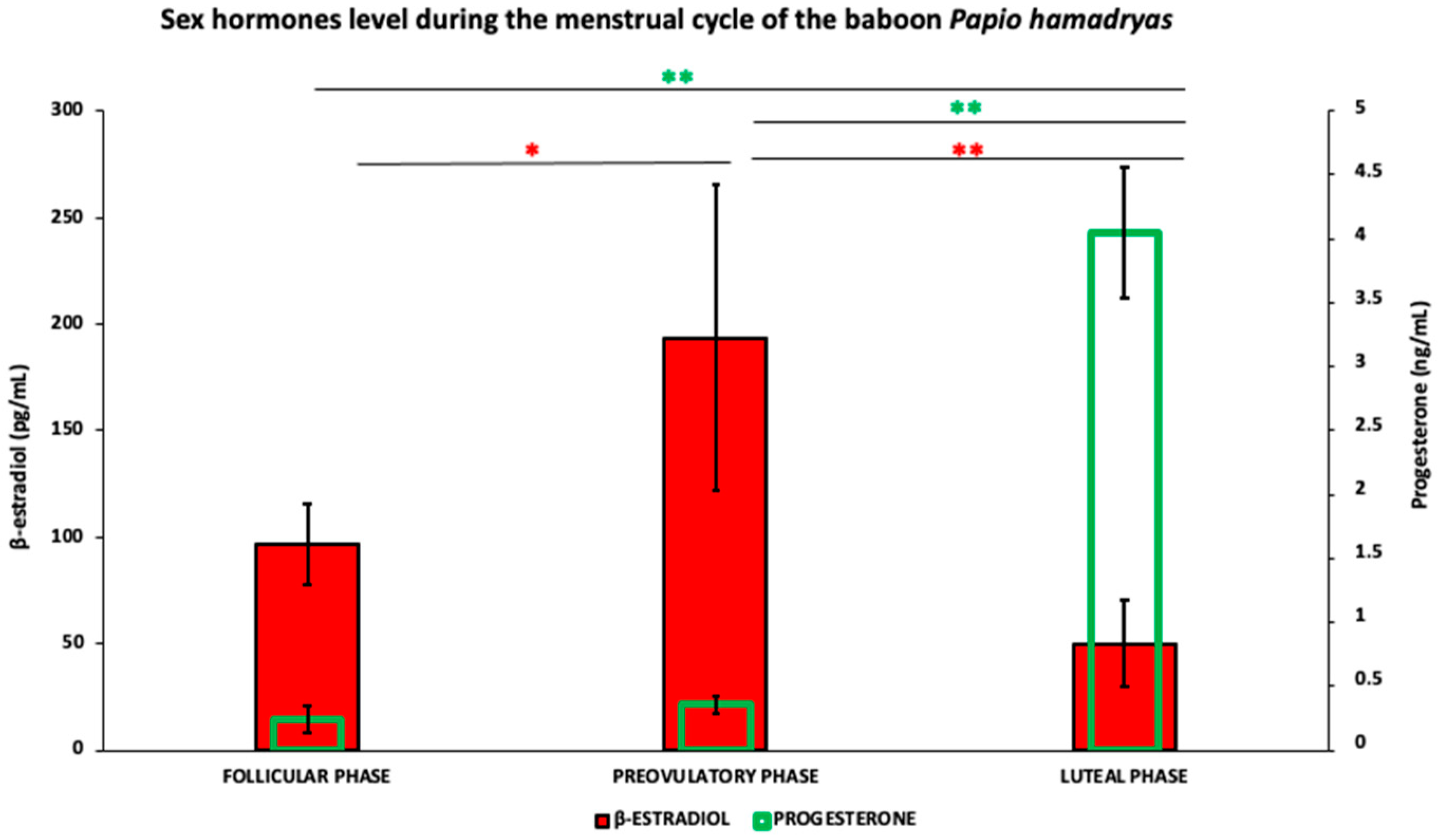



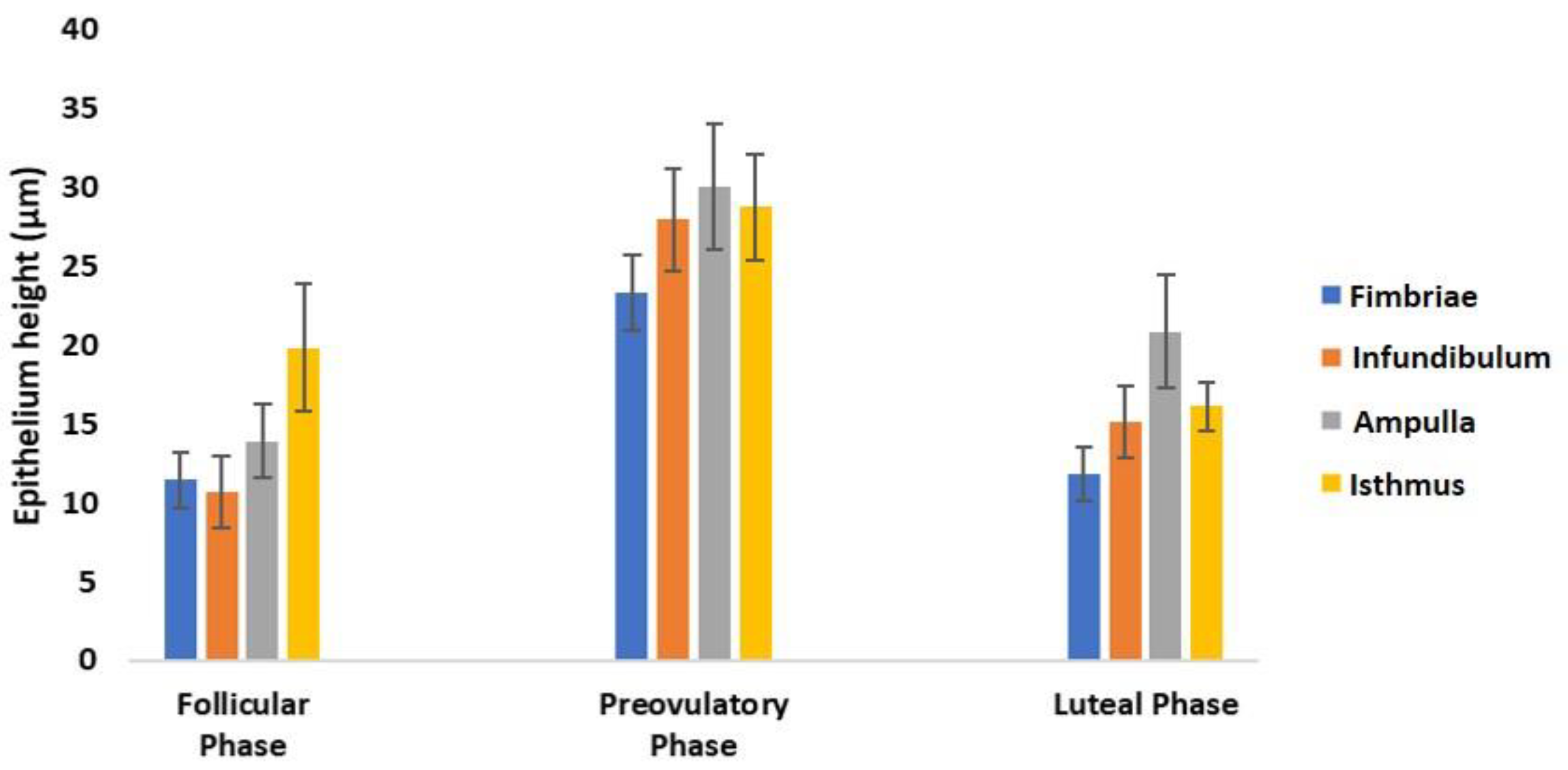
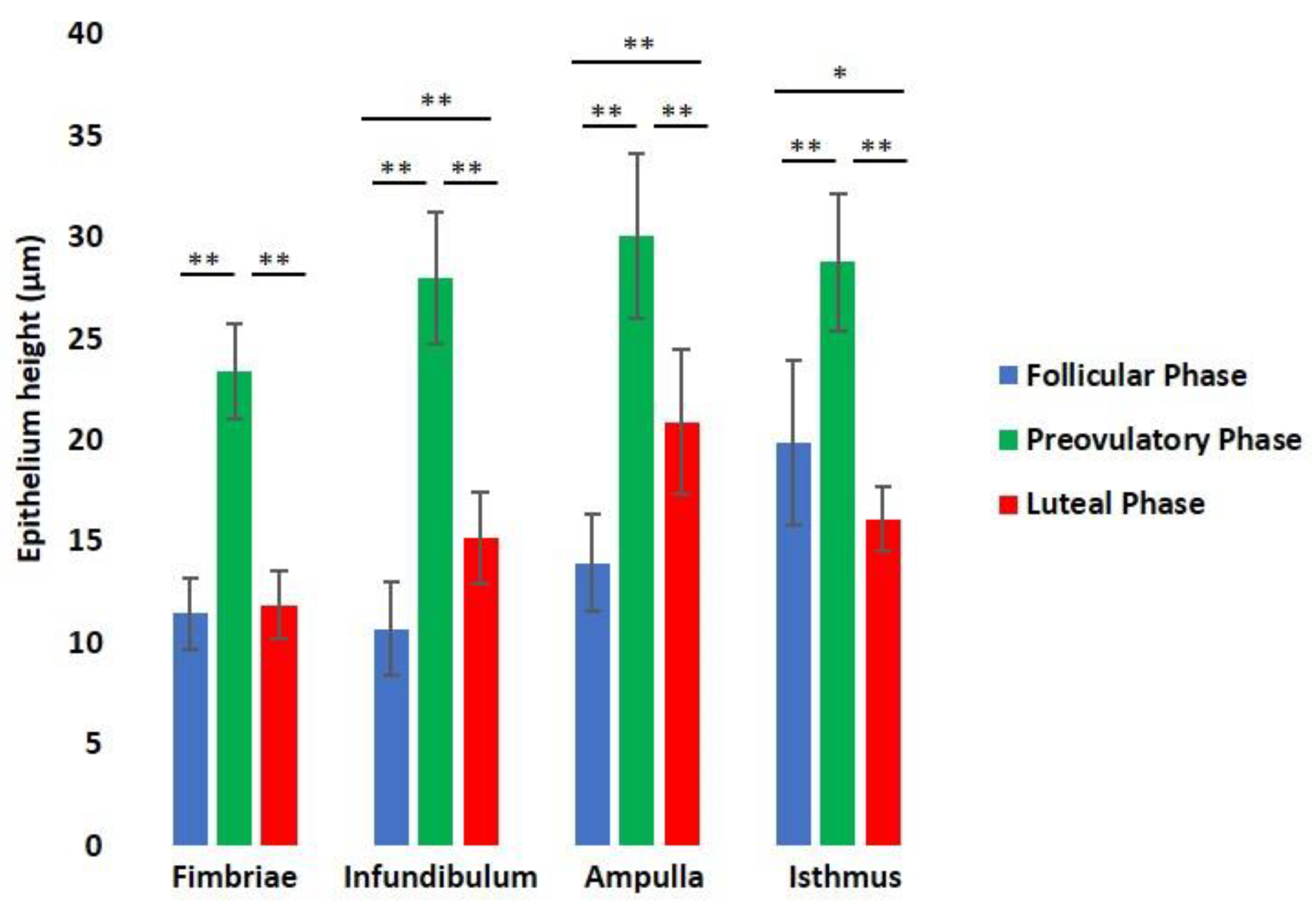


| Lectin Abbreviation | Source of Lectin | Concentration (µg/mL) | Sugar Specificity | Inhibitory Sugar (0.5 M) |
|---|---|---|---|---|
| MAL II | Maackia amurensis | 30 | NeuNAcα2,3Galβ1-3GalNAc | NeuNAc |
| SNA | Sambucus nigra | 30 | Neu5Acα2,6Gal/GalNAc | NeuNAc |
| PNA * | Arachis hypogaea | 30 | Galβl,3GalNAc | Galactose |
| HPA | Helix pomatia | 25 | αGalNAc | GalNAc |
| SBA * | Glycine max | 30 | α/βGalNAc | GalNAc |
| DBA | Dolichos biflorus | 30 | αGalNAc | GalNAc |
| Con A | Canavalia ensiformis | 25 | αMan > αGlc | Mannose |
| LTA | Lotus tetragonolobus | 30 | αL-Fuc | Fucose |
| UEA I | Ulex europaeus | 30 | L-Fucαl,2Galβl,4GlcNAcβ | Fucose |
| Lectin | Follicular Phase | Preovulatory Phase | Luteal Phase | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fim | Inf | Amp | Isth | Fim | Inf | Amp | Isth | Fim | Inf | Amp | Isth | |
| Con A | c,ac,bl | c,as,bl | c,ac,bl | c,ac,bl | c,ac | ac | ac,ab | ac | c,ac,bl | c,ac,bl | c,ac,bl | c,ac,bl |
| MAL II | ||||||||||||
| sMAL II | - | - | - | - | - | - | - | - | - | - | - | - |
| SNA | - | - | ±as | ±as | sn,as | sn,as | sn,as | ci | ac,as | ac,as | ac,as | ac,as |
| sSNA | - | - | - | - | - | - | - | - | - | - | - | - |
| PNA | - | - | - | - | - | - | - | - | - | - | - | - |
| sPNA | as | as | as | as | as | as | as | as | as | as | as | as |
| HPA | ±as,c | - | - | - | ac | ac | ac | ab | ±as | as | as | as |
| DBA | - | - | - | - | ±as | - | - | - | - | ±as | - | ±as |
| SBA | - | - | - | - | - | - | - | - | - | - | - | - |
| sSBA | ±as | ±as | ±as | ±as | as | as | as | as | - | - | - | - |
| LTA | - | - | - | ± c,as | - | - | ab | as | - | - | - | - |
| UEA I | - | - | - | - | - | - | - | - | as | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desantis, S.; Albrizio, M.; Lacitignola, L.; Laricchiuta, P.; Cinone, M. Modification of Morphology and Glycan Pattern of the Oviductal Epithelium of Baboon Papio hamadryas during the Menstrual Cycle. Animals 2022, 12, 2769. https://doi.org/10.3390/ani12202769
Desantis S, Albrizio M, Lacitignola L, Laricchiuta P, Cinone M. Modification of Morphology and Glycan Pattern of the Oviductal Epithelium of Baboon Papio hamadryas during the Menstrual Cycle. Animals. 2022; 12(20):2769. https://doi.org/10.3390/ani12202769
Chicago/Turabian StyleDesantis, Salvatore, Maria Albrizio, Luca Lacitignola, Pietro Laricchiuta, and Mario Cinone. 2022. "Modification of Morphology and Glycan Pattern of the Oviductal Epithelium of Baboon Papio hamadryas during the Menstrual Cycle" Animals 12, no. 20: 2769. https://doi.org/10.3390/ani12202769
APA StyleDesantis, S., Albrizio, M., Lacitignola, L., Laricchiuta, P., & Cinone, M. (2022). Modification of Morphology and Glycan Pattern of the Oviductal Epithelium of Baboon Papio hamadryas during the Menstrual Cycle. Animals, 12(20), 2769. https://doi.org/10.3390/ani12202769







