Detection of Genetic Patterns in Endangered Marine Species Is Affected by Small Sample Sizes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. PCR Protocols
2.3. Statistical Analysis
3. Results
3.1. Patterns of Genetic Diversity
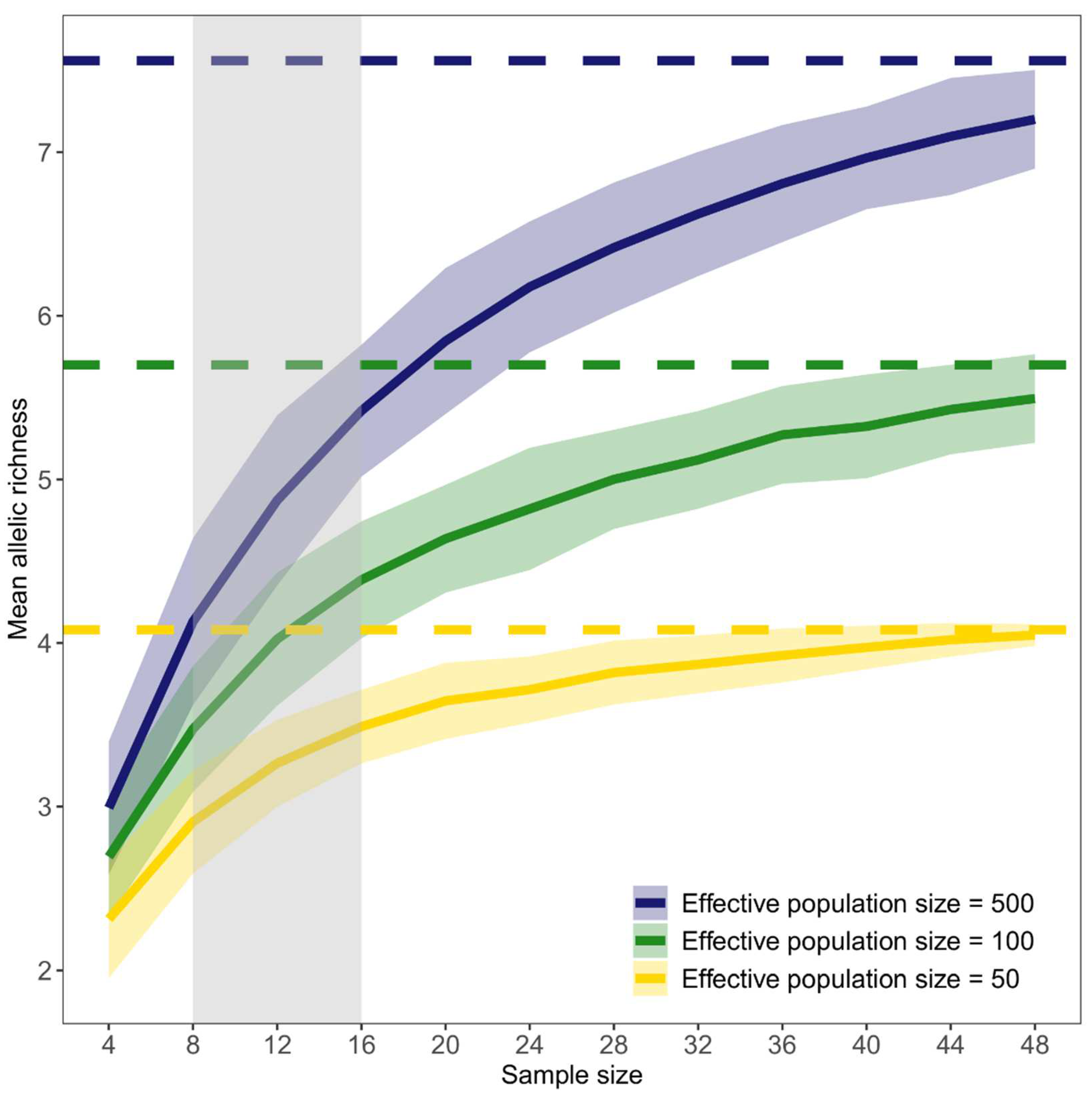
3.2. Effect of Sample Size on Genetic Diversity within Populations
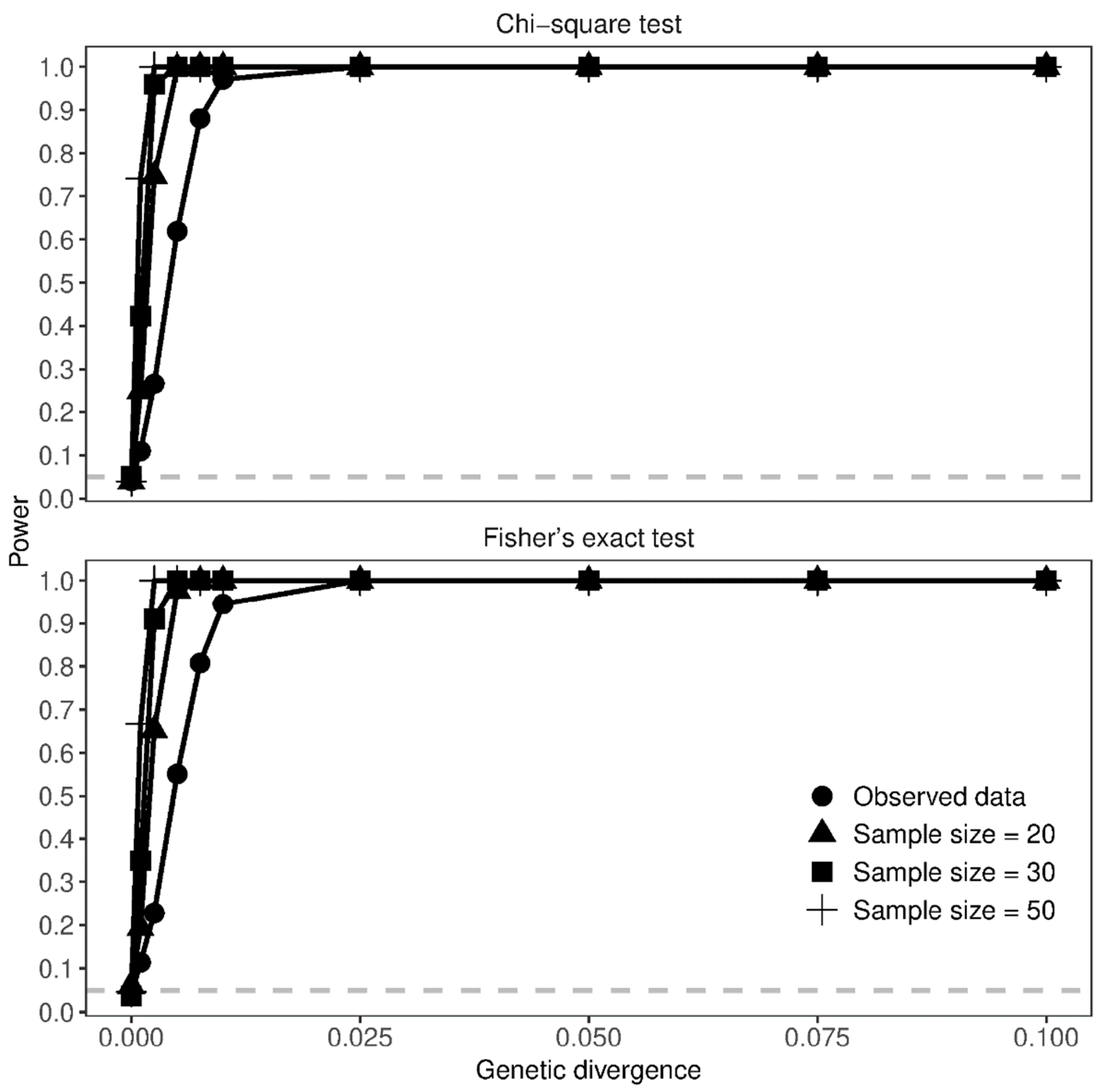
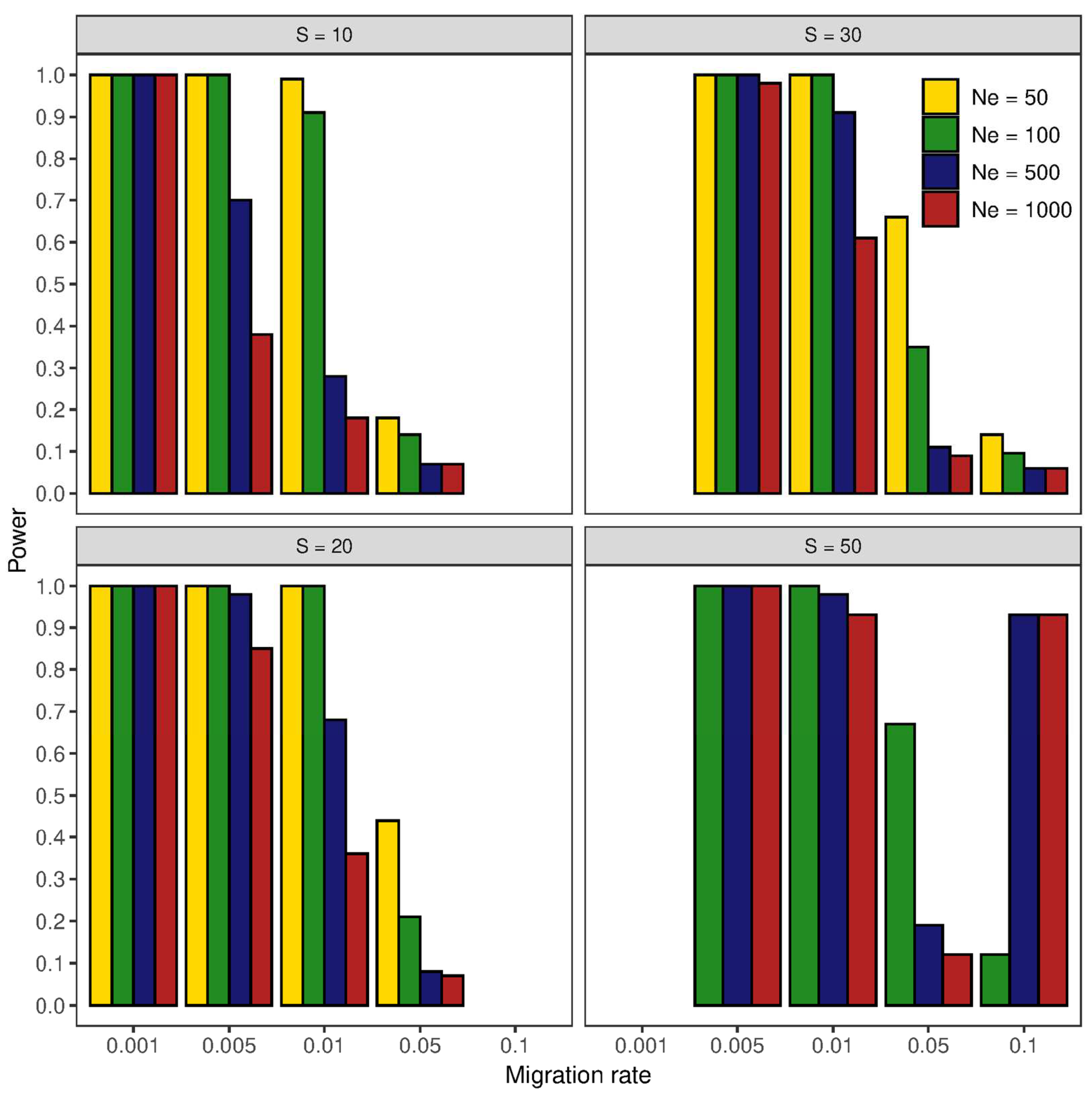
3.3. Effect of Sample Size on Genetic Differentiation
4. Discussion
4.1. Study Limitations
4.2. Effect of Sample Size on Genetic Diversity
4.3. Effect of Sample Size on Genetic Differentiation
4.4. Implications for the Conservation of Endangered Marine Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.J. Connectivity between island marine protected areas and the mainland. Biol. Conserv. 2008, 141, 2807–2820. [Google Scholar] [CrossRef]
- Lemer, S.; Planes, S. Effects of habitat fragmentation on the genetic structure and connectivity of the black-lipped pearl oyster Pinctada margaritifera populations in French Polynesia. Mar. Biol. 2014, 161, 2035–2049. [Google Scholar] [CrossRef]
- Johnson, M.P.; Crowe, T.P.; McAllen, R.; Allcock, A.L. Characterizing the marine Natura 2000 network for the Atlantic region. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 86–97. [Google Scholar] [CrossRef]
- McInerney, C.; Allcock, A.; Johnson, M.; Prodöhl, P. Understanding marine reserve function in a seascape genetics context: Nucella Lapillus in Strangford Lough (Northern Ireland) as an example. Aquat. Biol. 2009, 7, 45–58. [Google Scholar] [CrossRef][Green Version]
- McInerney, C.E.; Louise Allcock, A.; Johnson, M.P.; Prodöhl, P.A. Ecological coherence in marine reserve network design: An empirical evaluation of sequential site selection using genetic structure. Biol. Conserv. 2012, 152, 262–270. [Google Scholar] [CrossRef]
- Iacchei, M.; Ben-Horin, T.; Selkoe, K.A.; Bird, C.E.; García-Rodríguez, F.J.; Toonen, R.J. Combined analyses of kinship and FST suggest potential drivers of chaotic genetic patchiness in high gene-flow populations. Mol. Ecol. 2013, 22, 3476–3494. [Google Scholar] [CrossRef]
- Johnson, M.S.; Black, R. Islands increase genetic subdivision and disrupt patterns of connectivity of intertidal snails in a complex archipelago. Evolution 2006, 60, 2498. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Gardner, J. Small spatial scale population genetic structure in two limpet species endemic to the Kermadec islands, New Zealand. Mar. Ecol. Prog. Ser. 2007, 349, 159–170. [Google Scholar] [CrossRef]
- Shanks, A.L. Pelagic larval duration and dispersal distance revisited. Biol. Bull. 2009, 216, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.P.; Palumbi, S.R. Genetic structure among 50 species of the northeastern Pacific rocky intertidal community. PLoS ONE 2010, 5, e8594. [Google Scholar] [CrossRef] [PubMed]
- Krueger-Hadfield, S.A.; Roze, D.; Mauger, S.; Valero, M. Intergametophytic selfing and microgeographic genetic structure shape populations of the intertidal red seaweed Chondrus crispus. Mol. Ecol. 2013, 22, 3242–3260. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.L.; Montes, H.R., Jr.; Palumbi, S.R. Using isolation by distance and effective density to estimate dispersal scales in anemonefish. Evolution 2010, 64, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Puebla, O.; Bermingham, E.; McMillan, W.O. On the spatial scale of dispersal in coral reef fishes. Mol. Ecol. 2012, 21, 5675–5688. [Google Scholar] [CrossRef] [PubMed]
- Hauser, L.; Carvalho, G.R. Paradigm shifts in marine fisheries genetics: Ugly hypotheses slain by beautiful facts. Fish. Fish. 2008, 9, 333–362. [Google Scholar] [CrossRef]
- Björklund, M.; Bergek, S. On the relationship between population differentiation and sampling effort: Is more always better? Oikos 2009, 118, 1127–1129. [Google Scholar] [CrossRef]
- Kalinowski, S.T. Do polymorphic loci require large sample sizes to estimate genetic distances? Heredity 2005, 94, 33–36. [Google Scholar] [CrossRef]
- Leberg, P.L. Estimating allelic richness: Effects of sample size and bottlenecks. Mol. Ecol. 2002, 11, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.L.; Burg, T.M.; Steeves, T.E. Sampling for microsatellite-based population genetic studies: 25 to 30 individuals per population is enough to accurately estimate allele frequencies. PLoS ONE 2012, 7, e45170. [Google Scholar] [CrossRef] [PubMed]
- Pruett, C.L.; Winker, K. The effects of sample size on population genetic diversity estimates in song sparrows Melospiza melodia. J. Avian Biol. 2008, 39, 252–256. [Google Scholar] [CrossRef]
- Machordom, A.; Ramírez-Escobar, U.; Acevedo, I.; García-Jiménez, R.; Cabezas, P.; Calvo, M.; Toledo, C.; Bloor, P. Isolation and characterisation of polymorphic microsatellite markers for the endangered ferreous limpet Patella ferruginea (Gastropoda, Patellidae). Conserv. Genet. 2010, 11, 1083–1086. [Google Scholar] [CrossRef]
- Coleman, R.A.; Underwood, A.J.; Benedetti-Cecchi, L.; Åberg, P.; Arenas, F.; Arrontes, J.; Castro, J.; Hartnoll, R.G.; Jenkins, S.R.; Paula, J.; et al. A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia 2006, 147, 556–564. [Google Scholar] [CrossRef]
- Martins, G.M.; Thompson, R.C.; Neto, A.I.; Hawkins, S.J.; Jenkins, S.R. Exploitation of intertidal grazers as a driver of community divergence. J. Appl. Ecol. 2010, 47, 1282–1289. [Google Scholar] [CrossRef]
- Burgos-Rubio, V.; De la Rosa, J.; Altamirano, M.; Espinosa, F. The role of patellid limpets as omnivorous grazers: A new insight into intertidal ecology. Mar. Biol. 2015, 162, 2093–2106. [Google Scholar] [CrossRef]
- Faria, J.; Rivas, M.; Martins, G.M.; Hawkins, S.J.; Ribeiro, P.; Pita, A.; Neto, A.I.; Presa, P. A new multiplexed microsatellite tool for metapopulation studies in the overexploited endemic limpet Patella aspera (Röding, 1798). Anim. Genet. 2015, 46, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Pita, A.; Rivas, M.; Martins, G.M.; Hawkins, S.J.; Ribeiro, P.; Neto, A.I.; Presa, P. A multiplex microsatellite tool for conservation genetics of the endemic limpet Patella candei in the Macaronesian archipelagos. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 775–781. [Google Scholar] [CrossRef]
- Martins, G.; Jenkins, S.; Hawkins, S.; Neto, A.; Thompson, R. Exploitation of rocky intertidal grazers: Population status and potential impacts on community structure and functioning. Aquat. Biol. 2008, 3, 1–10. [Google Scholar] [CrossRef]
- Coppa, S.; De Lucia, G.A.; Massaro, G.; Camedda, A.; Marra, S.; Magni, P.; Perilli, A.; Di Bitetto, M.; García-Gómez, J.C.; Espinosa, F. Is the establishment of MPAs enough to preserve endangered intertidal species? The case of Patella ferruginea in Mal di ventre island (W Sardinia, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 623–638. [Google Scholar] [CrossRef]
- Casu, M.; Rivera-Ingraham, G.A.; Cossu, P.; Lai, T.; Sanna, D.; Dedola, G.L.; Sussarellu, R.; Sella, G.; Cristo, B.; Curini-Galletti, M.; et al. Patterns of spatial genetic structuring in the endangered limpet Patella ferruginea: Implications for the conservation of a Mediterranean endemic. Genetica 2011, 139, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Romdhane, M.S.; Espinosa, F. Reintroduction of the highly endangered mollusk Patella ferruginea Gmelin, 1791 in an MPA: A novel approach to achieve high survival rates. Estuar. Coast. Shelf Sci. 2018, 202, 259–269. [Google Scholar] [CrossRef]
- Guallart, J.; Peña, J.B.; Pérez-Larruscaín, J.; Luque, A.A.; Templado, J. Filling Gaps: Closing the life cycle of the endangered Mediterranean limpet Patella ferruginea Gmelin, 1791 (Gastropoda, Patellidae). Mediterr. Mar. Sci. 2020, 21, 400–419. [Google Scholar] [CrossRef]
- Espinosa, F.; Rivera-Ingraham, G.A.; Maestre, M.; González, A.R.; Bazairi, H.; García-Gómez, J.C. Updated global distribution of the threatened marine limpet Patella ferruginea (Gastropoda: Patellidae): An example of biodiversity loss in the Mediterranean. Oryx 2014, 48, 266–275. [Google Scholar] [CrossRef]
- Ferranti, M.P.; Monteggia, D.; Asnaghi, V.; Dagnino, A.; Gaino, F.; Moretto, P.; Parodi, V.; Tixi, L.; Cappanera, V.; Valerani, C.; et al. Distribution of the Mediterranean ribbed limpet Patella ferruginea Gmelin, 1791 along the ligurian coast and implications for conservation actions. Medit. Mar. Sci. 2019, 20, 496. [Google Scholar] [CrossRef]
- Guallart, J.; Peña, J.B.; Luque, Á.A.; Templado, J. Where have all the youngest gone? The postlarval and young stages of the Mediterranean endangered limpet Patella ferruginea Gmelin, 1791. Mediterr. Mar. Sci. 2017, 18, 385–392. [Google Scholar] [CrossRef]
- Zarrouk, A.; Romdhane, M.S.; Espinosa, F. Usefulness of marine protected areas as tools for preserving the highly endangered limpet, Patella ferruginea, in the Mediterranean Sea. Mar. Biol. Res. 2016, 12, 917–931. [Google Scholar] [CrossRef]
- Coppa, S.; De Lucia, G.A.; Massaro, G.; Magni, P. Density and distribution of Patella ferruginea in a marine protected area (western Sardinia, Italy): Constraint analysis for population conservation. Mediterr. Mar. Sci. 2012, 13, 108. [Google Scholar] [CrossRef]
- Cossu, P.; Scarpa, F.; Dedola, G.L.; Sanna, D.; Lai, T.; Cristo, B.; Curini-Galletti, M.; Panzalis, P.; Navone, A.; Careddu, G.; et al. Surviving at the edge of a fragmented range: Patterns of genetic diversity in isolated populations of the endangered giant Mediterranean limpet (Patella ferruginea). Mar. Biol. 2017, 164, 41. [Google Scholar] [CrossRef]
- Espinosa, F. Populational status of the endangered mollusc Patella ferruginea Gmelin, 1791 (Gastropoda, Patellidae) on algerian islands (SW Mediterranean). Anim. Biodivers. Conserv. 2009, 32, 19–28. [Google Scholar] [CrossRef]
- Rivera-Ingraham, G.A.; Espinosa, F.; Garcia-Gomez, J.C. Environmentally mediated sex change in the endangered limpet Patella ferruginea (Gastropoda: Patellidae). J. Molluscan Stud. 2011, 77, 226–231. [Google Scholar] [CrossRef]
- Rivera-Ingraham, G.A.; Espinosa, F.; Garcia-Gomez, J.C. Long-term monitoring of the critically endangered limpet Patella ferruginea Gmelin, 1791: New ecological insights and first demographic results. J. Molluscan Stud. 2015, 81, 124–130. [Google Scholar] [CrossRef]
- Espinosa, F.; Guerra-García, J.M.; Fa, D.; García-Gómez, J.C. Aspects of reproduction and their implications for the conservation of the endangered limpet, Patella ferruginea. Invertebr. Reprod. Dev. 2006, 49, 85–92. [Google Scholar] [CrossRef]
- Espinosa, F.; Rivera-Ingraham, G.; García-Gómez, J.C. Influence of habitat structure and nature of substratum on limpet recruitment: Conservation implications for endangered species. Estuar. Coast. Shelf Sci. 2011, 94, 164–171. [Google Scholar] [CrossRef]
- Guallart, J.; Calvo, M.; Acevedo, I.; Templado, J. Two-way sex change in the endangered limpet Patella ferruginea (Mollusca, Gastropoda). Invertebr. Reprod. Dev. 2013, 57, 247–253. [Google Scholar] [CrossRef]
- Espinosa, F.; Pavón-Paneque, A.; García-Gómez, J.C. Disentangling the impact of artificial substrata on the trophic ecology of the highly endangered marine invertebrate Patella ferruginea Gmelin, 1791. Molluscan Res. 2021, 41, 41–45. [Google Scholar] [CrossRef]
- Espinosa, F.; González, A.R.; Maestre, M.J.; Fa, D.; Guerra-García, J.M.; García-Gómez, J.C. Responses of the endangered limpet Patella ferruginea to reintroduction under different environmental conditions: Survival, growth rates and life-history. Ital. J. Zool. 2008, 75, 371–384. [Google Scholar] [CrossRef]
- Fa, D.A.; Finlayson, G.; Sempere-Valverde, J.; García-Gómez, J.C. ‘Impossible’ re-introduction of the endangered limpet Patella ferruginea Gmelin, 1791? Progress in resolving translocation mortality. Endanger. Species Res. 2018, 37, 219–232. [Google Scholar] [CrossRef]
- Espinosa, F.; Ozawa, T. Population genetics of the endangered limpet Patella ferruginea (Gastropoda: Patellidae): Taxonomic, conservation and evolutionary considerations. J. Zool. Syst. 2006, 44, 8–16. [Google Scholar] [CrossRef]
- Casu, M.; Casu, D.; Lai, T.; Cossu, P.; Curini-Galletti, M. Inter-simple sequence repeat markers reveal strong genetic differentiation among populations of the endangered mollusc Patella ferruginea (Gastropoda: Patellidae) from two Sardinian marine protected areas. Mar. Biol. 2006, 149, 1163–1174. [Google Scholar] [CrossRef]
- Bouzaza, Z.; Vera, M.; Mezali, K. Population genetic structure of the endangered limpet Patella ferruginea (Gastropoda: Patellidae) in the Western Mediterranean: New evidence implicating marine barriers’ effects. Molluscan. Res. 2021, 41, 32–40. [Google Scholar] [CrossRef]
- Cossu, P.; Dedola, G.L.; Scarpa, F.; Sanna, D.; Lai, T.; Maltagliati, F.; Curini-Galletti, M.; Casu, M. Patterns of spatial genetic variation in Patella ulyssiponensis: Insights from the Western Mediterranean marine ecoregion. Hydrobiologia 2015, 755, 39–55. [Google Scholar] [CrossRef]
- Casu, M.; Sanna, D.; Cristo, B.; Lai, T.; Dedola, G.L.; Curini-Galletti, M. COI sequencing as tool for the taxonomic attribution of Patella spp. (Gastropoda): The case of morphologically undistinguishable juveniles settled on a Patella ferruginea adult. J. Mar. Biol. Assoc. 2010, 90, 1449–1454. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2020. Available online: http://www.r-project.org/ (accessed on 10 October 2022).
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodöhl, P.A. DiveRsity: An R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol. Evol. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Hedrick, P.W. A standardized genetic differentiation measure. Evolution 2005, 59, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Waples, R.S.; Gaggiotti, O. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 2006, 15, 1419–1439. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.R. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conserv. Genet. 2006, 7, 783–787. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Janzen, F.J. Bottlesim: A bottleneck simulation program for long-lived species with overlapping generations. Mol. Ecol. Notes 2003, 3, 669–673. [Google Scholar] [CrossRef]
- Paquette, S.R. PopGenKit: Useful Functions for (Batch) File Conversion and Data Resampling in Microsatellite Datasets. 2012. Available online: https://CRAN.R-project.org/package=PopGenKit (accessed on 10 October 2022).
- Ryman, N.; Palm, S. POWSIM: A computer program for assessing statistical power when testing for genetic differentiation. Mol. Ecol. Notes 2006, 6, 600–602. [Google Scholar] [CrossRef]
- Hoban, S.; Gaggiotti, O.; Bertorelle, G.; ConGRESS Consortium. Sample planning optimization tool for conservation and population genetics (SPOTG): A software for choosing the appropriate number of markers and samples. Methods Ecol. Evol. 2013, 4, 299–303. [Google Scholar] [CrossRef]
- Laval, G.; Excoffier, L. SIMCOAL 2.0: A program to simulate genomic diversity over large recombining regions in a subdivided population with a complex history. Bioinformatics 2004, 20, 2485–2487. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Cadiou, G.; Diréac’h, L. Marine protected areas: A tool for coastal areas management. In Strategic Management of Marine Ecosystems; Levner, E., Linkov, I., Proth, J.-M., Eds.; NATO Science Series IV: Earth and Environmental Series; Springer: Berlin/Heidelberg, Germany, 2005; Volume 50, pp. 29–52. ISBN 978-1-4020-3157-1. [Google Scholar]
- Coleman, R.A.; Weeks, A.R.; Hoffmann, A.A. Balancing genetic uniqueness and genetic variation in determining conservation and translocation strategies: A comprehensive case study of threatened dwarf galaxias, Galaxiella pusilla (Mack) (Pisces: Galaxiidae). Mol. Ecol. 2013, 22, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Ryman, N.; Laikre, L.; Hössjer, O. Do estimates of contemporary effective population size tell us what we want to know? Mol. Ecol. 2019, 28, 1904–1918. [Google Scholar] [CrossRef] [PubMed]
- Palstra, F.P.; Ruzzante, D.E. Genetic estimates of contemporary effective population size: What can they tell us about the importance of genetic stochasticity for wild population persistence? Mol. Ecol. 2008, 17, 3428–3447. [Google Scholar] [CrossRef]
- Waples, R.S. Tiny estimates of the Ne/N ratio in marine fishes: Are they real? J. Fish. Biol. 2016, 89, 2479–2504. [Google Scholar] [CrossRef] [PubMed]
- Waples, R.S.; England, P.R. Estimating contemporary effective population size on the basis of linkage disequilibrium in the face of migration. Genetics 2011, 189, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Fauvelot, C.; Bertozzi, F.; Costantini, F.; Airoldi, L.; Abbiati, M. Lower genetic diversity in the limpet Patella caerulea on urban coastal structures compared to natural rocky habitats. Mar. Biol. 2009, 156, 2313–2323. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Branco, M.; Hawkins, S.J.; Santos, A.M. Recent changes in the distribution of a marine gastropod, Patella rustica, across the Iberian Atlantic coast did not result in diminished genetic diversity or increased connectivity. J. Biogeogr. 2010, 37, 1782–1796. [Google Scholar] [CrossRef]
- Faria, J.; Martins, G.M.; Pita, A.; Ribeiro, P.A.; Hawkins, S.J.; Presa, P.; Neto, A.I. Disentangling the genetic and morphological structure of Patella candei complex in Macaronesia (NE Atlantic). Ecol. Evol. 2017, 7, 6125–6140. [Google Scholar] [CrossRef]
- Faria, J.; Pita, A.; Martins, G.M.; Ribeiro, P.A.; Hawkins, S.J.; Presa, P.; Neto, A.I. Inbreeding in the exploited limpet Patella aspera across the Macaronesia archipelagos (NE Atlantic): Implications for conservation. Fish. Res. 2018, 198, 180–188. [Google Scholar] [CrossRef]
- Waples, R.S. Testing for Hardy–Weinberg proportions: Have we lost the plot? J. Hered. 2015, 106, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bashalkhanov, S.; Pandey, M.; Rajora, O.P. A simple method for estimating genetic diversity in large populations from finite sample sizes. BMC Genet. 2009, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Koen, E.L.; Bowman, J.; Garroway, C.J.; Wilson, P.J. The sensitivity of genetic connectivity measures to unsampled and under-sampled sites. PLoS ONE 2013, 8, e56204. [Google Scholar] [CrossRef] [PubMed]
- Landguth, E.L.; Cushman, S.A.; Schwartz, M.K.; McKelvey, K.S.; Murphy, M.; Luikart, G. Quantifying the lag time to detect barriers in landscape genetics. Mol. Ecol. 2010, 19, 4179–4191. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Bradshaw, C.J.A.; Brook, B.W. Genetics in conservation management: Revised recommendations for the 50/500 rules, red list criteria and population viability analyses. Biol. Conserv. 2014, 170, 56–63. [Google Scholar] [CrossRef]

| Primer | Sequence | Repeat Motif | Multiplex (Fluorescent Dye) | Size Range (bp) | Genbank Accession Number |
|---|---|---|---|---|---|
| Pf-31AH8 | F: GGGTGTGGCTCTGCCTATTA R: TGGTTACCCCAGATATACGC | (CA)2 GA (CA)8 | MpxB (6-FAM) | 123–131 | FJ436433 |
| Pf-31IF2 | F: TATCCTACATACCATCCATAATGC R: TAGTCCATAGTGCCGCTGTC | (CA)12 | (NED) 1 | 188–216 | FJ436435 |
| Pf-D11A | F: ACAACGAAGCCACCGACTAC R: AGCGCACTTCTTGACCTGAC | (CT)13 | MpxA (VIC) | 386–426 | FJ436436 |
| Pf-G1M | F: GGCTCAGTTCGAGAATCCAC R: TAACCGACCATTCACGTGTT | (CA)15 | MpxA (6-FAM) | 143–177 | FJ436437 |
| Pf-C10 | F: TCTATGCTAATATTTGTGTCTGTCG R: TTCACCCGGCTAAAGAATCA | (TYTG)3 TCG (TYTG)4 TTG (TYTG)4 | MpxB (6-FAM) | 312–328 | FJ436438 |
| Pf-G6A | F: CCAAATAGTCTTCGTGGTTGG | (TG)15 GG (TG)8 | MpxB (VIC) | 225–251 | FJ436439 |
| Pf-31IB2 | F: TGGATAGTGGGTATGTGTTGC R: GTAGCCACCAATCCATTAGC | (GT)14 | MpxA (NED) | 90–114 | FJ436441 |
| Pf-31IB1 | F: GTTGTTGCGATTTCATGTGG R: TTAAGAATTGTGGCCTGTTGG | (TG)21 (TATG)3 (TG)2 (TC) (TG)2 | MpxA (VIC) | 124–192 | FJ436443 |
| Population | A ± SE | AR ± SE | AE ± SE | HE ± SE | HO ± SE | uHE ± SE | FIS ± SE |
|---|---|---|---|---|---|---|---|
| APS | 5.12 ± 1.11 | 3.86 ± 0.69 | 3.37 ± 0.73 | 0.55 ± 0.11 | 0.53 ± 0.11 | 0.58 ± 0.12 | 0.02 ± 0.05 |
| ACS | 4.37 ± 0.71 | 3.72 ± 0.52 | 3.15 ± 0.50 | 0.58 ± 0.10 | 0.60 ± 0.11 | 0.62 ± 0.11 | −0.05 ± 0.06 |
| APB | 5.62 ± 1.05 | 4.51 ± 0.77 | 4.13 ± 0.86 | 0.63 ± 0.10 | 0.64 ± 0.11 | 0.68 ± 0.11 | −0.03 ± 0.07 |
| CDN | 6.25 ± 1.25 | 4.64 ± 0.65 | 4.21 ± 1.00 | 0.62 ± 0.10 | 0.67 ± 0.11 | 0.65 ± 0.11 | −0.07 ± 0.04 |
| IPO | 5.00 ± 0.91 | 3.98 ± 0.56 | 3.44 ± 0.61 | 0.61 ± 0.10 | 0.67 ± 0.12 | 0.64 ± 0.10 | −0.11 ± 0.07 |
| ARG | 6.12 ± 1.06 | 4.35 ± 0.43 | 3.62 ± 0.62 | 0.62 ± 0.10 | 0.63 ± 0.11 | 0.64 ± 0.10 | −0.02 ± 0.04 |
| MVE | 5.75 ± 1.11 | 4.52 ± 0.71 | 4.27 ± 0.85 | 0.65 ± 0.10 | 0.73 ± 0.11 | 0.70 ± 0.11 | −0.14 ± 0.04 |
| CPA | 5.37 ± 1.02 | 4.09 ± 0.57 | 3.66 ± 0.68 | 0.60 ± 0.11 | 0.64 ± 0.12 | 0.64 ± 0.11 | −0.08 ± 0.05 |
| CPC | 5.75 ± 1.08 | 4.34 ± 0.61 | 3.93 ± 0.78 | 0.64 ± 0.09 | 0.66 ± 0.11 | 0.67 ± 0.09 | 0.01 ± 0.10 |
| PLF | 5.25 ± 0.96 | 4.22 ± 0.69 | 3.94 ± 0.82 | 0.61 ± 0.11 | 0.69 ± 0.13 | 0.65 ± 0.12 | −0.13 ± 0.06 |
| MAD | 5.00 ± 0.84 | 4.09 ± 0.64 | 3.64 ±0.71 | 0.59 ± 0.11 | 0.66 ± 0.12 | 0.63 ± 0.11 | −0.12 ± 0.06 |
| CAM | 5.50 ± 0.96 | 4.16 ± 0.56 | 3.63 ± 0.61 | 0.61 ± 0.11 | 0.62 ± 0.11 | 0.65 ± 0.11 | −0.03 ± 0.06 |
| MLA | 5.25 ± 1.01 | 4.31 ± 0.60 | 3.78 ± 0.65 | 0.63 ± 0.10 | 0.63 ± 0.11 | 0.67 ± 0.11 | 0.00 ± 0.05 |
| MLT | 5.37 ± 1.02 | 4.31 ± 0.64 | 3.54 ± 0.73 | 0.59 ± 0.10 | 0.62 ± 0.11 | 0.63 ± 0.11 | −0.05 ± 0.05 |
| APS | ACS | APB | CDN | IPO | ARG | MVE | CPA | CPC | PLF | MAD | CAM | MLA | MLT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APS | — | 0.041 | 0.018 | −0.004 | 0.014 | 0.037 | 0.013 | 0.035 | −0.008 | 0.010 | 0.042 | 0.006 | 0.017 | 0.066 |
| ACS | 0.016 | — | −0.060 | 0.019 | 0.009 | 0.026 | −0.009 | 0.047 | 0.028 | −0.024 | −0.044 | −0.016 | 0.008 | 0.103 |
| APB | 0.003 | −0.029 | — | −0.045 | −0.008 | 0.015 | −0.069 | −0.042 | −0.042 | −0.041 | −0.039 | −0.086 | −0.059 | 0.038 |
| CDN | −0.003 | 0.008 | −0.018 | — | −0.064 | −0.016 | −0.014 | 0.011 | −0.030 | −0.052 | −0.002 | −0.040 | −0.048 | 0.036 |
| IPO | 0.005 | 0.005 | −0.003 | −0.026 | — | −0.006 | −0.016 | 0.016 | 0.006 | −0.027 | 0.021 | −0.036 | 0.000 | 0.027 |
| ARG | 0.015 | 0.010 | 0.005 | −0.007 | −0.002 | — | 0.012 | 0.077 | −0.009 | 0.005 | 0.022 | 0.007 | −0.020 | 0.041 |
| MVE | 0.005 | −0.002 | −0.026 | −0.003 | −0.002 | 0.006 | — | −0.001 | −0.010 | 0.001 | −0.006 | −0.057 | −0.019 | 0.052 |
| CPA | 0.014 | 0.021 | −0.018 | 0.006 | 0.009 | 0.033 | 0.002 | — | 0.012 | −0.010 | 0.000 | −0.032 | 0.023 | 0.069 |
| CPC | −0.007 | 0.011 | −0.019 | −0.012 | 0.003 | −0.004 | −0.002 | 0.005 | — | −0.032 | 0.005 | −0.019 | −0.008 | 0.009 |
| PLF | 0.004 | −0.010 | −0.016 | −0.020 | −0.008 | 0.003 | 0.005 | −0.002 | −0.012 | — | −0.050 | −0.019 | −0.018 | 0.050 |
| MAD | 0.018 | −0.020 | −0.017 | 0.001 | 0.013 | 0.010 | 0.002 | 0.001 | 0.003 | −0.019 | — | −0.039 | −0.013 | 0.069 |
| CAM | −0.002 | −0.011 | −0.039 | −0.018 | −0.016 | 0.002 | −0.023 | −0.015 | −0.009 | −0.008 | −0.018 | — | −0.058 | 0.041 |
| MLA | 0.004 | 0.001 | −0.026 | −0.020 | 0.000 | −0.009 | −0.007 | 0.009 | −0.005 | −0.007 | −0.006 | −0.027 | — | 0.017 |
| MLT | 0.029 | 0.045 | 0.014 | 0.016 | 0.013 | 0.018 | 0.023 | 0.030 | 0.003 | 0.023 | 0.031 | 0.016 | 0.006 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, P.; Mura, L.; Dedola, G.L.; Lai, T.; Sanna, D.; Scarpa, F.; Azzena, I.; Fois, N.; Casu, M. Detection of Genetic Patterns in Endangered Marine Species Is Affected by Small Sample Sizes. Animals 2022, 12, 2763. https://doi.org/10.3390/ani12202763
Cossu P, Mura L, Dedola GL, Lai T, Sanna D, Scarpa F, Azzena I, Fois N, Casu M. Detection of Genetic Patterns in Endangered Marine Species Is Affected by Small Sample Sizes. Animals. 2022; 12(20):2763. https://doi.org/10.3390/ani12202763
Chicago/Turabian StyleCossu, Piero, Laura Mura, Gian Luca Dedola, Tiziana Lai, Daria Sanna, Fabio Scarpa, Ilenia Azzena, Nicola Fois, and Marco Casu. 2022. "Detection of Genetic Patterns in Endangered Marine Species Is Affected by Small Sample Sizes" Animals 12, no. 20: 2763. https://doi.org/10.3390/ani12202763
APA StyleCossu, P., Mura, L., Dedola, G. L., Lai, T., Sanna, D., Scarpa, F., Azzena, I., Fois, N., & Casu, M. (2022). Detection of Genetic Patterns in Endangered Marine Species Is Affected by Small Sample Sizes. Animals, 12(20), 2763. https://doi.org/10.3390/ani12202763










