Effects of Cage and Floor Rearing Systems on the Metabolic Components of the Uropygial Gland in Ducks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Sampling
2.2. Metabolite Extraction
2.3. Chromatographic Conditions
2.4. Mass Spectrometry Condition
2.5. Qualitative and Quantitative Analysis of Metabolites
2.6. Differential Metabolite Screening and Functional Analysis
2.7. Statistical Analysis
3. Results
3.1. Changes in the Uropygial Glands’ Weight
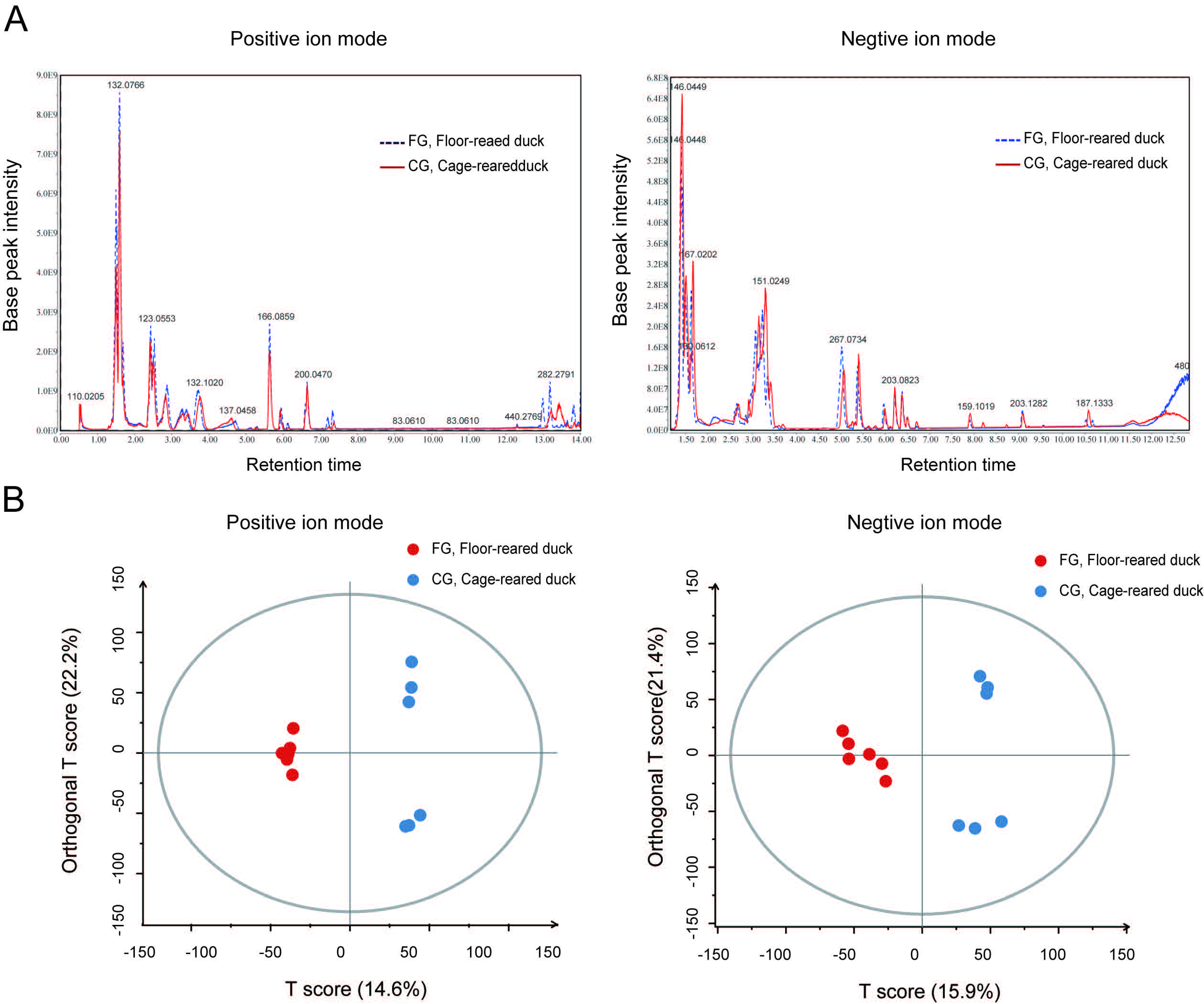
3.2. The Metabolic Components of Uropygial Glands Differed Significantly in Ducks from Different Rearing Systems
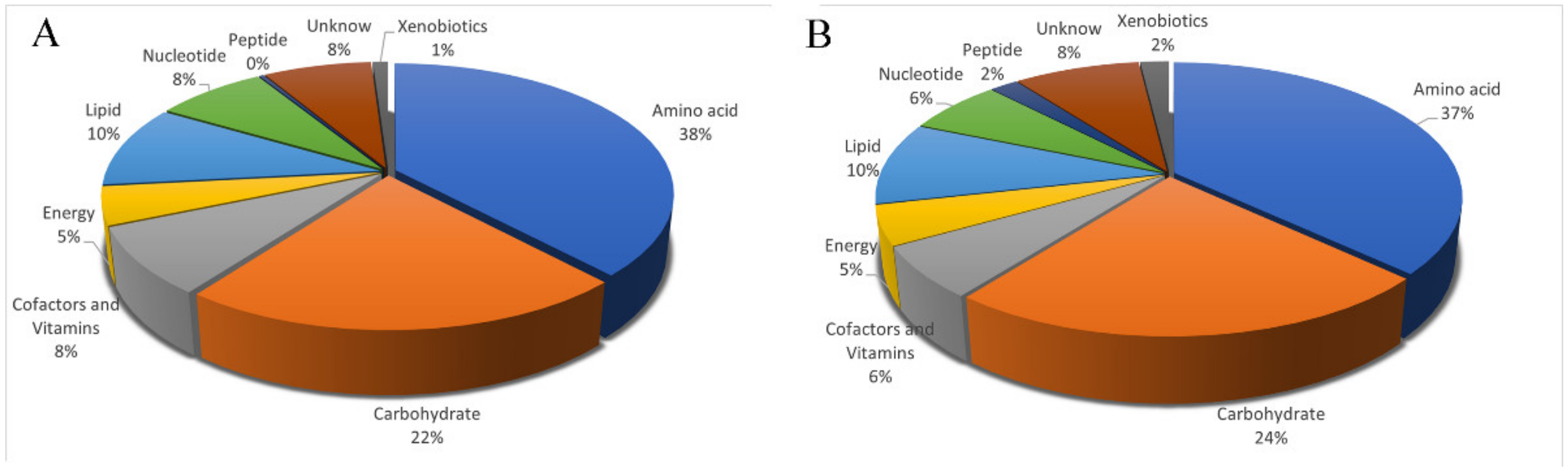
3.3. Identification and Classification of Metabolites in the Uropygial Glands
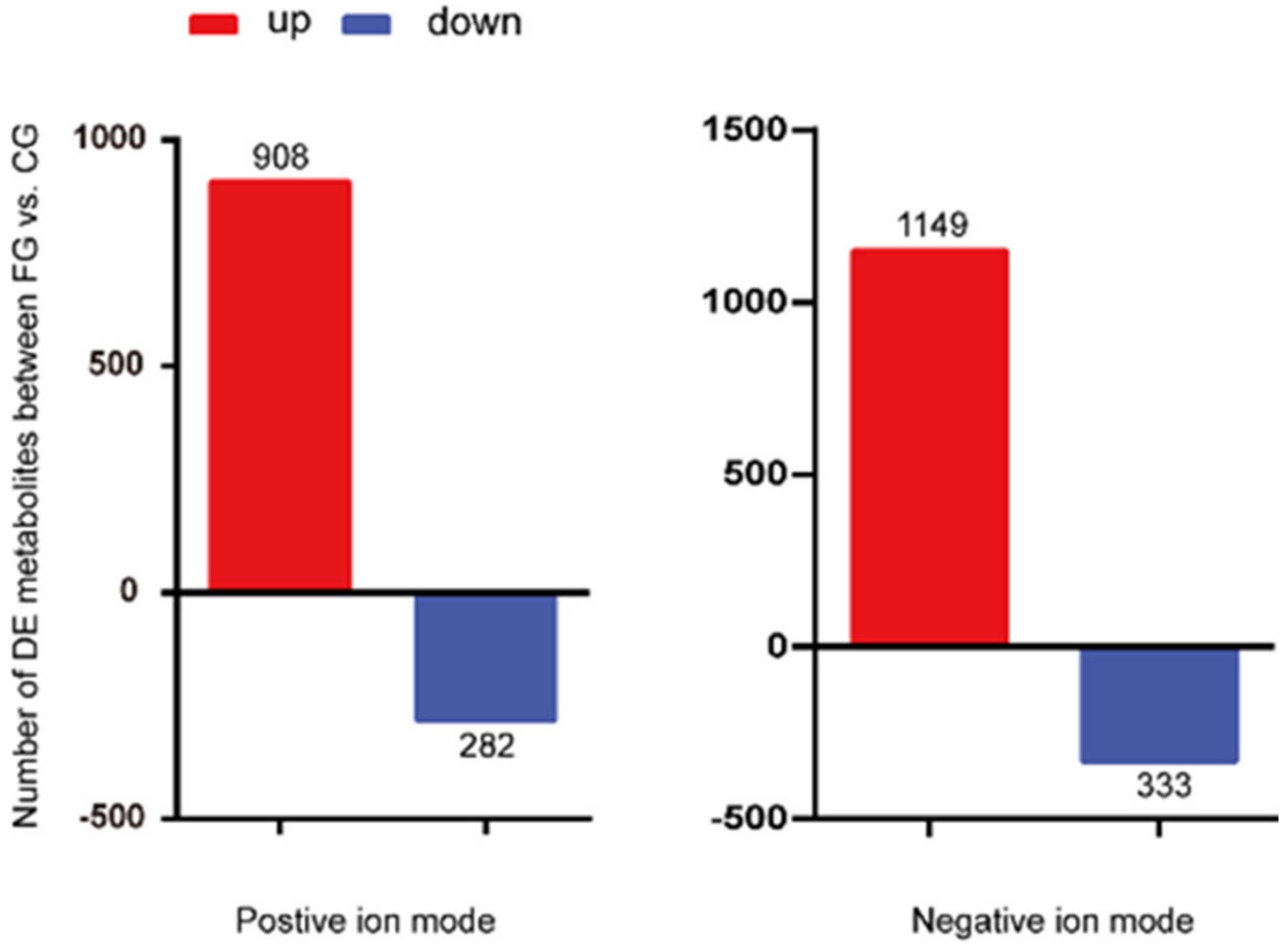
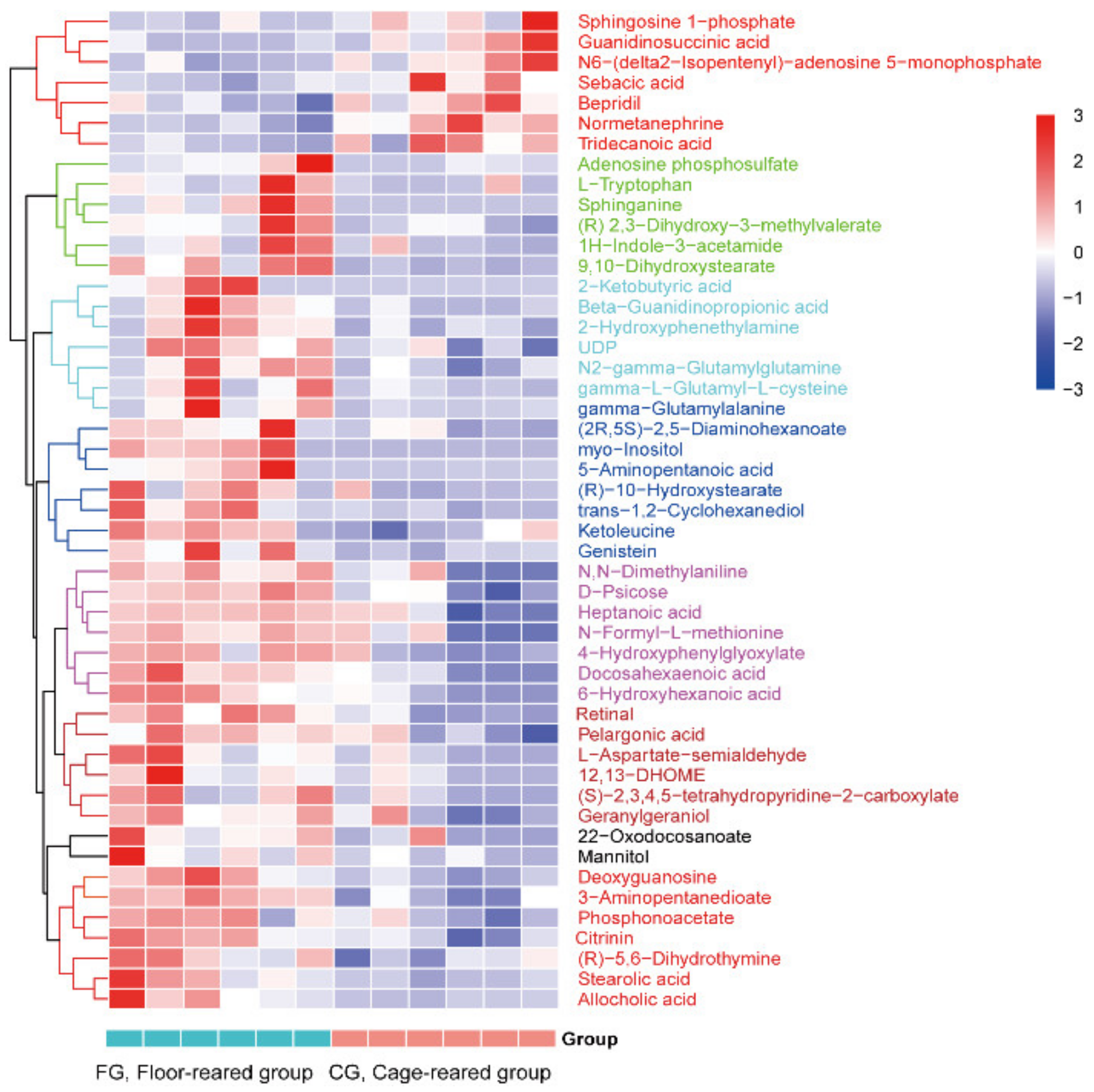
3.4. Screening and Identification of Differential Metabolites
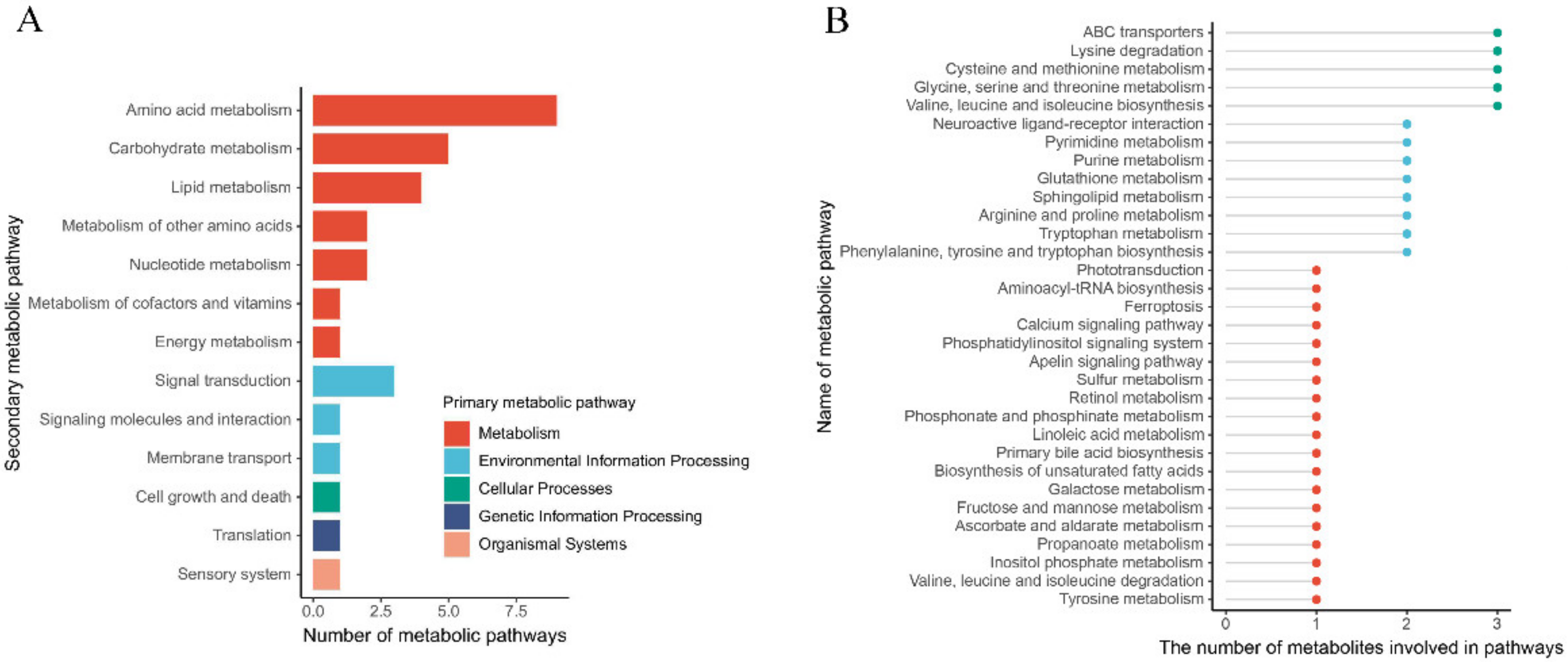
3.5. The Metabolic Pathway in the Gland Is Affected by Rearing Modes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lovette, I.J.; Fitzpatrick, J.W. Handbook of Bird Biology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Vincze, O.; Vágási, C.I.; Kovács, I.; Galván, I.; Pap, P.L. Sources of variation in uropygial gland size in European birds. Biol. J. Linn. Soc. 2013, 110, 543–563. [Google Scholar] [CrossRef]
- Montalti, D.; Salibián, A. Uropygial gland size and avian habitat. Ornitol. Neotrop. 2000, 11, 297–306. [Google Scholar]
- Yilmaz, B.; Yilmaz, R. Macroanatomical and histological features of uropygial Gland in mallard ducks (Anas platyrhynchos). Harran Üniv. Vet. Fak. Derg. 2019, 8, 214–220. [Google Scholar] [CrossRef]
- Hassanin, A.; Shoeib, M.; Massoud, D. Micro−and macroanatomical features of the uropygial gland of duck (Anas platyrhynchos) and pigeon (Columba livia). Biotech. Histochem. 2020, 96, 213–222. [Google Scholar] [CrossRef]
- Campagna, S.; Mardon, J.; Celerier, A.; Bonadonna, F. Potential Semiochemical Molecules from Birds: A Practical and Comprehensive Compilation of the Last 20 Years Studies. Chem. Senses 2011, 37, 3–25. [Google Scholar] [CrossRef]
- Burger, B.V.; Reiter, B.; Borzyk, O.; du Plessis, M.A. Avian Exocrine Secretions. I. Chemical Characterization of the Volatile Fraction of the Uropygial Secretion of the Green Woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 2004, 30, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Luisa Amo, J.M.A.D. Sex recognition by odour and variation in the uropygial gland secretion in starlings. J. Anim. Ecol. 2012, 3, 605–613. [Google Scholar]
- Hirao, A.; Aoyama, M.; Sugita, S. The role of uropygial gland on sexual behavior in domestic chicken Gallus gallus domesticus. Behav. Process. 2008, 80, 115–120. [Google Scholar] [CrossRef]
- Giraudeau, M.; Duval, C.; Guillon, N.; Bretagnolle, V.; Gutierrez, C.; Heeb, P. Effects of access to preen gland secretions on mallard plumage. Sci. Nat. 2010, 97, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, M.; Czirják, G.Á.; Duval, C.; Bretagnolle, V.; Eraud, C.; McGraw, K.J.; Heeb, P. Effect of restricted preen-gland access on maternal self maintenance and reproductive investment in mallards. PLoS ONE 2010, 10, e13555. [Google Scholar] [CrossRef]
- Moyer, B.; Rock, A.N.; Clayton, D.H. Experimental test of the importance of preen oil in rock doves (Columba livia). Auk 2003, 120, 490–496. [Google Scholar] [CrossRef]
- Moreno-Rueda, G. Preen oil and bird fitness: A critical review of the evidence. Biol. Rev. 2017, 92, 2131–2143. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Immer, A.; Leclaire, S.; Parthuisot, N.; Ducamp, C.; Espinasse, G.; Heeb, P. Uropygial gland size and composition varies according to experimentally modified microbiome in Great tits. BMC Evol. Biol. 2014, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Magallanes, S.; Møller, A.P.; García-Longoria, L.; De Lope, F.; Marzal, A. Volume and antimicrobial activity of secretions of the uropygial gland are correlated with malaria infection in house sparrows. Parasites Vectors 2016, 9, 232. [Google Scholar] [CrossRef]
- Nørgaard-Nielsen, G.; Vestergaard, K.; Simonsen, H. Effects of rearing experience and stimulus enrichment on feather damage in laying hens. Appl. Anim. Behav. Sci. 1993, 38, 345–352. [Google Scholar] [CrossRef]
- Drake, K.; Donnelly, C.; Dawkins, M.S. Influence of rearing and lay risk factors on propensity for feather damage in laying hens. Br. Poult. Sci. 2010, 51, 725–733. [Google Scholar] [CrossRef]
- Decina, C.; Berke, O.; Van Staaveren, N.; Baes, C.F.; Widowski, T.M.; Harlander-Matauschek, A. A cross-sectional study on feather cover damage in Canadian laying hens in non-cage housing systems. BMC Vet. Res. 2019, 15, 435. [Google Scholar] [CrossRef]
- Decina, C.; Berke, O.; Van Staaveren, N.; Baes, C.F.; Widowski, T.M.; Harlander-Matauschek, A. An Investigation of Associations Between Management and Feather Damage in Canadian Laying Hens Housed in Furnished Cages. Animals 2019, 9, 135. [Google Scholar] [CrossRef]
- Richards, S.A. The influence of loss of plumage on temperature regulation in laying hens. J. Agric. Sci. 1977, 89, 393–398. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, D.; Singh, Y. Potential usefulness of the plumage reducing Naked Neck (Na) gene in poultry production at normal and high ambient temperatures. Worlds Poult. Sci. J. 2001, 57, 139–156. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Forsyth, M.H.; Swaddle, J.P. Evidence that plumage bacteria influence feather coloration and body condition of eastern bluebirds Sialia sialis. J. Avian Biol. 2009, 40, 440–447. [Google Scholar] [CrossRef]
- Savory, C.J. Feather pecking and cannibalism. World’s Poult. Sci. J. 1995, 51, 215–219. [Google Scholar] [CrossRef]
- Mills, A.D.; Faure, J.M.; Williams, J.B. Feather loss and egg production in broiler breeders and layers. Anim. Res. 1988, 37, 133–142. [Google Scholar] [CrossRef]
- Glatz, P.C. Effect of Poor Feather Cover on Feed Intake and Production of Aged Laying Hens. Asian-Australas. J. Anim. Sci. 2001, 14, 553–558. [Google Scholar] [CrossRef]
- Yamak, U.S.; Sarica, M. Relationships between feather score and egg production and feed consumption of different layer hybrids kept in conventional cages. Archiv. Geflugelkd. 2012, 76, 31–37. [Google Scholar]
- Mi, J.; Wang, H.; Chen, X.; Hartcher, K.; Wang, Y.; Wu, Y.; Liao, X. Lack of access to an open water source for bathing inhibited the development of the preen gland and preening behavior in Sanshui White ducks. Poult. Sci. 2020, 99, 5214–5221. [Google Scholar] [CrossRef]
- Sangster, T.; Major, H.; Plumb, R.; Wilson, A.J.; Wilson, I.D. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Jsy Ng, U.R.R.T. Development of an untargeted metabolomics method for the analysis of human faecal samples using Cryptosporidium-infected samples. Mol. Biochem. 2012, 2, 145–150. [Google Scholar]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to convert raw mass spectrometry data. Curr. Protoc. Bioinform. 2014, 46, 13.24.1–13.24.9. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, K.; Fahy, E.; Subramaniam, S.; Dennis, E.A. The Lipid Maps Initiative in Lipidomics. Methods Enzymol. 2007, 432, 171–183. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef]
- Møller, A.P.; Laursen, K. Function of the uropygial gland in eiders (Somateria mollissima). Avian Res. 2019, 1, 24. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, E.V.J.J.M. Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J. Exp. Biol. 2009, 22, 3621–3626. [Google Scholar] [CrossRef]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Y.; Hansen, I.A. Lipogenesis in Chicken Uropygial Glands. JBIC J. Biol. Inorg. Chem. 1972, 31, 372–377. [Google Scholar] [CrossRef]
- Murphy, M.E.; King, J.R. Amino Acid Composition of the Plumage of the White-Crowned Sparrow. Condor 1982, 84, 435. [Google Scholar] [CrossRef]
- Kubińska, M.; Mróz, E.; Jankowski, J.; Zduńczyk, Z. Growth performance and plumage development of young turkeys fed on diets with graded levels of sulfur-containing amino acids. Eur. Poultry Sci. 2015, 79, 100. [Google Scholar]
- Van Emous, R.A.; van Krimpen, M.M. Effects of Nutritional Interventions on Feathering of Poultry—A Review; CABI: Wallingford, UK, 2019; pp. 133–150. [Google Scholar] [CrossRef]
- Elder, W.H. The oil gland of birds. Wilson Bull. 1954, 66, 6–31. [Google Scholar]
- Rajput, R. A Scientific Hypothesis on the Role of Nutritional Supplements for Effective Management of Hair Loss and Promoting Hair Regrowth. J. Nutr. Health Food Sci. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Parthasarathy, L.K.; Ratnam, L.; Seelan, S.; Tobias, C.; Casanova, M.F.; Parthasarathy, R.N. Mammalian Inositol 3-phosphate Synthase: Its Role in the Biosynthesis of Brain Inositol and its Clinical Use as a Psychoact Agent. Biol. Inositols Phosphoinositides 2006, 39, 293–314. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Herwig, E.; Classen, H.L.; Walk, C.L.; Bedford, M.; Schwean-Lardner, K. Dietary Inositol Reduces Fearfulness and Avoidance in Laying Hens. Animals 2019, 9, 938. [Google Scholar] [CrossRef]
- Van Hierdenabjaap, M.K.; Mechielkortea, Y.M. Chronic increase of dietary l-tryptophan decreases gentle feather pecking behaviour. Appl. Anim. Behav. Sci. 2004, 12, 71–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Min, L.; Ruan, D.; Kim, H.; Hong, Y.; Chen, W.; Wang, S.; Xia, W.; Luo, X.; et al. Effects of L-methionine on growth performance, carcass quality, feather traits, and small intestinal morphology of Pekin ducks compared with conventional DL-methionine. Poult. Sci. 2019, 12, 6866–6872. [Google Scholar] [CrossRef]
- Cuvillier, O. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2002, 2–3, 153–162. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Qi, J.; Yang, Q.; Tang, Q.; Qi, J.; Li, Y.; Wang, J.; Han, C.; Li, L. Effects of Cage and Floor Rearing Systems on the Metabolic Components of the Uropygial Gland in Ducks. Animals 2022, 12, 214. https://doi.org/10.3390/ani12020214
Liu H, Qi J, Yang Q, Tang Q, Qi J, Li Y, Wang J, Han C, Li L. Effects of Cage and Floor Rearing Systems on the Metabolic Components of the Uropygial Gland in Ducks. Animals. 2022; 12(2):214. https://doi.org/10.3390/ani12020214
Chicago/Turabian StyleLiu, Hehe, Jiawen Qi, Qinglan Yang, Qian Tang, Jingjing Qi, Yanying Li, Jiwen Wang, Chunchun Han, and Liang Li. 2022. "Effects of Cage and Floor Rearing Systems on the Metabolic Components of the Uropygial Gland in Ducks" Animals 12, no. 2: 214. https://doi.org/10.3390/ani12020214
APA StyleLiu, H., Qi, J., Yang, Q., Tang, Q., Qi, J., Li, Y., Wang, J., Han, C., & Li, L. (2022). Effects of Cage and Floor Rearing Systems on the Metabolic Components of the Uropygial Gland in Ducks. Animals, 12(2), 214. https://doi.org/10.3390/ani12020214



