Maternal Supplementation of Saccharomyces cerevisiae boulardii during Late-Gestation through Lactation Differentially Modulated Immune Status and Stress Responsiveness of the Progeny to Farrowing and Weaning Stressors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Total White Blood Cell Counts (WBC) and Leukocyte Differentials
2.3. Cell Isolation and Plasma Analysis
2.4. Immune Assays
2.5. Piglet Body Weight and Litter Processing Practices
2.6. Statistical Analysis
3. Results
3.1. Maternal Gestation Treatment on Progeny
3.1.1. Interactive Effect on Cortisol and Immune Profile
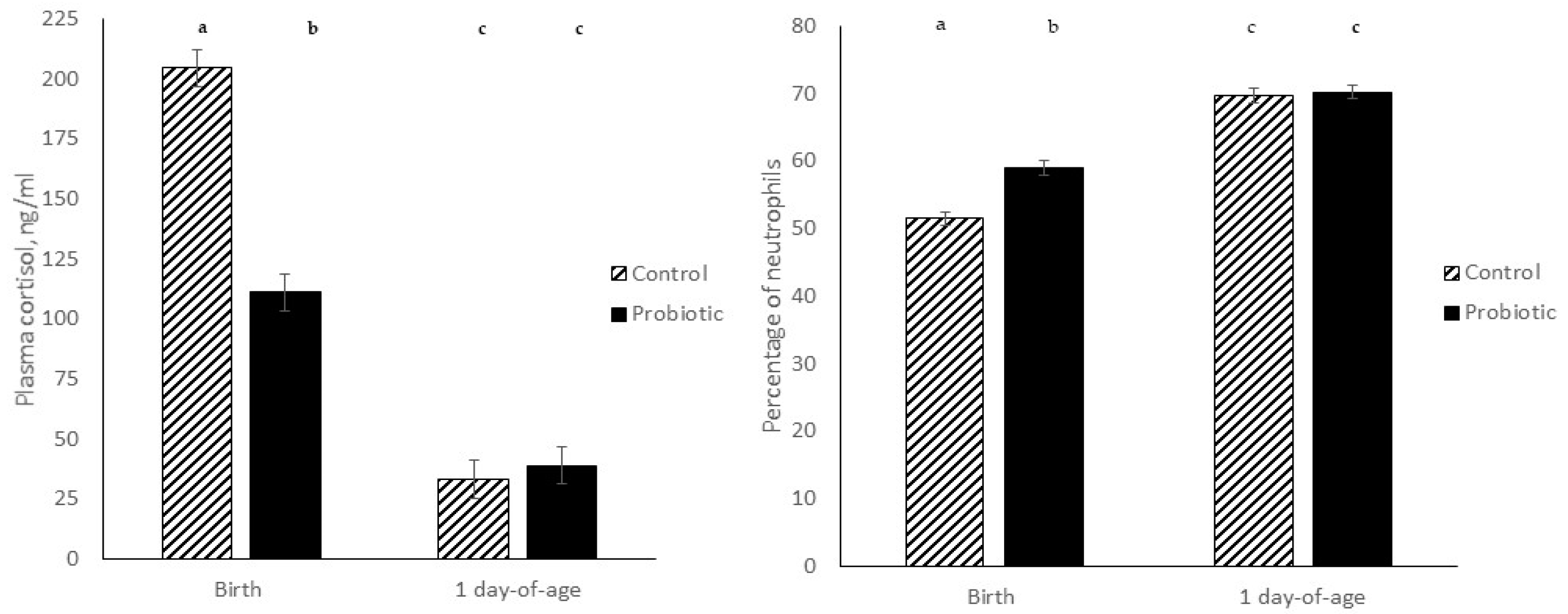
3.1.2. Treatment Effects at Birth
3.1.3. Overall Treatment Effect on the Neonate
3.2. Effects of Maternal Treatment on Piglets during Suckling
3.3. Effects of Maternal Treatment on Pigs to Weaning Stress
3.4. Effects of Maternal Treatment on Body Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salak-Johnson, J.L.; McGlone, J.J. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 2007, 85, E81–E88. [Google Scholar] [CrossRef] [PubMed]
- Clapperton, M.; Diack, A.B.; Matika, O.; Glass, E.J.; Gladney, C.D.; Mellencamp, M.A.; Hoste, A.; Bishop, S.C. Traits associated with innate and adaptive immunity in pigs: Heritability and associations with performance under different health status conditions. Genet. Sel. Evol. 2009, 41, 54. [Google Scholar] [CrossRef] [PubMed]
- Romano-Keeler, J.; Weitkamp, J.-H. Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 2015, 77, 189–195. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Kanitz, E.; Otten, W.; Tuchscherer, A. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet. Immunol. Immunopathol. 2002, 86, 195–203. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament and the Council: Action Plan against the Rising Threats from Antimicrobial Resistance; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Millet, S.; Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, V.; di Giancamillo, A.; Savoini, G.; Dell’Orto, V.; Domeneghini, C. Live yeast dietary supplementation acts upon intestinal morpho-functional aspects and growth in weanling piglets. Anim. Feed. Sci. Technol. 2006, 129, 224–236. [Google Scholar] [CrossRef]
- Le Bon, M.; Davies, H.E.; Glynn, C.; Thompson, C.; Madden, M.; Wiseman, J.; Dodd, C.E.; Hurdidge, L.; Payne, G.; Le Treut, Y.; et al. Influence of probiotics on gut health in the weaned pig. Livest. Sci. 2010, 133, 179–181. [Google Scholar] [CrossRef]
- Rajput, I.R.; Li, L.; Xin, X.; Wu, B.; Juan, Z.; Cui, Z.; Yu, D.; Li, W. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013, 92, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Hancox, L.R.; Le Bon, M.; Richards, P.J.; Guillou, D.; Dodd, C.E.R.; Mellits, K.H. Effect of a single dose of Saccharomyces cerevisiae var. boulardii on the occurrence of porcine neonatal diarrhoea. Animal 2015, 9, 1756–1759. [Google Scholar] [CrossRef]
- Brousseau, J.-P.; Talbot, G.; Beaudoin, F.; Lauzon, K.; Roy, D.; Lessard, M. Effects of probiotics Pediococcus acidilactici strain MA18/5M and Saccharomyces cerevisiae subsp. boulardii strain SB-CNCM I-1079 on fecal and intestinal microbiota of nursing and weanling piglets. J. Anim. Sci. 2015, 93, 5313–5326. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Cottrez, F.; Imbert, V.; Lagadec, P.; Peyron, J.-F.; Rampal, P.; Czerucka, D.; Groux, H. Saccharomyces boulardii Inhibits Inflammatory Bowel Disease by Trapping T Cells in Mesenteric Lymph Nodes. Gastroenterology 2006, 131, 1812–1825. [Google Scholar] [CrossRef]
- Collier, C.T.; Carroll, J.A.; Ballou, M.A.; Starkey, J.D.; Sparks, J.C. Oral administration of Saccharomyces cerevisiae boulardii reduces mortality associated with immune and cortisol responses to Escherichia coli endotoxin in pigs. J. Anim. Sci. 2011, 89, 52–58. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [CrossRef]
- Salak, J.L.; McGlone, J.J.; Lyte, M. Effects of in vitro adrenocorticotrophic hormone, cortisol and human recombinant inter-leukin-2 on porcine neutrophil migration and luminol-dependent chemiluminescence. Vet. Immunol. Immunopathol. 1993, 39, 327–337. [Google Scholar] [CrossRef]
- Jolie, R.; Backstrom, L.; Olson, L.; Chase, C. Respiratory and systemic health parameters in pigs raised in a conventional farm or in isolation. Swine Health Prod. 1999, 7, 269–275. [Google Scholar]
- Niekamp, S.R.; Sutherland, M.A.; Dahl, G.E.; Salak-Johnson, J.L. Photoperiod influences the immune status of multiparous pregnant sows and their piglets. J. Anim. Sci. 2006, 84, 2072–2082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutherland, M.A.; Niekamp, S.R.; Rodriguez-Zas, S.L.; Salak-Johnson, J.L. Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J. Anim. Sci. 2006, 84, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S.; Miller, A.H.; McEwen, B.S.; Spencer, R.L. Stress-induced changes in blood leukocyte distribution: Role of adrenal steroid hormones. J. Immunol. 1996, 157, 1638–1644. [Google Scholar] [PubMed]
- Salak-Johnson, J.L.; Webb, S.R. Short- and Long-Term Effects of Weaning Age on Pig Innate Immune Status. Open J. Anim. Sci. 2018, 8, 137–150. [Google Scholar] [CrossRef]
- Kanitz, E.; Otten, W.; Tuchscherer, M. Changes in endocrine and neurochemical profiles in neonatal pigs prenatally exposed to increased maternal cortisol. J. Endocrinol. 2006, 191, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Jarvis, S.; Moinard, C.; Robson, S.K.; Baxter, E.; Ormandy, E.; Douglas, A.J.; Seckl, J.R.; Russell, J.A.; Lawrence, A. Programming the offspring of the pig by prenatal social stress: Neuroendocrine activity and behaviour. Horm. Behav. 2006, 49, 68–80. [Google Scholar] [CrossRef]
- Couret, D.; Jamin, A.; Kuntz-Simon, G.; Prunier, A.; Merlot, E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Vet. Immunol. Immunopathol. 2009, 131, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Maskal, J.M.; Duttlinger, A.W.; Kpodo, K.R.; McConn, B.R.; Byrd, C.J.; Richert, B.T.; Marchant-Forde, J.N.; Lay, D.C.; Perry, S.D.; et al. In utero heat stress alters the postnatal innate immune response of pigs. J. Anim. Sci. 2020, 98, 1–13. [Google Scholar] [CrossRef]
- Pravieux, J.J.; Poulet, H.; Charreyre, C.; Juillard, V. Protection of Newborn Animals through Maternal Immunization. J. Comp. Pathol. 2007, 137, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Kick, A.R.; Wolfe, Z.C.; Amaral, A.F.; Cortes, L.M.; Almond, G.W.; Crisci, E.; Gauger, P.C.; Pittman, J.; Käser, T. Maternal Autogenous Inactivated Virus Vaccination Boosts Immunity to PRRSV in Piglets. Vaccines 2021, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Anil, L.; Anil, S.S.; Deen, J.; Baidoo, S.K. Cortisol, behavioral responses, and injury scores of sows housed in gestation stalls. J. Swine Health Prod. 2006, 14, 196–201. [Google Scholar]
- Tsuma, V.T.; Einarsson, S.; Madej, A.; Lundeheim, N. Cortisol and β-endorphin levels in peripheral circulation around weaning in primiparous sows. Anim. Reprod. Sci. 1995, 37, 175–182. [Google Scholar] [CrossRef]
- Chevaux, E.; Guillou, D.; Keith, E. Meta-analysis of the influence of live yeast addition on feed intake in lactating sows. J. Anim. Sci. 2015, 93, 415. [Google Scholar]
- Jurgens, M.H.; Rikabi, R.A.; Zimmerman, D.R. The effect of dietary active dry yeast supplement on performance of sows during gestation-lactation and their pigs. J. Anim. Sci. 1997, 75, 593–597. [Google Scholar] [CrossRef]
- Domingos, R.L.; Silva, B.A.N.; de Laguna, F.B.; Araujo, W.A.G.; Gonçalves, M.F.; Rebordões, F.I.G.; Evangelista, R.P.; de Alkmim, T.C.C.; Miranda, H.A.F.; Cardoso, H.M.C.; et al. Saccharomyces Cerevisiae var. Boulardii CNCM I-1079 during late gestation and lactation improves voluntary feed intake, milk production and litter performance of mixed-parity sows in a tropical humid climate. Anim. Feed Sci. Technol. 2021, 272, 114785. [Google Scholar] [CrossRef]
- Rist, V.T.S.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Merska, M.; Ognik, K. Blood Immunological and Biochemical Indicators in Turkey Hens Fed Diets with a Different Content of the Yeast Yarrowia Lipolytica. Ann. Anim. Sci. 2014, 14, 935–946. [Google Scholar] [CrossRef]
- Shen, Y.B.; Piao, X.S.; Kim, S.W.; Wang, L.; Liu, P.; Yoon, I.; Zhen, Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 2009, 87, 2614–2624. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of Yeast Culture in Broiler Diets on Performance and Immunomodulatory Functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.; Dlamini, Z.; Okoh, A.; Langa, R. Effects of probiotics on growth performance, blood parameters, and antibody stimulation in piglets. S. Afr. J. Anim. Sci. 2017, 47, 765. [Google Scholar] [CrossRef]
- Shen, Y.B.; Carroll, J.A.; Yoon, I.; Mateo, R.D.; Kim, S.W. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 2011, 89, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Nakayama, D.; Toda, K.; Inagaki, S.; Tanaka, K.; Yasui, H. Suppressive Effect of Wild Saccharomyces cerevisiae and Saccharomyces paradoxus Strains on Ige Production by Mouse Spleen Cells. Food Sci. Technol. Res. 2013, 19, 1019–1027. [Google Scholar] [CrossRef][Green Version]
- Actor, J.K. Innate Immunity. In Elsevier’s Integrated Review Immunology and Microbiology, 2nd ed.; Actor, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 43–51. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Chapter 24: The Adaptive Immune System. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]

| Measures | Control | Probiotic | SEM | p-Value |
|---|---|---|---|---|
| Plasma cortisol, ng/mL | 205.8 | 111.1 | 9.21 | <0.000 |
| White blood cell, 107/10 µL | 1.67 | 2.37 | 0.180 | 0.006 |
| Lymphocytes, % | 47.1 | 38.9 | 1.09 | 0.002 |
| Neutrophils, % | 51.5 | 59.0 | 1.17 | 0.004 |
| Monocytes, % | 0.98 | 1.57 | 0.180 | 0.019 |
| Eosinophils, % | 0.44 | 0.29 | 0.073 | 0.149 |
| Neutrophil-to-lymphocyte ratio | 1.16 | 1.84 | 0.122 | <0.001 |
| Interleukin-12 pg/mL | 172.4 | 170.0 | 9.15 | 0.736 |
| Measures | Control | Probiotic | SEM | p-Value |
|---|---|---|---|---|
| Plasma cortisol, ng/mL | 119.0 | 75.1 | 6.20 | <0.000 |
| White Blood Cell, 107/10µL | 2.03 | 2.51 | 0.142 | 0.019 |
| Lymphocytes, % | 37.6 | 32.2 | 1.20 | <0.005 |
| Neutrophils, % | 60.6 | 64.6 | 1.02 | 0.023 |
| Monocytes, % | 1.31 | 2.11 | 0.182 | 0.002 |
| Eosinophils, % | 0.46 | 0.51 | 0.078 | 0.626 |
| Neutrophil-to-lymphocyte ratio | 2.34 | 2.62 | 0.285 | 0.507 |
| Interluekin-12, pg/mL | 183.5 | 190.1 | 7.49 | 0.531 |
| 7D | 14D | 21D | ||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Control | Probiotic | Control | Probiotic | Control | Probiotic | SEM | Treatment × Age |
| Plasma cortisol, ng/mL | 43.4 | 42.4 | 37.1 | 45.6 | 44.2 | 50.8 | 6.00 | 0.493 |
| White blood cell, 107/10 µL | 6.29 | 6.10 | 3.49 | 3.34 | 1.41 | 2.24 | 0.70 | 0.697 |
| Lymphocyte, 107/mL | 3.96 a | 4.86 a | 3.70 a | 4.68 a | 8.94 b | 4.03 a | 2.01 | 0.005 |
| Neutrophil, 106/mL | 4.43 | 3.86 | 2.49 | 2.71 | 2.64 | 2.04 | 0.53 | 0.432 |
| Lymphocytes, % | 43.9 a | 53.0 b | 60.4 c | 64.4 cd | 65.7 d | 65.4 cd | 2.23 | 0.060 |
| Neutrophils, % | 53.7 a | 44.4 b | 37.4 c | 32.7 c | 33.0 c | 32.5 c | 2.25 | 0.088 |
| Monocytes, % | 2.47 a | 2.20 ab | 1.88 a | 1.17 ad | 1.69 ac | 2.13 ab | 0.43 | 0.165 |
| Eosinophils, % | 0.27 | 0.40 | 0.46 | 0.53 | 0.42 | 0.36 | 0.13 | 0.758 |
| Neutrophil-to-lymphocyte ratio | 1.62 a | 0.97 b | 0.69 c | 0.61 c | 0.57 c | 0.61 c | 0.11 | 0.001 |
| Chemotaxis-C5a, no./5 fields | 31.2 a | 31.6 a | 39.9 a | 68.2 b | 110.2 c | 115.4 c | 4.94 | 0.052 |
| Chemotaxis- IL8, no./5 fields | 34.9 a | 32.1 a | 56.3 b | 84.6 c | 125.1 d | 92.4 c | 6.21 | 0.007 |
| Neutrophil phagocytosis, % | 67.4 ab | 68.7 a | 63.4 ab | 61.1 bc | 56.0 c | 62.2 b | 3.09 | 0.036 |
| Natural killer cell cytotoxicity, % | 54.9 | 53.4 | 58.6 | 66.9 | 49.7 | 67.7 | 7.81 | 0.605 |
| Concanavalin-A proliferation | 1.02 a | 1.25 ab | 1.18 ab | 1.27 ab | 2.47 c | 1.75 d | 0.12 | 0.053 |
| Lipopolysaccharide proliferation | 1.39 a | 1.76 b | 1.31 a | 1.14 c | 1.46 a | 1.72 b | 0.20 | 0.067 |
| Interleukin-12, pg/mL | 165.6 | 158.1 | 259.7 | 276.8 | 296.3 | 317.6 | 17.95 | 0.425 |
| D0 | D1 | D7 | D14 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Control | Probiotic | Control | Probiotic | Control | Probiotic | Control | Probiotic | SEMp | Treatment × Day |
| Plasma cortisol, ng/mL | 44.4 a | 50.8 a | 36.4 b | 53.3 a | 24.7 c | 31.1 bc | 25.8 c | 25.1 c | 4.00 | 0.023 |
| White Blood Cell (WBC), 108/mL | 1.41 a | 2.21 a | 2.19 a | 1.35 b | 2.30 b | 2.08 ab | 3.37 c | 3.51 c | 0.28 | <0.001 |
| Lymphocyte, 107/mL | 8.94 a | 4.03 b | 3.44 bc | 1.58 c | 3.80 b | 2.51 bc | 5.25 b | 3.72 b | 1.31 | 0.052 |
| Neutrophil, 106/mL | 2.64 a | 2.04 a | 1.76 a | 1.64 a | 2.26 a | 2.02 a | 7.29 b | 5.13 c | 0.47 | 0.061 |
| Lymphocytes, % | 65.7 | 65.2 | 56.1 | 57.5 | 59.0 | 61.0 | 47.7 | 56.3 | 2.40 | 0.143 |
| Neutrophils, % | 33.0 | 32.1 | 41.1 | 39.6 | 37.1 | 33.9 | 49.7 | 40.9 | 2.51 | 0.196 |
| Monocytes, % | 1.69 | 2.13 | 2.54 | 2.44 | 3.04 | 3.92 | 1.71 | 1.87 | 0.59 | 0.504 |
| Eosinophils, % | 0.41 | 0.35 | 0.38 | 0.30 | 0.52 | 1.04 | 0.96 | 0.87 | 0.15 | 0.116 |
| Neutrophil-to-lymphocyte ratio | 0.57 a | 0.60 a | 0.85 b | 0.87 b | 0.72 ab | 0.63 a | 1.23 c | 0.81 b | 0.10 | 0.012 |
| Chemotaxis-C5a, no. | 110.2 | 115.4 | 60.1 | 64.5 | 75.3 | 58.3 | 67.8 | 64.1 | 13.2 | 0.858 |
| Chemotaxis-IL-8, no. | 125.1 a | 92.4 b | 118.8 a | 143.8 c | 65.0 d | 66.7 d | 113.4 a | 173.1 e | 9.3 | 0. 053 |
| Neutrophil phagocytosis, % | 56.0 a | 62.3 b | 70.1 c | 65.3 bc | 60.5 b | 62.3 b | 66.2 c | 68.3 c | 1.91 | <0.008 |
| Natural killer cell cytotoxicity, % | 49.7 | 67.7 | 33.2 | 35.7 | 70.7 | 71.5 | 47.7 | 45.0 | 5.75 | 0.237 |
| ConcanavalinA proliferation | 2.47 a | 1.75 b | 1.72 b | 2.32 a | 2.36 a | 2.43 a | 2.18 ab | 2.06 ab | 0.23 | 0.021 |
| Lipopolysaccharide proliferation | 1.45 ab | 1.72 a | 1.65 a | 1.89 a | 1.10 b | 1.63 a | 1.65 a | 3.07 c | 0.38 | 0.072 |
| Interluekin-12, pg/mL | 296.3 | 317.4 | 230.0 | 258.7 | 419.3 | 411.8 | 312.9 | 353.5 | 23.7 | 0.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salak-Johnson, J.L.; Reddout, C.; Hernandez, L.; Visconti, A. Maternal Supplementation of Saccharomyces cerevisiae boulardii during Late-Gestation through Lactation Differentially Modulated Immune Status and Stress Responsiveness of the Progeny to Farrowing and Weaning Stressors. Animals 2022, 12, 164. https://doi.org/10.3390/ani12020164
Salak-Johnson JL, Reddout C, Hernandez L, Visconti A. Maternal Supplementation of Saccharomyces cerevisiae boulardii during Late-Gestation through Lactation Differentially Modulated Immune Status and Stress Responsiveness of the Progeny to Farrowing and Weaning Stressors. Animals. 2022; 12(2):164. https://doi.org/10.3390/ani12020164
Chicago/Turabian StyleSalak-Johnson, Janeen L., Cassidy Reddout, Lily Hernandez, and Anne Visconti. 2022. "Maternal Supplementation of Saccharomyces cerevisiae boulardii during Late-Gestation through Lactation Differentially Modulated Immune Status and Stress Responsiveness of the Progeny to Farrowing and Weaning Stressors" Animals 12, no. 2: 164. https://doi.org/10.3390/ani12020164
APA StyleSalak-Johnson, J. L., Reddout, C., Hernandez, L., & Visconti, A. (2022). Maternal Supplementation of Saccharomyces cerevisiae boulardii during Late-Gestation through Lactation Differentially Modulated Immune Status and Stress Responsiveness of the Progeny to Farrowing and Weaning Stressors. Animals, 12(2), 164. https://doi.org/10.3390/ani12020164






