Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Methods
2.2.1. Collection of Records
2.2.2. Environmental Descriptions
2.2.3. Model Calibration and Evaluation
2.2.4. Conservation and Threat Issues
3. Results
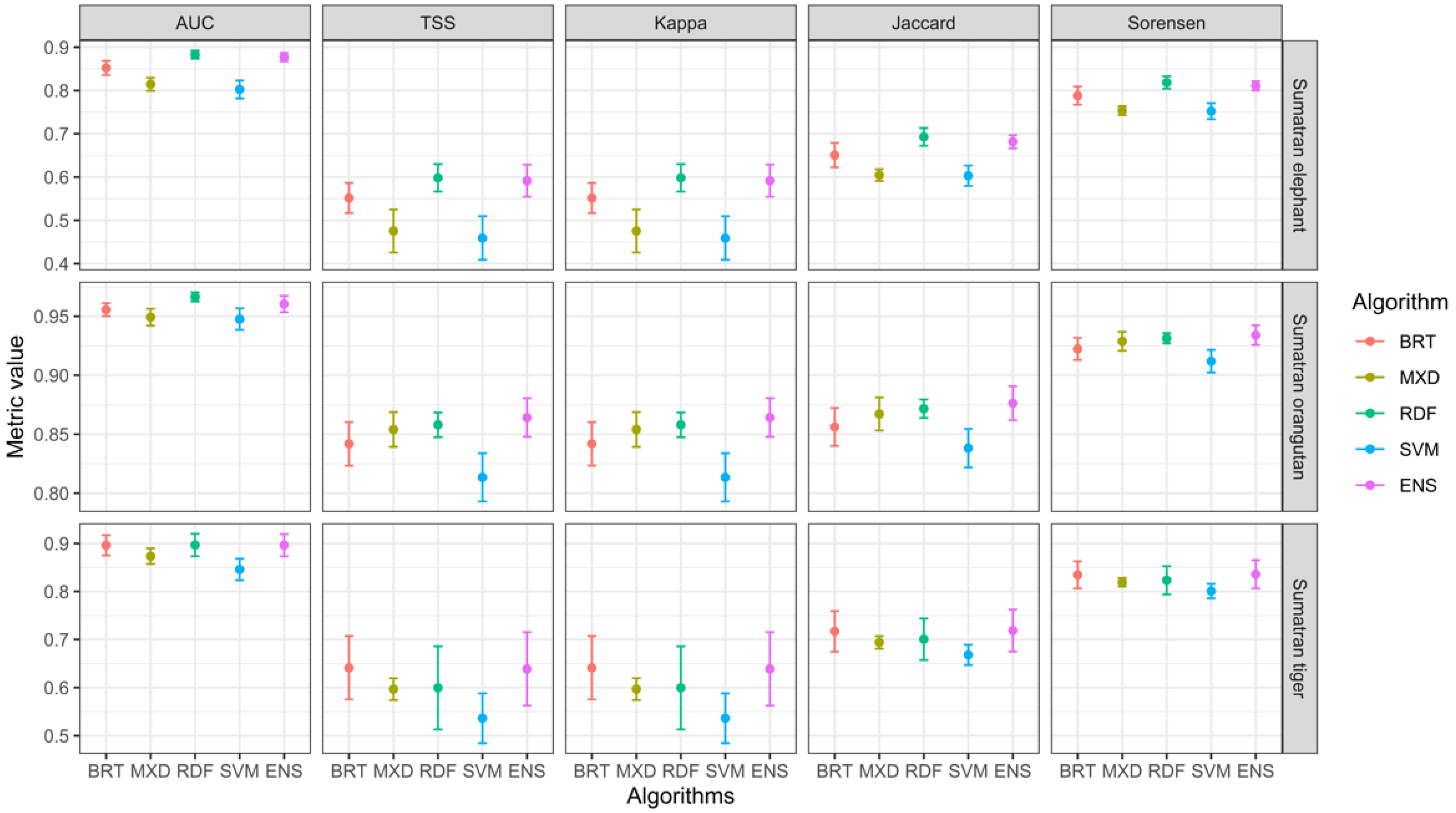
3.1. Models Performance Evaluation
3.2. Environmental Variables and Habitat Suitability
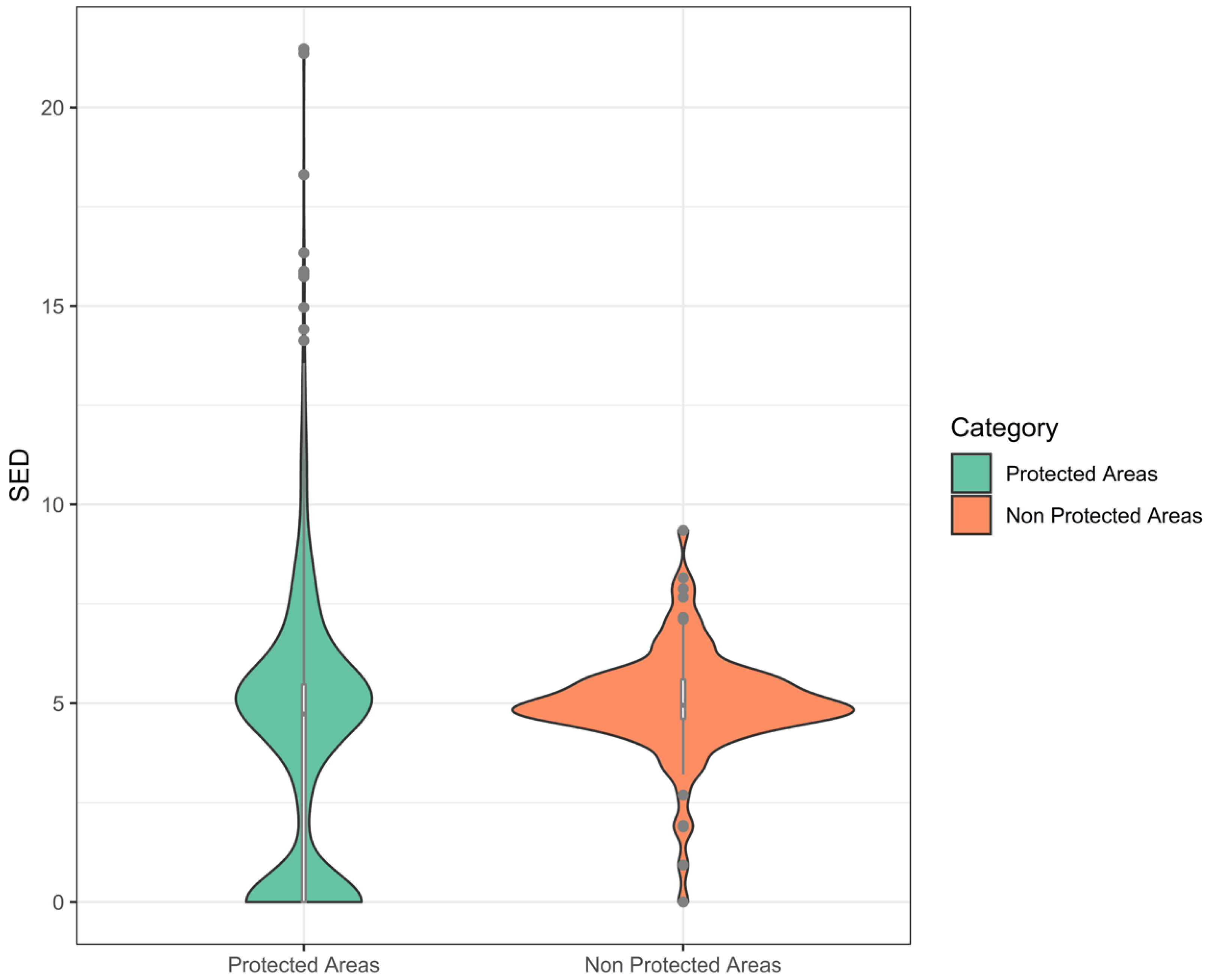
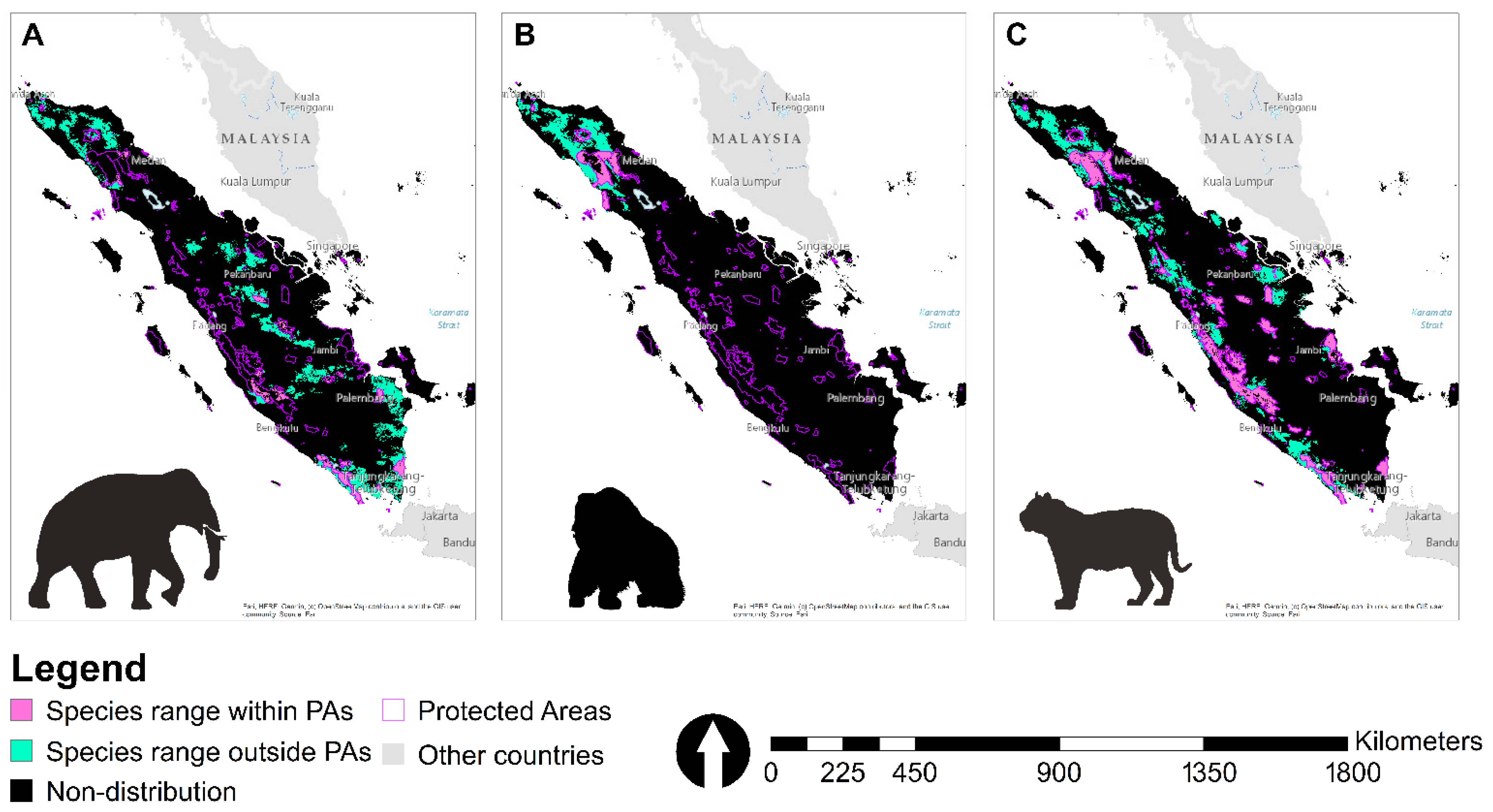
3.3. Suitable Landscape, Protected Areas, and Concessions
4. Discussion
4.1. Model Performance and Utility of Models to Identify Area of Suitable Habitats
4.2. Effect of Environmental Variables on the Three Charismatic Species Occurrence
4.3. Contribution of Different Land-Use Types to Three Charismatic Species Conservation in Sumatra
4.4. Habitat Conservation and Management Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, D.A.; Sitorus, A.B.Y.; Condro, A.A. From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics. Drones 2022, 6, 6. [Google Scholar] [CrossRef]
- Whitmore, T.C. Tropical Rain Forests of the Far East; Clarendon Press: Oxford, UK, 1984. [Google Scholar]
- Phillips, O.L.; Gentry, A.H. Increasing Turnover Through Time in Tropical Forests. Science 1994, 263, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.; Rajpar, M.N.; Ozdemir, I.; Rosli, Z. Fauna Diversity in Tropical Rainforest: Threats from Land-Use Change. In Tropical Forests—The Challenges of Maintaining Ecosystem Services While Managing the Landscape; Intech: London, UK, 2016; pp. 11–49. [Google Scholar]
- Gopala, A.; Hadian, O.; Sunarto, S.; Sitompul, A.; Williams, A.; Leimgruber, P.; Chambliss, S.E.; Gunaryadi, D. Elephas maximus ssp. Sumatranus. The IUCN Red List of Threatened Species 2011: E.T199856A9129626; IUCN: Geneva, Switzerland, 2011. [Google Scholar] [CrossRef]
- Singleton, I.; Wich, S.A.; Nowak, M.; Usher, G.; Utami-Atmoko, S.S. Pongo abelii (Errata Version Published in 2018). The IUCN Red List of Threatened Species 2017: E.T121097935A123797627; IUCN: Geneva, Switzerland, 2007. [Google Scholar] [CrossRef]
- Linkie, M.; Wibisono, H.T.; Martyr, D.J.; Sunarto, S. Panthera tigris ssp. Sumatrae. The IUCN Red List of Threatened Species 2008: E.T15966A5334836; IUCN: Geneva, Switzerland, 2008. [Google Scholar] [CrossRef]
- Ministry of Environment and Forestry. Peraturan Menteri Lingkungan Hidup dan Kehutanan Republik Indonesia No. P.106/MENLHK/SETJEN/KUM.1/12/2018 Tentang Perubahan Kedua Atas Peraturan Menteri Lingkungan Hidup dan Kehutanan No. P.20/MENLHK/SETJEN/KUM.1/6/2018 Tentang Jenis Tumbuhan dan Satwa Yang Dilindungi; Sekretariat Kabinet RI: Jakarta, Indonesia, 2018.
- Gaveau, D.L.A.; Wandonoc, H.; Setiabudi, F. Three Decades of Deforestation in Southwest Sumatra: Have Protected Areas Halted Forest Loss and Logging, and Promoted Re-growth? Biol. Conserv. 2007, 134, 495–504. [Google Scholar] [CrossRef]
- Sitompul, A.F.; Griffin, C.R.; Rayl, N.D.; Fuller, T.K. Spatial and Temporal Habitat Use of an Asian Elephant in Sumatra. Animals 2013, 3, 670–679. [Google Scholar] [CrossRef]
- Rao, M.; van Schaik, C.P. The Behavioural Ecology of Sumatran Orangutans in Logged and Unlogged Forest. Trop. Biodivers. 1997, 4, 173–185. [Google Scholar]
- Hardus, M.E.; Lameira, A.R.; Menken, S.B.J.; Wich, S.A. Effects of Logging on Orangutan Behavior. Biol. Conserv. 2012, 146, 177–187. [Google Scholar] [CrossRef]
- Hardus, M.E.; Lameira, A.R.; Zulfa, A.; Utami Atmoko, S.; de Vries, J.; Wich, S.A. Behavioral, Ecological and Evolutionary Aspects of Meat-eating by Sumatran Orangutans (Pongo abelii). Int. J. Primatol. 2012, 33, 287–304. [Google Scholar] [CrossRef]
- Wibisono, H.T. Population Ecology of Sumatran Tigers (Panthera tigris sumatrae) and Their Prey in Bukit Barisan Selatan National Park, Sumatra, Indonesia. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2005; p. 90. [Google Scholar]
- Wibisono, H.T.; Pusparini, W. Sumatran Tiger (Panthera tigris sumatrae): A Review of Conservation Status. Integr. Zool. 2010, 5, 309–318. [Google Scholar] [CrossRef]
- Wibisono, H.T.; Linkie, M.; Guillera-Arroita, G.; Smith, J.A.; Sunarto; Pusparini, W.; Asriadi; Baroto, P.; Brickle, N.; Dinata, Y.; et al. Population Status of a Cryptic Top Predator: An Island-Wide Assessment of Tigers in Sumatran Rainforests. PLoS ONE 2011, 6, e25931. [Google Scholar] [CrossRef]
- Hedges, S.; Tyson, M.J.; Sitompul, A.F.; Kinnaird, M.F.; Gunaryadi, D.; Aslan. Distribution, Status, and Conservation Needs of Asian Elephants (Elephas maximus) in Lampung Province, Sumatra, Indonesia. Biol. Conserv. 2005, 124, 35–48. [Google Scholar] [CrossRef]
- Linkie, M.; Chapron, G.; Martyr, D.J.; Holden, J.; Leader-Williams, N. Assessing the Viability of Tiger Subpopulations in a Fragmented Landscape. J. Appl. Ecol. 2006, 43, 576–586. [Google Scholar] [CrossRef]
- Wich, S.A.; Meijaard, E.; Marshall, A.J.; Husson, S.; Ancrenaz, M.; Lacy, R.C.; van Schaik, C.P.; Sugardjito, J.; Simorangkir, T.; Traylor-Holzer, K.; et al. Distribution and Conservation Status of the Orangutan (Pongo spp.) on Borneo and Sumatra: How Many Remain? Oryx 2008, 42, 329–339. [Google Scholar] [CrossRef]
- Wich, S.A.; Singleton, I.; Nowak, M.G.; Utami-Atmoko, S.S.; Nisam, G.; Arif, S.M.; Putra, R.H.; Ardi, R.; Fredriksson, G.; Usher, G.; et al. Land-cover Changes Predict Steep Declines for the Sumatran Orangutan (Pongo abelii). Sci. Adv. 2016, 2, e1500789. [Google Scholar] [CrossRef]
- Sukmantoro, Y.W.; Alikodra, H.S.; Kartono, A.P.; Efransjah. Distribution and Habitat Preferences of Sumatran Elephant (Elephas maximus sumatranus) in Riau, Indonesia. Biodiversitas 2019, 20, 226–235. [Google Scholar] [CrossRef]
- Priatna, D. Habitat Suitability Model to Determine a Suitable Area for Translocation of Sumatran Tiger (Panthera tigris sumatrae Pocock, 1929). Asian J. Conserv. Biol. 2020, 9, 39–55. [Google Scholar]
- Monterroso, P.; Brito, J.C.; Ferreras, P.; Alves, P.C. Spatial Ecology of the European Wildcat in a Mediterranean Ecosystem: Dealing with Small Radiotracking Datasets in Species Conservation. J. Zool. 2009, 279, 27–35. [Google Scholar] [CrossRef]
- Angelieri, C.C.S.; Adams-Hosking, C.; Ferraz, K.M.P.M.d.B.; de Souza, M.P.; McAlpine, C.A. Using Species Distribution Models to Predict Potential Landscape Restoration Effects on Puma Conservation. PLoS ONE 2016, 11, e0145232. [Google Scholar] [CrossRef]
- Rahman, D.A.; Gonzalez, G.; Haryono, M.; Muhtarom, A.; Firdaus, A.Y.; Aulagnier, S. Factors Affecting Seasonal Habitat Use and Predicted Range of Two Tropical Deer in Indonesian Rainforest. Acta Oecol. 2017, 82, 41–51. [Google Scholar] [CrossRef]
- Wibisono, H.T.; Wahyudi, H.A.; Wilianto, E.; Pinondang, I.M.R.; Primajati, M.; Liswanto, D.; Linkie, M. Identifying Priority Conservation Landscapes and Actions for the Critically Endangered Javan Leopard in Indonesia: Conserving the Last Large Carnivore in Java Island. PLoS ONE 2018, 13, e0198369. [Google Scholar] [CrossRef]
- Croft, S.; Ward, A.I.; Aegerter, J.N.; Smith, G.C. Modeling Current and Potential Distributions of Mammal Species Using Presence-only Data: A Case Study on British Deer. Ecol. Evol. 2019, 9, 8724–8735. [Google Scholar] [CrossRef]
- Rahman, D.A.; Rinaldi, D.; Kuswanda, W.; Siregar, R.; Noorchasanatun, F.; Hakim, F.; Arief, H.; Putro, H.R. Determining the Landscape Priority and Their Threats for the Critically Endangered Pongo tapanuliensis Population in Indonesia. Biodiversitas 2019, 20, 3584–3592. [Google Scholar] [CrossRef]
- Rahman, D.A. Ecological Niche and Potential Distribution of the Endangered Bos javanicus in Southwestern Java, Indonesia. Therya 2020, 11, 57–68. [Google Scholar] [CrossRef]
- Rahman, D.A.; Condro, A.A.; Rianti, P.; Masy’ud, B.; Aulagnier, S.; Semiadi, G. Geographical Analysis of the Javan Deer Distribution in Indonesia and Priorities for Landscape Conservation. J. Nat. Conserv. 2020, 54, 125795. [Google Scholar] [CrossRef]
- Graham, C.H.; Ferrier, S.; Huettman, F.; Moritz, C.; Peterson, A.T. New Developments in Museum-Based Informatics and Applications in Biodiversity Analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Dai, L.; Fu, D.; Liu, B.; Wang, H.; Chen, H. Factors Influencing the Geographical Distribution of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytidae) in China. Forests 2019, 10, 425. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The Effect of Sample Size and Species Characteristics on Performance of Different Species Distribution Modeling Methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Navia-Vazquez, A.; Gutierrez-Gonzalez, D.; Parrado-Hernandez, E.; Navarro-Abellan, J.J. Distributed Support Vector Machines. IEEE Trans. Neural. Netw. 2006, 17, 1091–1097. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- De’ath, G. Boosted Trees for Ecological Modeling and Prediction. Ecology 2007, 88, 243–251. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of Consensus Methods in Predictive Species Distribution Modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species Distribution from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Cianfrani, C.; LeLay, G.; Hirzel, A.H.; Loy, A. Do Habitat Suitability Models Reliably Predict the Recovery Areas of Threatened Species? J. Appl. Ecol. 2010, 47, 421–430. [Google Scholar] [CrossRef]
- Uryu, Y.; Mott, C.; Foead, N.; Yulianto, K.; Budiman, A.; Setiabudi; Takakai, F.; Nursamsu.; Sunarto; Purastuti, E.; et al. Deforestation, Forest Degradation, Biodiversity Loss and CO2 Emissions in Riau, Sumatra, Indonesia; WWF Indonesia Technical Report: Jakarta, Indonesia, 2008. [Google Scholar]
- GBIF, 2022. GBIF Occurrence Download [WWW Document]. Available online: https://www.gbif.org/occurrence/download/0258363-210914110416597 (accessed on 5 January 2022).
- IUCN. IUCN Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN: Geneva, Switzerland, 2012; Available online: https://portals.iucn.org/library/node/10315 (accessed on 10 January 2022).
- Velazco, S.J.E.; Villalobos, F.; Galvão, F.; De Marco Júnior, P. A Dark Scenario for Cerrado Plant Species: Effects of Future Climate, Land Use and Protected Areas Ineffectiveness. Divers. Distrib. 2019, 25, 660–673. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The Shuttle Radar Topography Mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- Georgievski, G.; Hagemann, S. Characterizing uncertainties in the ESA-CCI land cover map of the epoch 2010 and their impacts on MPI-ESM climate simulations. Theor. Appl. Climatol. 2019, 137, 1587–1603. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with it and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in Ecological Niche Modeling: Confusions and Challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Karatzoglou, A.; Hornik, K.; Smola, A.; Zeileis, A. Kernlab—An S4 Package for Kernel Methods in R. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef]
- Andrade, A.F.A.; de Velazco, S.J.E.; De Marco Júnior, P. ENMTML: An R Package for a Straightforward Construction of Complex Ecological Niche Models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- Thuiller, W.; Brotons, L.; Araújo, M.B.; Lavorel, S. Effects of Restricting Environmental Range of Data to Project Current and Future Species Distributions. Ecography 2004, 27, 165–172. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without Quality Presence–Absence Data, Discrimination Metrics such as TSS can be Misleading Measures of Model Performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Kremen, C.; Cameron, A.; Moilanen, A.; Phillips, S.J.; Thomas, C.D.; Beentje, H.; Dransfield, J.; Fisher, B.L.; Glaw, F.; Good, T.C.; et al. Aligning Conservation Priorities Across Taxa in Madagascar with High-Resolution Planning Tools. Science 2008, 320, 222–226. [Google Scholar] [CrossRef]
- Mendes, P.; Velazco, S.J.E.; de Andrade, A.F.A.; De Marco, P. Dealing with Overprediction in Species Distribution Models: How Adding Distance Constraints Can Improve Model Accuracy. Ecol. Modell. 2020, 431, 109180. [Google Scholar] [CrossRef]
- Yudhistira, P. Sang Pelopor Peranan Dr. SH. Koorders Dalam Sejarah Perlindungan Alam Di Indonesia. Direktorat Kawasan Konservasi Dan Bina Hutan Lindung Ditjen Perlindungan Hutan Dan Konservasi Alam Kementerian Kehutanan. 2014. Available online: http://ksdae.menlhk.go.id/sejarah-ksdae.html (accessed on 10 November 2021).
- Ditjen KSDAE. Statistik Direktorat Jenderal Konservasi Sumberdaya Alam dan Ekosistem Tahun 2020; Kementerian Lingkungan Hidup dan Kehutanan Republik Indonesia: Jakarta, Indonesia, 2020.
- Condro, A.A.; Prasetyo, L.B.; Rushayati, S.B.; Santikayasa, I.P.; Iskandar, E. Measuring Metrics of Climate Change and Its Implication on the Endangered Mammal Conservation in the Leuser Ecosystem. Front. Environ. Sci. 2021, 9, 713837. [Google Scholar] [CrossRef]
- Ditjen PHKA. Strategi dan Rencana Aksi Konservasi Gajah Indonesia 2007–2017; Kementerian Kehutanan Republik Indonesia: Jakarta, Indonesia, 2007.
- Ditjen PHKA. Strategi dan Rencana Aksi Konservasi Orangutan Indonesia 2007–2017; Kementerian Kehutanan Republik Indonesia: Jakarta, Indonesia, 2007.
- Ditjen PHKA. Strategi dan Rencana Aksi Konservasi Harimau Sumatera Indonesia 2007–2017; Kementerian Kehutanan Republik Indonesia: Jakarta, Indonesia, 2007.
- Lawler, J.J.; Wiersma, Y.F.; Huettmann, F. Using Species Distribution Models for Conservation Planning and Ecological Forecasting. In Predictive Species and Habitat Modeling in Landscape Ecology; Drew, C., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Segurado, P.; Araújo, M.B. An Evaluation of Methods for Modelling Species Distributions. J. Biogeogr. 2004, 31, 1555–1568. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C. Do They? How do They? Why do They? On Finding Reasons for Differing Performances of Species Distribution Models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Syphard, A.D.; Franklin, J. Differences in Spatial Predictions Among Species Distribution Modeling Methods Vary with Species Traits and Environmental Predictors. Ecography 2009, 32, 907–918. [Google Scholar] [CrossRef]
- Ready, J.; Kaschner, K.; South, A.B.; Eastwood, P.D.; Rees, T.; Rius, J.; Agbayani, E.; Kullander, S.; Froese, R. Predicting the Distributions of Marine Organisms at the Global Scale. Ecol. Modell. 2010, 221, 467–478. [Google Scholar] [CrossRef]
- Arcos, J.M.; Bécares, J.; Rodríguez, B.; Ruiz, A. Áreas Importantes para laConservación de las Aves marinas en España. LIFE04NAT/ES/000049-Sociedad Española de Ornitología (SEO/BirdLife). Madrid. 2009. Available online: http://www.seo.org/avesmarinas/flash.html#/1/ (accessed on 30 March 2022).
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike. Springer Series in Statistics (Perspectives in Statistics); Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What Matters for Predicting the Occurrences of Trees: Techniques, Data, or Species’ Characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Gritti, E.S.; Gaucherel, C.; Crespo-Perez, M.-V.; Chuine, I. How Can Model Comparison Help Improving Species Distribution Models? PLoS ONE 2013, 8, e68823. [Google Scholar] [CrossRef]
- Lee, S.; Jung, H.; Choi, J. Projecting the Impact of Climate Change on the Spatial Distribution of Six Subalpine Tree Species in South Korea Using a Multi-Model Ensemble Approach. Forests 2021, 12, 37. [Google Scholar] [CrossRef]
- Linkie, M.; Martyr, D.J.; Holden, J.; Yanuar, A.; Hartana, A.; Sugardjito, J.; Leader-Williams, N. Habitat Destruction and Poaching Threaten the Sumatran Tiger in Kerinci Seblat National Park, Sumatra. Oryx 2003, 37, 41–48. [Google Scholar] [CrossRef]
- Songer, M.; Sampson, C.; Williams, C.; Forrest, J.; Gyeltshen, K.; Huy, K.; Sharma, A.; Gopala, A.; Budiman, A.; Sadikin, H.; et al. Mapping Habitat and Deforestation in WWF Elephant Priority Landscapes. Gajah 2012, 36, 3–10. [Google Scholar]
- Wich, S.A.; Fredriksson, G.M.; Usher, G.; Peters, H.H.; Priatna, D.; Basalamah, F.; Susanto, W.; Kühl, H.S. Hunting of Sumatran Orangutans and Its Importance in Determining Distribution and Density. Biol. Conserv. 2012, 146, 163–169. [Google Scholar] [CrossRef]
- Utami-Atmoko, S.S.; Traylor-Hozler, K.; Rifqi, M.A.; Siregar, P.; Achmad, B.; Priadjati, A.; Husson, S.; Wich, S.A.; Hadisiswoyo, P.; Saputra, F. (Eds.) The Orangutan Population and Habitat Viability Assessment 2017. In Bogor (ID): The Directorate General of Natural Resources and Ecosystem Conservation; Ministry of Environment and Forestry of Indonesia: Jakarta, Indonesia, 2017. [Google Scholar]
- Luskin, M.S.; Albert, W.R.; Tobler, M.W. Sumatran Tiger Survival Threatened by Deforestation Despite Increasing Densities in Parks. Nat. Commun. 2017, 8, 1783. [Google Scholar] [CrossRef]
- Widodo, F.A.; Imron, M.A.; Sunarto, S.; Giordano, A.J. Carnivores and Their Prey in Sumatra: Occupancy and Activity in Human-Dominated Forests. PLoS ONE 2022, 17, e0265440. [Google Scholar] [CrossRef] [PubMed]
- Blouch, R.A.; Haryanto. Elephants in Southern Sumatra; Unpublished Report; IUCN/WWF Project 3033; IUCN: Bogor, Indonesia, 1984. [Google Scholar]
- Blouch, R.A.; Simbolon, K. Elephants in Northern Sumatra; Unpublished Report; IUCN/WWF Project 3033; IUCN: Bogor, Indonesia, 1985. [Google Scholar]
- Santiapillai, C.; Jackson, P. The Asian Elephant: An Action Plan for its Conservation; IUCN/SSC, Asian Elephant Specialist Group: Gland, Switzerland, 1990. [Google Scholar]
- Azmi, W.; Gunaryadi, D. Current Status of Asian Elephants in Indonesia. Gajah 2011, 35, 55–61. [Google Scholar]
- Poor, E.E.; Shao, Y.; Kelly, M. Mapping and Predicting Forest Loss in a Sumatran Tiger Landscape from 2002 to 2050. J. Environ. Manag. 2019, 231, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gaveau, D.L.A.; Epting, J.; Lyne, O.; Linkie, M.; Kumara, I.; Kanninen, M.; Leader-Williams, N. Evaluating whether Protected Areas Reduce Tropical Deforestation in Sumatra. J. Biogeogr. 2009, 36, 2165–2175. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science 2014, 344, 6332. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Detecting Tropical Forests’ Responses to Global Climatic and Atmospheric Change: Current Challenges and a Way Forward. Biotropica 2004, 39, 4–19. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A Global Overview of Drought and Heat-induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The Velocity of Climate Change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Palomo, I. Climate Change Impacts on Ecosystem Services in High Mountain Areas: A Literature Review. Mt. Res. Dev. 2017, 37, 179–187. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lin, Y.-P.; Lien, W.-Y.; Wang, Y.-C.; Lin, C.-T.; Chiou, C.-R.; Anthony, J.; Crossman, N.D. Expansion of Protected Areas under Climate Change: An Example of Mountainous Tree Species in Taiwan. Forests 2014, 5, 2882–2904. [Google Scholar] [CrossRef]
- Soares-Filho, B.; Moutinho, P.; Nepstad, D.; Anderson, A.; Rodrigues, H.; Garcia, R.; Dietzsch, L.; Merry, F.; Bowman, M.; Hissa, L.; et al. Role of Brazilian Amazon Protected Areas in Climate Change Mitigation. PNAS 2010, 107, 10821–10826. [Google Scholar] [CrossRef]
- Nantha, H.S.; Tisdell, C. The Orangutan–Oil Palm Conflict: Economic Constraints and Opportunities for Conservation. Biodivers. Conserv. 2009, 18, 487–502. [Google Scholar] [CrossRef]
- Lubis, M.I.; Pusparini, W.; Prabowo, S.A.; Marthy, W.; Tarmizi; Andayani, N.; Linkie, M. Unraveling the Complexity of Human–Tiger Conflicts in the Leuser Ecosystem, Sumatra. Anim. Conserv. 2020, 23, 741–749. [Google Scholar] [CrossRef]
- Kuswanda, W.; Garsetiasih, R.; Gunawan, H.; Situmorang, R.O.P.; Hutapea, F.J.; Kwatrina, R.T.; Karlina, E.; Atmoko, T.; Zahrah, M.; Takandjandji, M.; et al. Can Humans and Elephants Coexist? A Review of the Conflict on Sumatra Island, Indonesia. Diversity 2022, 14, 420. [Google Scholar] [CrossRef]
- Fimbel, R.; Robinson, J.G.; Grajal, R. The Cutting Edge: Conserving Wildlife in Logged Tropical Forests; Columbia University Press: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Mayor, P.; Pérez-Peña, P.; Bowler, M.; Puertas, P.E.; Kirkland, M.; Bodmer, R. Effects of Selective Logging on Large Mammal Populations in a Remote Indigenous Territory in the Northern Peruvian Amazon. Ecol. Soc. 2015, 20, 36. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Curran, L.M.; Paoli, G.D.; Carlson, K.M.; Wells, P.; Besse-Rimba, A.; Ratnasari, D.; Leader-Williams, N. Examining Protected Area Effectiveness in Sumatra: Importance of Regulations Governing Unprotected Lands. Conserv. Lett. 2012, 5, 142–148. [Google Scholar] [CrossRef]
- Kebanyakan Orangutan Hidup di Luar Kawasan Konservasi. Available online: http://lipi.go.id/berita/single/kebanyakan-orangutan-hidup-di-luar-kawasan-konservasi/4181 (accessed on 11 September 2022).
- Chiaverini, L.; Macdonald, D.W.; Bothwell, H.M.; Hearn, A.J.; Cheyne, S.M.; Haidir, I.; Hunter, L.T.B.; Kaszta, Ż.; Macdonald, E.A.; Ross, J.; et al. Multi-Scale, Multivariate Community Models Improve Designation of Biodiversity Hotspots in the Sunda Islands. Anim. Conserv. 2022. [Google Scholar] [CrossRef]
- Dudley, N.; Jonas, H.; Nelson, F.; Parrish, J.; Pyhälä, A.; Stolton, S.; Watson, J.E.M. The essential role of other effective area-based conservation measures in achieving big bold conservation targets. Glob. Ecol. Conserv. 2018, 15, e00424. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, J.; Shi, Y.; Li, Y.; Guo, Z.; Wang, S.; Song, S. Effects of Climate Change on the Habitat of the Leopard (Panthera pardus) in the Liupanshan National Nature Reserve of China. Animals 2022, 12, 1866. [Google Scholar] [CrossRef]
- Pan, D.; Song, Y.-L.; Zeng, Z.-G.; Bravery, B.D. Habitat Selection by Eld’s Deer Following Relocation to Patchy Landscape. PLoS ONE 2014, 9, e91158. [Google Scholar] [CrossRef]
- Mori, G.M.; Rojas-Briceño, N.B.; Sánchez, A.C.; Oliva-Cruz, M.; Tarifeño, C.M.O.; Cerna, M.Y.H.; Sandoval, J.D.R.; Guzmán, C.T. Potential Current and Future Distribution of the Long-Whiskered Owlet (Xenoglaux loweryi) in Amazonas and San Martin, NW Peru. Animals 2022, 12, 1794. [Google Scholar] [CrossRef]
| Group | Variables | Species | ||
|---|---|---|---|---|
| E.m. sumatranus | P. abelii | P.t. sumatrae | ||
| Topographic | Slope | 3% | 9% | 6% |
| Topographic | Eastness | 3% | 1% | 3% |
| Topographic | Northness | 3% | 1% | 2% |
| Biophysical | NDWI | 4% | 9% | 9% |
| Biophysical | SAVI | 2% | 1% | 2% |
| Biophysical | Protected areas | 14% | 4% | 15% |
| Anthropogenic | Land cover | 1% | 11% | 6% |
| Anthropogenic | Access to FPC | 8% | 13% | 14% |
| Anthropogenic | Access to LC | 21% | 5% | 8% |
| Anthropogenic | Access to IOPP | 8% | 10% | 8% |
| Anthropogenic | Access to SFC | 7% | 6% | 4% |
| Climatic | Mean temperature | 6% | 11% | 8% |
| Climatic | Temperature seasonality | 7% | 14% | 6% |
| Climatic | Annual precipitation | 6% | 2% | 4% |
| Climatic | Precipitation seasonality | 6% | 4% | 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, D.A.; Santosa, Y.; Purnamasari, I.; Condro, A.A. Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia. Animals 2022, 12, 2722. https://doi.org/10.3390/ani12192722
Rahman DA, Santosa Y, Purnamasari I, Condro AA. Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia. Animals. 2022; 12(19):2722. https://doi.org/10.3390/ani12192722
Chicago/Turabian StyleRahman, Dede Aulia, Yanto Santosa, Intan Purnamasari, and Aryo Adhi Condro. 2022. "Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia" Animals 12, no. 19: 2722. https://doi.org/10.3390/ani12192722
APA StyleRahman, D. A., Santosa, Y., Purnamasari, I., & Condro, A. A. (2022). Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia. Animals, 12(19), 2722. https://doi.org/10.3390/ani12192722






