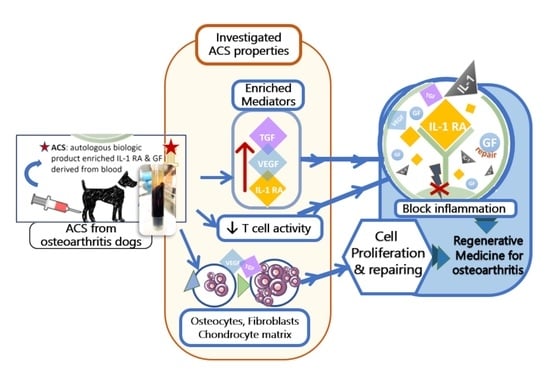
In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Osteoarthritis Dogs
2.3. ACS Sample Collection
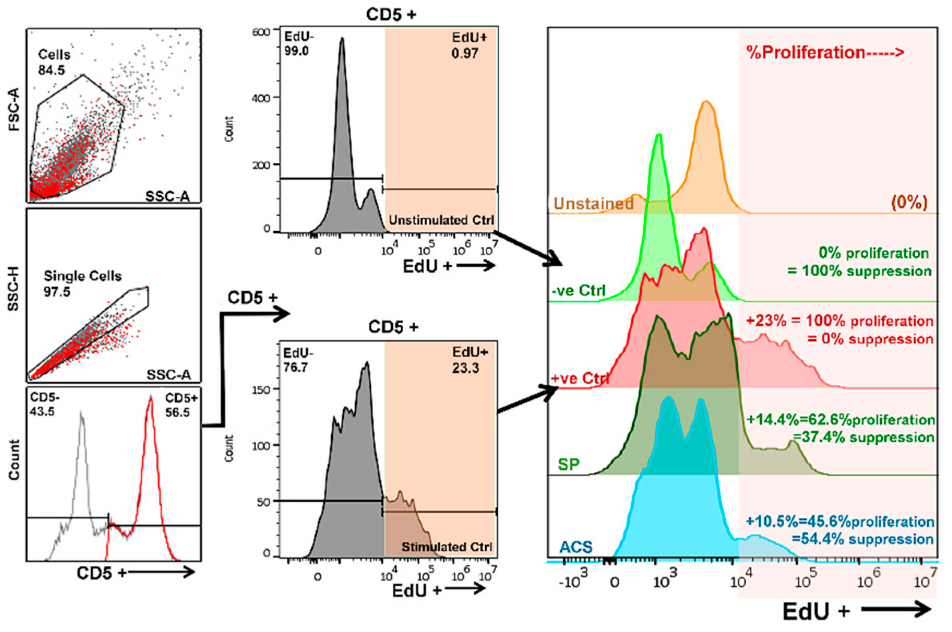
2.4. T Cell Proliferation Assay
2.5. ELISA for Cytokine Production
2.6. Cell Proliferation Assay
2.7. Analysis of Chondrocyte Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Demographic of Study Dogs
3.2. ACS Enriched Mediators for Regenerative and Anti-Inflammation
3.3. Impact of ACS on T Cell Proliferation Activity
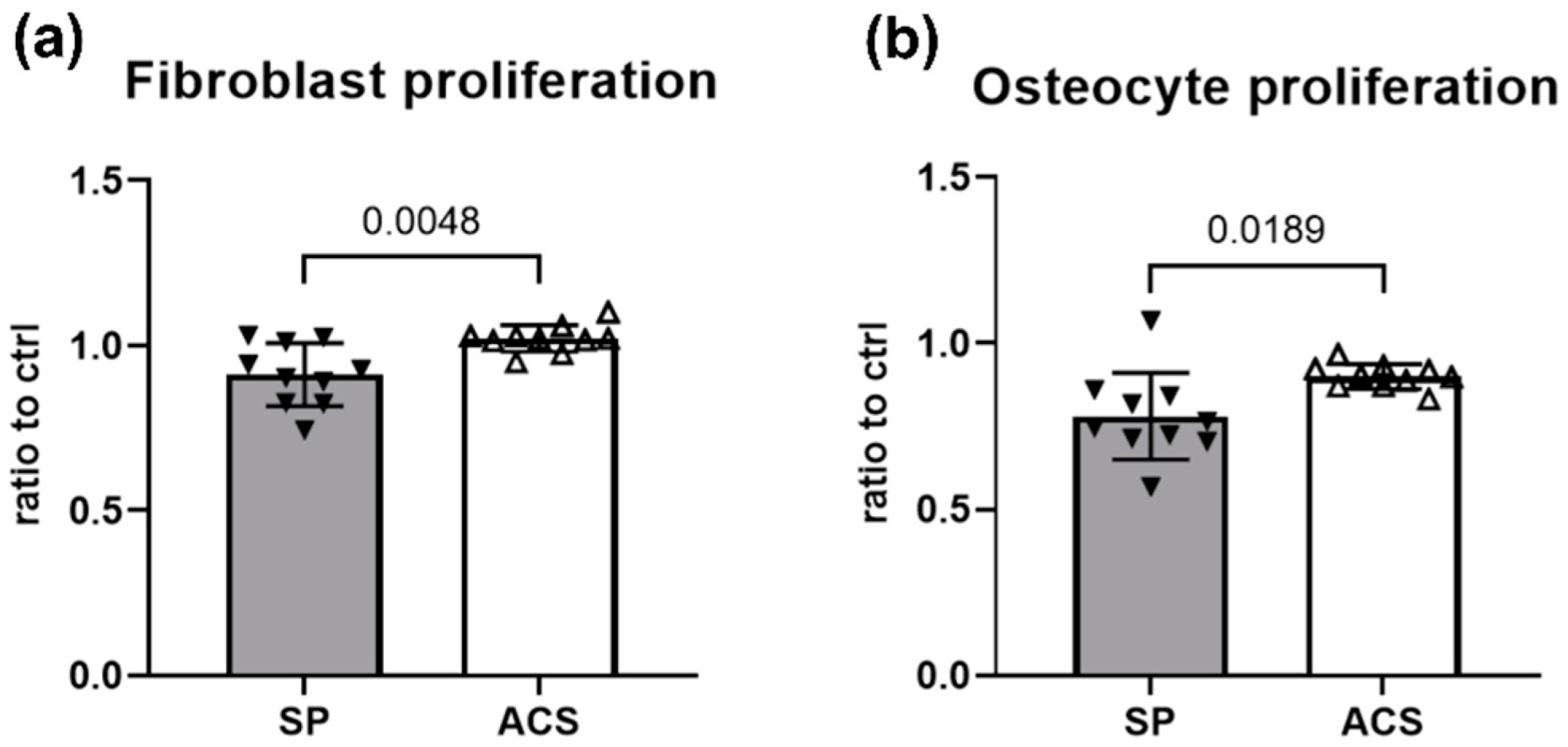
3.4. Promotion of Fibroblast and Osteocyte Proliferation by ACS
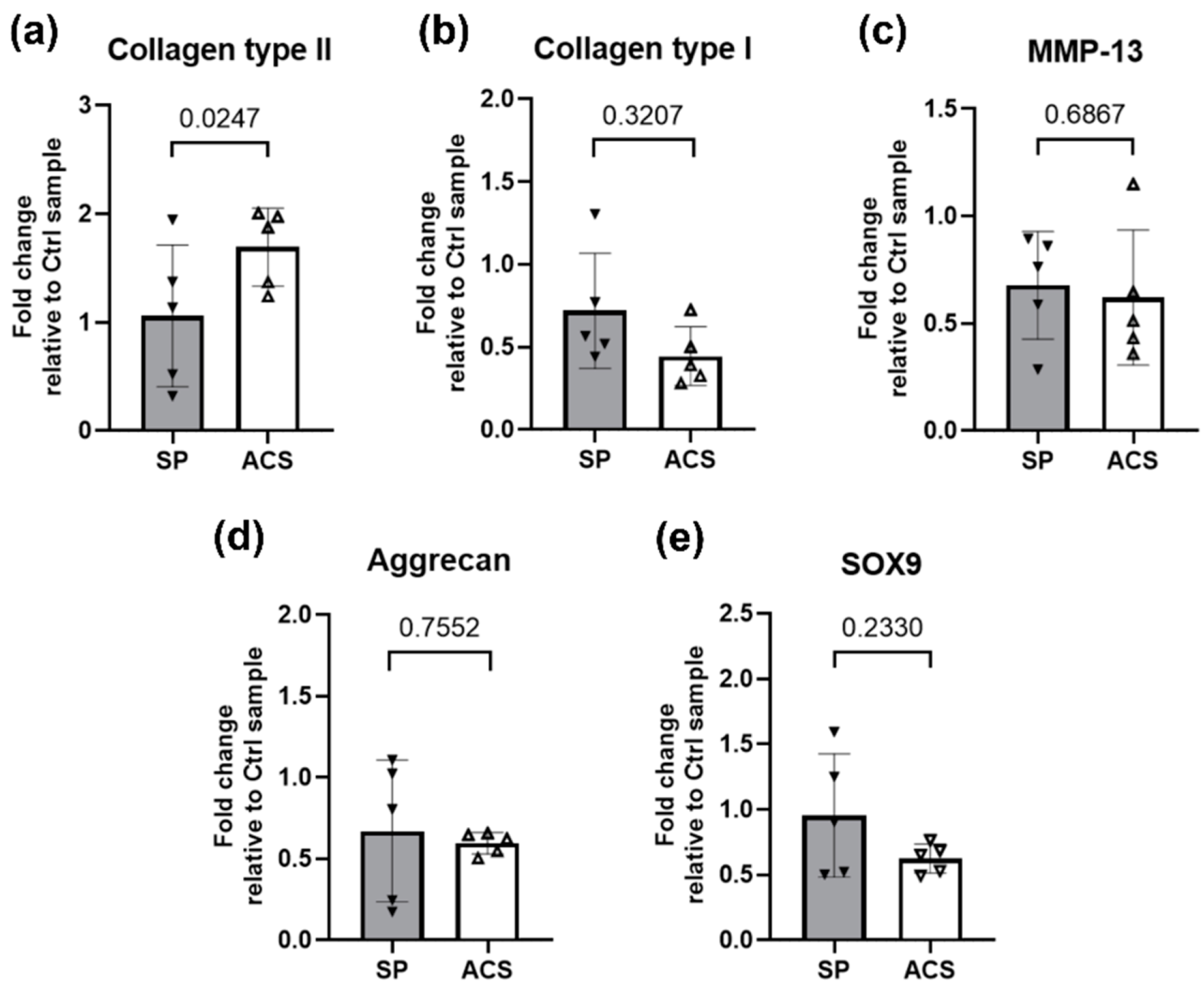
3.5. ACS Effects on Cartilage Matrix mRNA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Waring, N. Canine osteoarthritis: Pathophysiology and management. Vet. Nurse 2014, 5, 462–467. [Google Scholar] [CrossRef]
- Boehme, K.A.; Rolauffs, B. Onset and Progression of Human Osteoarthritis—Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int. J. Mol. Sci. 2018, 19, 2282. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; Zulch, H.; O’Neill, D.G.; Meeson, R.L.; Collins, L.M. Risk Factors for Canine Osteoarthritis and Its Predisposing Arthropathies: A Systematic Review. Front. Vet. Sci. 2020, 7, 220. [Google Scholar] [CrossRef]
- Gagnon, A.; Brown, D.; Moreau, M.; Lussier, B.; Otis, C.; Troncy, E. Therapeutic response analysis in dogs with naturally occurring osteoarthritis. Vet. Anaesth. Analg. 2017, 44, 1373–1381. [Google Scholar] [CrossRef]
- Stephanie, D.B. Canine osteoarthritis and treatments: A review. Vet. Sci. Dev. 2015, 5, 84–89. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; Morris, M.J. A Comparative Review of Autologous Conditioned Serum and Autologous Protein Solution for Treatment of Osteoarthritis in Horses. Front. Vet. Sci. 2021, 8, 602978. [Google Scholar] [CrossRef]
- Frizziero, A.; Giannotti, E.; Oliva, F.; Masiero, S.; Maffulli, N. Autologous conditioned serum for the treatment of osteoarthritis and other possible applications in musculoskeletal disorders. Br. Med. Bull. 2013, 105, 169–184. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Wildi, L.M.; Pelletier, J.-P. Future therapeutics for osteoarthritis. Bone 2012, 51, 297–311. [Google Scholar] [CrossRef]
- Khella, C.M.; Horvath, J.M.; Asgarian, R.; Rolauffs, B.; Hart, M.L. Anti-Inflammatory Therapeutic Approaches to Prevent or Delay Post-Traumatic Osteoarthritis (PTOA) of the Knee Joint with a Focus on Sustained Delivery Approaches. Int. J. Mol. Sci. 2021, 22, 8005. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; Boyle, D.L.; Yu, C.; Paine, M.M.; Whisenand, T.D.; Zvaifler, N.J.; Arend, W.P. Synovial interleukin-1 receptor antagonist and interleukin-1 balance in rheumatoid arthritis. Arthritis Rheum. 1994, 37, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.; Reinecke, J.; Becker, C.; Tholen, G.; Wehling, P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm. Res. 2003, 52, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef]
- Sawyere, D.M.; Lanz, O.I.; Dahlgren, L.A.; Barry, S.L.; Nichols, A.C.; Werre, S.R. Cytokine and Growth Factor Concentrations in Canine Autologous Conditioned Serum. Vet. Surg. 2016, 45, 582–586. [Google Scholar] [CrossRef]
- Barreto, A. A short report on the effect of decreased incubation time on the architectural profile of autologous conditioned serum (ACS). Cytokine 2017, 94, 52–54. [Google Scholar] [CrossRef]
- Moreira, J.J.; Moraes, A.P.L.; Brossi, P.M.; Machado, T.S.L.; Michelacci, Y.M.; Massoco, C.O.; Baccarin, R.Y.A. Autologous processed plasma: Cytokine profile and effects upon injection into healthy equine joints. J. Vet. Sci. 2015, 16, 47–55. [Google Scholar] [CrossRef]
- Baltzer, A.W.A.; Moser, C.; Jansen, S.A.; Krauspe, R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr. Cartil. 2009, 17, 152–160. [Google Scholar] [CrossRef]
- Baselga García-Escudero, J.; Miguel Hernández Trillos, P. Treatment of Osteoarthritis of the Knee with a Combination of Autologous Conditioned Serum and Physiotherapy: A Two-Year Observational Study. PLoS ONE 2016, 10, e0145551. [Google Scholar] [CrossRef]
- Huggins, S.S.; Suchodolski, J.S.; Bearden, R.N.; Steiner, J.M.; Saunders, W.B. Serum concentrations of canine interleukin-1 receptor antagonist protein in healthy dogs after incubation using an autologous serum processing system. Res. Vet. Sci. 2015, 101, 28–33. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Kawcak, C.E.; Werpy, N.M.; Park, R.D.; McIlwraith, C.W. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am. J. Vet. Res. 2007, 68, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.G.A.; Raijmakers, N.J.H.; van Arkel, E.R.A.; Caron, J.J.; Rijk, P.C.; Willems, W.J.; Zijl, J.A.C.; Verbout, A.J.; Dhert, W.J.A.; Saris, D.B.F. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr. Cartil. 2008, 16, 498–505. [Google Scholar] [CrossRef]
- Bogers, S.H. Cell-Based Therapies for Joint Disease in Veterinary Medicine: What We Have Learned and What We Need to Know. Front. Vet. Sci. 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Su varu, C.; Anghel, M.; Georgescu, E.; Chichp, B.; Olìnescu, A. Shift toward T lymphocytes with Th1 and Tc1 cytokine-secterion profile in the joints of patients with osteoarthritis. Roum. Arch. Microbiol. Immunol. 1999, 58, 249–258. [Google Scholar]
- Estrada McDermott, J.; Pezzanite, L.; Goodrich, L.; Santangelo, K.; Chow, L.; Dow, S.; Wheat, W. Role of Innate Immunity in Initiation and Progression of Osteoarthritis, with Emphasis on Horses. Animals 2021, 11, 3247. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.; Bernsen, M.R.; van Osch, G.J. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Coy, J.; Regan, D.; Dow, S. Mechanisms of Immune Suppression Utilized by Canine Adipose and Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 374–389. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. A report on the use of a single intra-articular administration of autologous platelet therapy in a naturally occurring canine osteoarthritis model—A preliminary study. BMC Musculoskelet. Disord. 2020, 21, 127. [Google Scholar] [CrossRef]
- Rosshirt, N.; Trauth, R.; Platzer, H.; Tripel, E.; Nees, T.A.; Lorenz, H.-M.; Tretter, T.; Moradi, B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther. 2021, 23, 37. [Google Scholar] [CrossRef]
- Stief, M.; Gottschalk, J.; Ionita, J.-C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; Saris, D.B.; Dhert, W.J.; Creemers, L.B. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res. Ther. 2010, 12, R114. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4(+)CD25(+)Foxp3(+) regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Akaraphutiporn, E.; Sunaga, T.; Bwalya, E.C.; Echigo, R.; Okumura, M. Alterations in characteristics of canine articular chondrocytes in non-passaged long-term monolayer culture: Matter of differentiation, dedifferentiation and redifferentiation. J. Vet. Med. Sci. 2020, 82, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Velloso Alvarez, A.; Boone, L.H.; Pondugula, S.R.; Caldwell, F.; Wooldridge, A.A. Effects of Autologous Conditioned Serum, Autologous Protein Solution, and Triamcinolone on Inflammatory and Catabolic Gene Expression in Equine Cartilage and Synovial Explants Treated With IL-1β in Co-culture. Front. Vet. Sci. 2020, 7, 323. [Google Scholar] [CrossRef]
- Hraha, T.H.; Doremus, K.M.; McIlwraith, C.W.; Frisbie, D.D. Autologous conditioned serum: The comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet. J. 2011, 43, 516–521. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Li, S.; Wan, J.; Anderson, W.; Sun, H.; Zhang, H.; Peng, X.; Yu, Z.; Wang, T.; Yan, X.; Smith, W. Downregulation of IL-10 secretion by Treg cells in osteoarthritis is associated with a reduction in Tim-3 expression. Biomed. Pharmacother. 2016, 79, 159–165. [Google Scholar] [CrossRef]
- Wagner, U.; Rossol, M.; Kaltenhäuser, S.; Hauschild, S.; Häntzschel, H. T cell contact-mediated induction of tumor necrosis factor alpha in peripheral blood monocytes is inhibited by autologous serum in rheumatoid arthritis. Arthritis Res. Ther. 2003, 5, 120. [Google Scholar] [CrossRef]
- Wang, J.; Ioan-Facsinay, A.; van der Voort, E.I.; Huizinga, T.W.; Toes, R.E. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007, 37, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Peláez, P.; Damiá, E.; Torres-Torrillas, M.; Chicharro, D.; Cuervo, B.; Miguel, L.; Del Romero, A.; Carrillo, J.M.; Sopena, J.J.; Rubio, M. Cell and Cell Free Therapies in Osteoarthritis. Biomedicines 2021, 9, 1726. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- Darabos, N.; Haspl, M.; Moser, C.; Darabos, A.; Bartolek, D.; Groenemeyer, D. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19 (Suppl. 1), S36–S46. [Google Scholar] [CrossRef]
- Ayache, S.; Panelli, M.C.; Byrne, K.M.; Slezak, S.; Leitman, S.F.; Marincola, F.M.; Stroncek, D.F. Comparison of proteomic profiles of serum, plasma, and modified media supplements used for cell culture and expansion. J. Transl. Med. 2006, 4, 40. [Google Scholar] [CrossRef]
- Kocaoemer, A.; Kern, S.; Klüter, H.; Bieback, K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 2007, 25, 1270–1278. [Google Scholar] [CrossRef]
- Muraglia, A.; Nguyen, V.T.; Nardini, M.; Mogni, M.; Coviello, D.; Dozin, B.; Strada, P.; Baldelli, I.; Formica, M.; Cancedda, R.; et al. Culture Medium Supplements Derived from Human Platelet and Plasma: Cell Commitment and Proliferation Support. Front. Bioeng. Biotechnol. 2017, 5, 66. [Google Scholar] [CrossRef]



| Breed | Age | Gender | Duration of OA | Affected Joint (s) | |
|---|---|---|---|---|---|
| 1 | Golden Retriever | 8 Y, 6 M | Male | 1 year | bilateral hips |
| 2 | Golden Retriever | 4 Y, 10 M | Female | 4 weeks | stifle |
| 3 | Labrador Retriever | 4 Y, 7 M | Female | 8 weeks | stifle |
| 4 | Labrador Retriever | 3 Y, 4 M | Male | 4 weeks | bilateral stifles |
| 5 | Crossbreed | 8 Y, 7 M | Male | 5 weeks | bilateral stifles |
| 6 | American Pitbull | 3 Y, 4 M | Female | 3 weeks | stifle |
| 7 | Golden Retriever | 2 Y, 2 M | Female | 3 weeks | bilateral stifles |
| 8 | Siberian Husky | 8 Y, 2 M | Male | 3 weeks | stifle |
| 9 | German Shepherd | 8 Y, 7 M | Male | 4 weeks | elbow |
| 10 | Labrador Retriever | 6 Y, 8 M | Female | 1 year | bilateral stifles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soontararak, S.; Ardaum, P.; Senarat, N.; Yangtara, S.; Lekcharoensuk, C.; Putchong, I.; Kashemsant, N.; Vijarnsorn, M.; Chow, L.; Dow, S.; et al. In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis. Animals 2022, 12, 2717. https://doi.org/10.3390/ani12192717
Soontararak S, Ardaum P, Senarat N, Yangtara S, Lekcharoensuk C, Putchong I, Kashemsant N, Vijarnsorn M, Chow L, Dow S, et al. In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis. Animals. 2022; 12(19):2717. https://doi.org/10.3390/ani12192717
Chicago/Turabian StyleSoontararak, Sirikul, Piyathida Ardaum, Napaporn Senarat, Sarawut Yangtara, Chalermpol Lekcharoensuk, Iyarath Putchong, Narudee Kashemsant, Monchanok Vijarnsorn, Lyndah Chow, Steven Dow, and et al. 2022. "In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis" Animals 12, no. 19: 2717. https://doi.org/10.3390/ani12192717
APA StyleSoontararak, S., Ardaum, P., Senarat, N., Yangtara, S., Lekcharoensuk, C., Putchong, I., Kashemsant, N., Vijarnsorn, M., Chow, L., Dow, S., & Lekcharoensuk, P. (2022). In Vitro Anti-Inflammatory and Regenerative Effects of Autologous Conditioned Serum from Dogs with Osteoarthritis. Animals, 12(19), 2717. https://doi.org/10.3390/ani12192717