Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
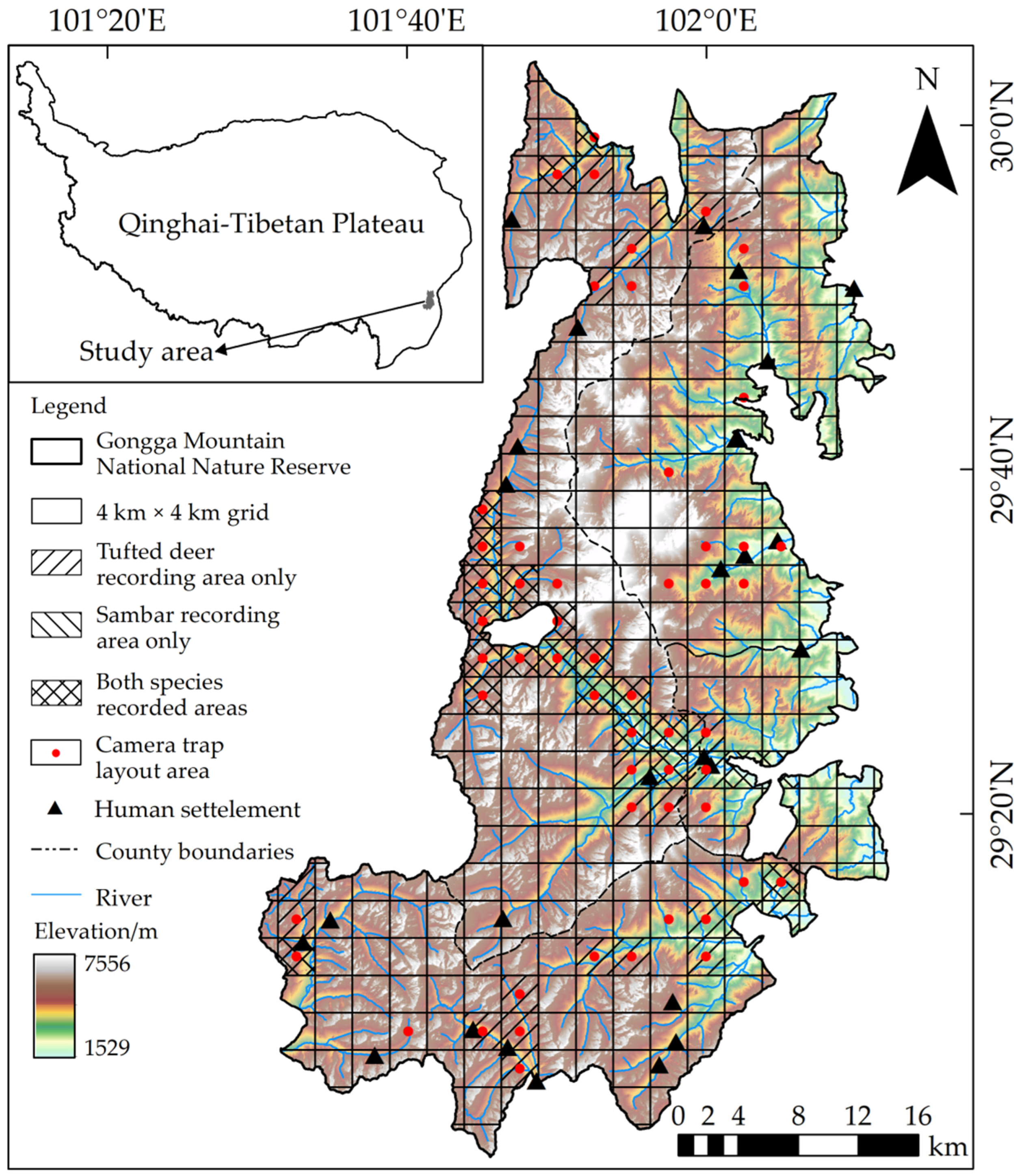
2.1. Study Area
2.2. Data Collection
2.2.1. Species Presence Data
2.2.2. Environmental Data and Data Processing
2.3. Data Analysis
2.3.1. MaxEnt Model
2.3.2. Spatial Overlap Analysis
2.3.3. Temporal Niche Analysis
3. Results
3.1. General Summary
3.2. Spatial Niche
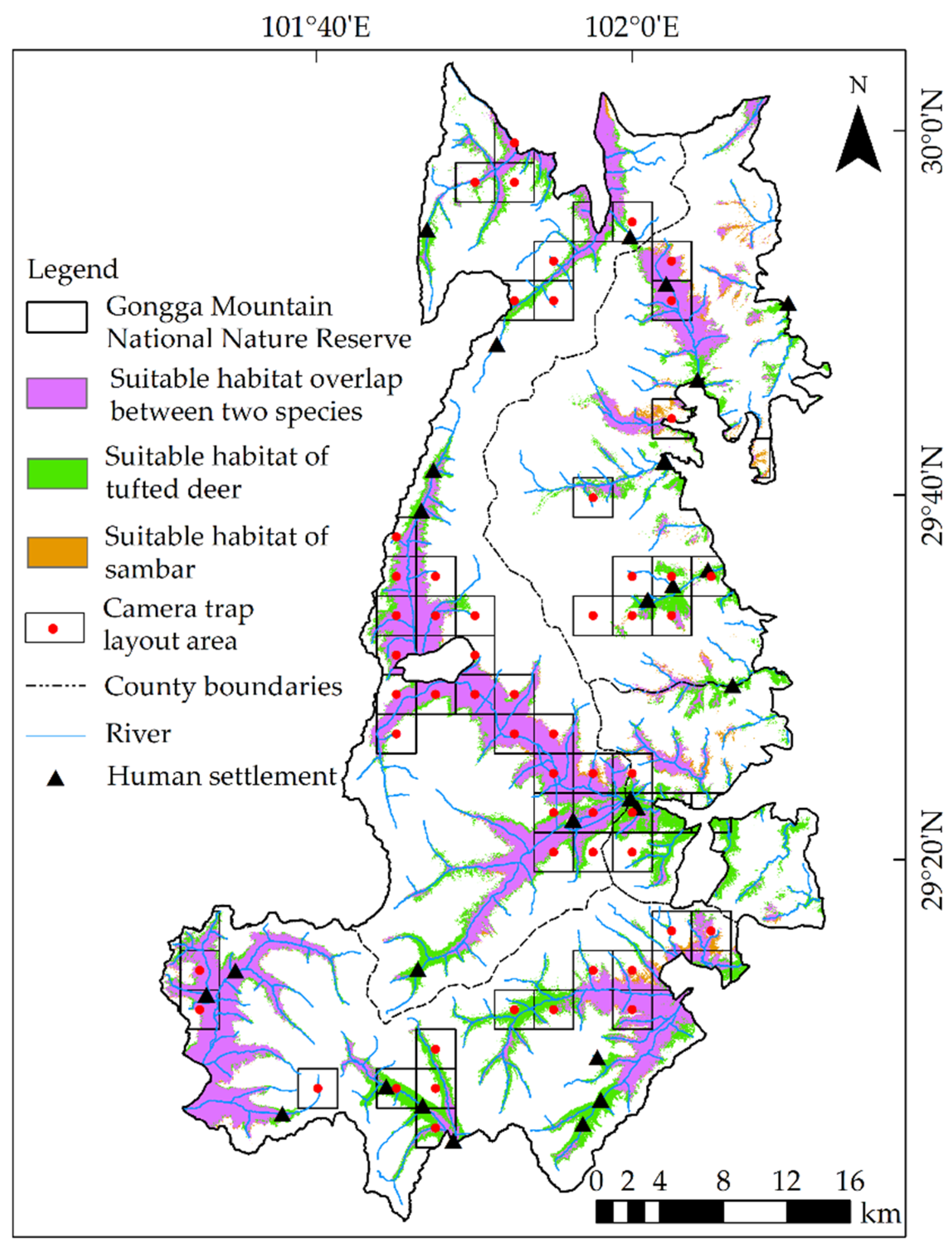
3.2.1. Suitable Habitats
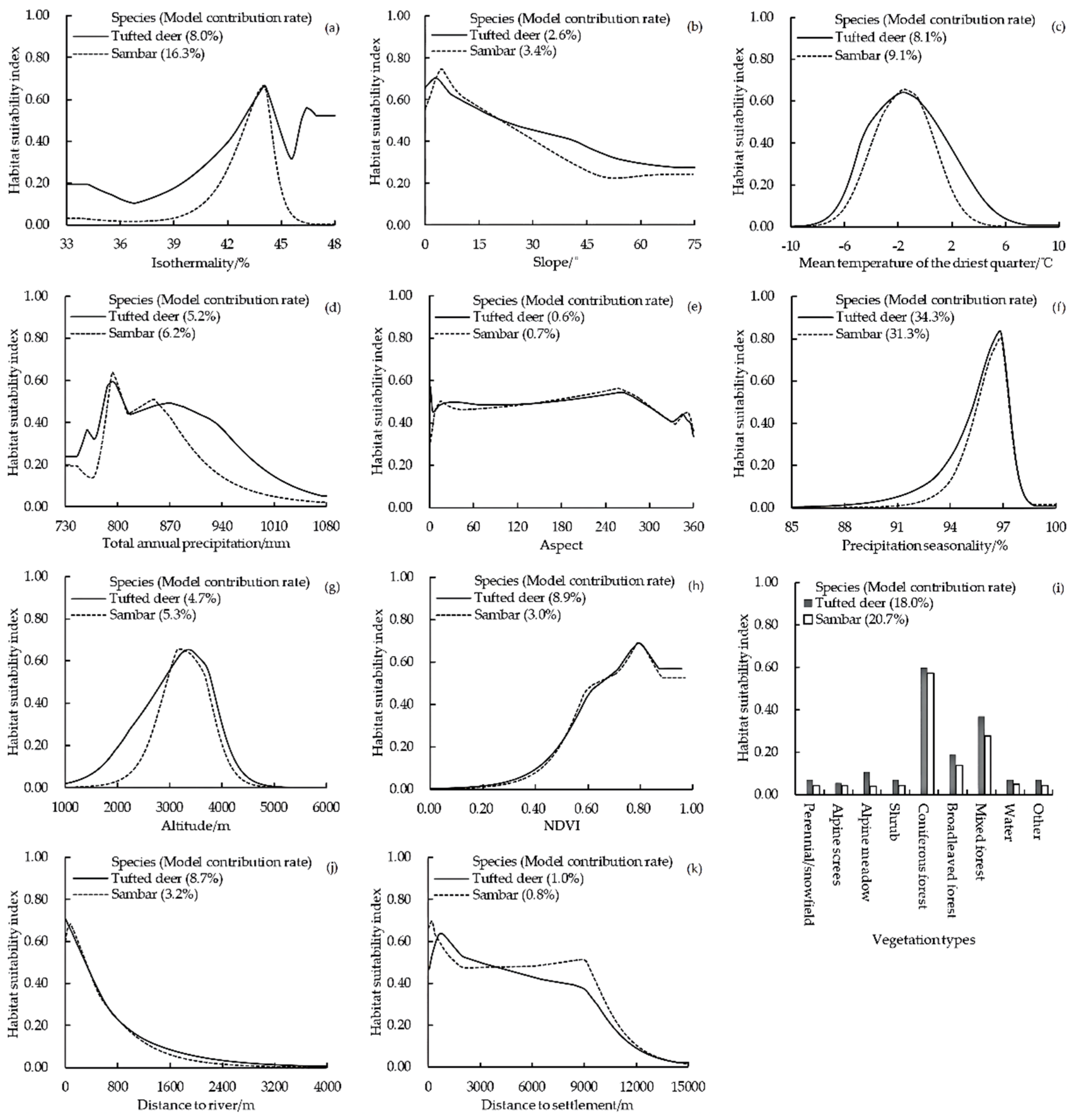
3.2.2. Important Environmental Variables
3.2.3. Spatial Overlaps
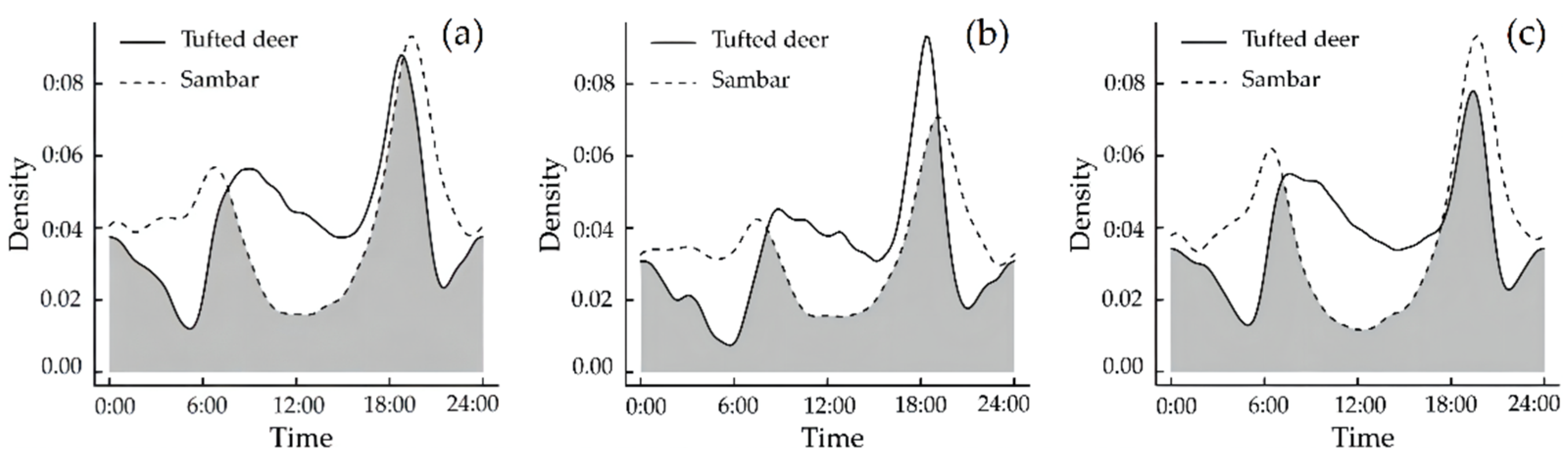
3.3. Temporal Niche
Daily Activity Rhythm Characteristics and Overlapping Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurlbert, S.H. The measurement of niche overlap and some relatives. Ecology 1978, 59, 67–77. [Google Scholar] [CrossRef]
- Grinnell, J. The niche-relationships of the California thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Hardin, G. The competitive exclusion principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Coudrat, C.N.Z.; Anne-Isola Nekaris, K. Modelling Niche Differentiation of Co-Existing, Elusive and Morphologically Similar Species: A Case Study of Four Macaque Species in Nakai-Nam Theun National Protected Area, Laos. Animals 2013, 3, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.W.; Lease, H.M.; Hetem, R.S.; Mitchell, D.; Fuller, A.; Woodborne, S. Stable isotope analysis of diet confirms niche separation of two sympatric species of Namib Desert lizard. Integr. Zool. 2016, 11, 60–75. [Google Scholar] [CrossRef]
- Ramos, J.A.A.; Barletta, M.; Dantas, D.V.; Lima, A.R.A.; Costa, M.F. Trophic niche and habitat shifts of sympatric Gerreidae. J. Fish Biol. 2014, 85, 1446–1469. [Google Scholar] [CrossRef] [PubMed]
- He, M.X.; Chen, L.X.; Luo, G.; Gu, X.D.; Wang, G.; Ran, J.H. Suitable habitat prediction and overlap analysis of two sympatric species, giant panda (Ailuropoda melanoleuca) and Asiatic black bear (Ursus thibetanus) in Liangshan Mountains. Biodivers. Sci. 2018, 26, 1180–1189. [Google Scholar] [CrossRef]
- Di, C.J.A.; Wickham, S.A. Does spatiotemporal nutrient variation allow more species to coexist? Oecologia 2020, 194, 695–707. [Google Scholar]
- Zhang, D.X.; An, B.; Chen, L.Y.; Sun, Z.Y.; Mao, R.R.; Zhao, C.M.; Zhang, L.X. Camera Trapping Reveals Spatiotemporal Partitioning Patterns and Conservation Implications for Two Sympatric Pheasant Species in the Qilian Mountains, Northwestern China. Animals 2022, 12, 1657. [Google Scholar] [CrossRef]
- Gawel, A.M.; Rogers, H.S.; Miller, R.H.; Kerr, A.M. Contrasting ecological roles of non-native ungulates in a novel ecosystem. R. Soc. Open Sci. 2018, 5, 170151. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, J.; Ewa, P.; Izabela, S. Endozoochory by the guild of ungulates in Europe’s primeval forest. For. Ecol. Manag. 2013, 305, 21–28. [Google Scholar]
- Xiao, W.H.; Hebblewhite, M.; Robinson, H.; Feng, L.M.; Zhou, B.; Mou, P.; Wang, T.M.; Ge, J.P. Relationships between humans and ungulate prey shape Amur tiger occurrence in a core protected area along the Sino-Russian border. Ecol. Evol. 2018, 8, 11677–11693. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.D.; Liao, M.F.; Dong, W.; Wu, B.; Li, D.Q. Temporal and Spatial Activity Patterns of Sympatric Wild Ungulates in Qinling Mountains, China. Animals 2022, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.H.; Feng, Y.; Yu, Y.Z.; Zhang, M.H.; Zhang, W.Q. Influence of High Dietary Overlap on Sympatric Species Habitat Selection Segregation: A Case Study of Red Deer and Roe Deer. J. Northeast. For. Univ. 2020, 48, 72–75. [Google Scholar]
- Chen, X.; Hu, X.X.; Liu, M.X.; Li, D.D.; Jiang, W.L.; Luo, C.P.; Zhao, L.J.; Jiang, S.W.; Guan, T.P. A preliminary analysis on the spatio-temporal dynamic of ungulate’s circadian activities: A case study of Bharal (Pseudois nayaur). Acta Ecol. Sin. 2021, 41, 1251–1258. [Google Scholar]
- Jiang, Z.G. China’s Red List Biodiversity: Vertebrates, Volum, Mammals (I); Science Press: Beijing, China, 2021. [Google Scholar]
- Liu, X.H.; Wu, P.F.; Songer, M.; Cai, Q.; He, X.B.; Zhu, Y.; Shao, X.M. Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol. Indic. 2013, 33, 121–128. [Google Scholar] [CrossRef]
- Zhang, Y.K.; He, X.B.; Liu, X.H.; Songer, M.; Dang, H.S.; Zhang, Q.F. Fine-scale activity patterns of large- and medium-sized mammals in a deciduous broadleaf forest in the Qinling Mountains, China. J. For. Res. 2021, 32, 2709–2717. [Google Scholar] [CrossRef]
- Davies, C.; Wright, W.; Hogan, F.E.; Davies, H. Detectability and activity patterns of Sambar deer (Rusa unicolor) in Baw Baw National Park, Victoria. Aust. Mammal. 2020, 42, 312–320. [Google Scholar] [CrossRef]
- Yen, S.C.; Wang, Y.; Ou, H.Y. Habitat of the Vulnerable Formosan Sambar deer Rusa unicolor swinhoii in Taiwan. Oryx 2014, 48, 232–240. [Google Scholar] [CrossRef]
- Forsyth, D.M.; McLeod, S.R.; Scroggie, M.P.; White, M.D. Modelling the abundance of wildlife using field surveys and GIS: Non-native Sambar deer (Cervus unicolor) in the Yarra Ranges, south-eastern Australia. Wildl. Res. 2009, 36, 231–241. [Google Scholar] [CrossRef]
- Tao, N.; Liu, D.; Wu, J. Evaluation study on ecosystem based on system dynamics: A case study of Gongga Mountain forest ecosystem. J. Interdiscip. Math. 2017, 20, 1415–1418. [Google Scholar] [CrossRef]
- Thomas, A. Overview of the geoecology of the Gongga Shan range, Sichuan Province, China. Mt. Res. Dev. 1999, 19, 17–30. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhao, W.J. The characteristics of the Mountain Ecosystem and Environment in the Gongga Mountain Region. Ambio 1999, 28, 648–654. [Google Scholar]
- Xie, S.Q.; Wang, Y.; Jia, G.Q.; Jiang, Y.; Lu, B.G.; Liu, W. Species diversity and fauna characteristics of mammals in the Gongga Mountain National Nature Reserve. J. Southwest Minzu Univ. (Nat. Sci. Ed.) 2021, 47, 445–454. [Google Scholar]
- Xiao, Z.S. An introduction to wildlife camera trapping monitoring from Chinese Forest Biodiversity Monitoring Network (CForBio). Biodivers. Sci. 2014, 22, 808. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Morris, D.W.; Clark, R.G.; Boyce, M.S. Habitat and habitat selection: Theory, tests, and implications. Isr. J. Ecol. Evol. 2008, 54, 287–294. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. A thoery of habitat selection. Ecology 1981, 62, 327–335. [Google Scholar] [CrossRef]
- Bergeson, S.M.; Carter, T.C.; Whitby, M.D. Partitioning of foraging resources between sympatric Indiana and little brown bats. J. Mammal. 2013, 94, 1311–1320. [Google Scholar] [CrossRef]
- Swanepoel, L.H. Extent and fragmentation of suitable leopard habitat in South Africa. Anim. Conserv. 2013, 16, 41–50. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 30 May 2019).
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.R.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Yasuda, M. Monitoring diversity and abundance of mammals with camera-traps: A case study on Mount Tsukuba, central Japan. Mammal Study 2004, 29, 37–46. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera-trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Zucco, C.A.; Agostinelli, C. Using conditional circular kernel density functions to test hypotheses on animal circadian activity. Anim. Behav. 2013, 85, 269–280. [Google Scholar] [CrossRef]
- Chen, L.J.; Shu, Z.F.; Xiao, Y.S. Application of camera-trapping data to study daily activity patterns of Galliformes in Guangdong Chebaling National Nature Reserve. Biodivers. Sci. 2019, 27, 266–272. [Google Scholar]
- Linkie, M.; Ridout, M.S. Assessing tiger-prey interactions in Sumatran rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns. Available online: https://cran.r-project.org/package=overlap (accessed on 12 May 2022).
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A.; Fisher, D. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Harrison, H.L. Temporal niche separation of desert cottontails (Sylvilagus audubonii) and black-tailed jackrabbits (Lepus californicus) in the chihuahuan desert. Southwest. Nat. 2019, 63, 153–160. [Google Scholar] [CrossRef]
- Yao, W.; Wang, G.H.; Lin, J.Z.; Long, J.F.; Li, J.Q.; Zhou, Q.H. Comparison of activity rhythms of sympatric Chinese ferret-badger (Melogale moschata) and crab-eating mongoose (Herpestes urva). Acta Theriol. Sin. 2021, 41, 128–135. [Google Scholar]
- Wang, B.; Xu, Y.; Price, M.; Yang, N.; Liu, W.; Zhu, B.W.; Zhong, X.; Ran, J.H. Niche partitioning among three montane ground-welling pheasant species along multiple ecological dimensions. Ibis 2020, 163, 171–182. [Google Scholar] [CrossRef]
- Viviano, A.; Mori, E.; Fattorini, N.; Mazza, G.; Lazzeri, L.; Panichi, A.; Strianese, L.; Mohamed, W.F. Spatiotemporal Overlap between the European Brown Hare and Its Potential Predators and Competitors. Animals 2021, 11, 562. [Google Scholar] [CrossRef]
- Liu, X.H.; Wu, P.F.; Shao, X.M.; Songer, M.; Cai, Q.; He, X.B.; Zhu, Y. Diversity and activity patterns of sympatric animals among four types of forest habitat in Guanyinshan Nature Reserve in the Qinling Mountains, China. Environ. Sci. Pollut. Res. 2017, 24, 16465–16477. [Google Scholar] [CrossRef]
- Espinosa, S.; Salvador, J. Hunters’ landscape accessibility and daily activity of ungulates in Yasuní Biosphere Reserve, Ecuador. Therya 2017, 8, 45–52. [Google Scholar] [CrossRef][Green Version]
- Corlatti, L.; Cotza, A.; Nelli, L. Linking alternative reproductive tactics and habitat selection in Northern chamois. Ecol. Evol. 2021, 11, 7057–7068. [Google Scholar] [CrossRef] [PubMed]
- Duparc, A.; Garel, M.; Marchand, P.; Dubray, D.; Maillard, D.; Loison, A. Revisiting the functional response in habitat selection for large herbivores: A matter of spatial variation in resource distribution? Behav. Ecol. 2019, 30, 1725–1733. [Google Scholar] [CrossRef]
- Northrup, J.M.; Anderson, C.R.; Hooten, M.B.; Wittemyer, G. Movement reveals scale dependence in habitat selection of a large ungulate. Ecol. Appl. 2016, 26, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.C.; Kowalczyk, R.; Kohler, M. Resting site selection by large herbivores-the case of European bison (Bison bonasus) in Bialowieza Primeval Forest. Mamm. Biol. 2013, 78, 438–445. [Google Scholar] [CrossRef]
- Frey, S.; Fisher, J.T.; Burton, A.C.; Volpe, J.P. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: Challenges and opportunities. Remote Sens. Ecol. Conserv. 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Seki, Y.; Hayama, S. Habitat Selection and Activity Patterns of Japanese Serows and Sika Deer with Currently Sympatric Distributions. Animals 2021, 11, 3398. [Google Scholar] [CrossRef] [PubMed]
- Cutler, T.L.; Swann, D.E. Using remote photography in wildlife ecology: A review. Wildl. Soc. Bull. 1999, 27, 571–581. [Google Scholar]
- Huang, G.P.; Ping, X.G.; Xu, W.H.; Hu, Y.B.; Chang, J.; Swaisgood, R.R.; Zhou, J.; Zhan, X.J.; Zhang, Z.J.; Nie, Y.G.; et al. Wildlife conservation and management in China: Achievements, challenges, and perspectives. Natl. Sci. Rev. 2021, 8, nwab042. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.J.; Xiao, Z.S.; Li, X.H.; Wang, T.M.; Feng, L.M.; Wang, Y. Camera-trapping in wildlife research and conservation in China: Review and outlook. Biodivers. Sci. 2014, 22, 685. [Google Scholar]
- Ahumada, J.A.; Fegraus, E.; Birch, T.; Flores, N.; Kays, R.; G O’Brien, T.; Palmer, J.; Schuttler, S.; Zhao, J.Y.; Jetz, W.; et al. Wildlife Insights: A platform to maximize the potential of camera trap and other passive sensor wildlife data for the planet. Environ. Conserv. 2020, 47, 1–6. [Google Scholar] [CrossRef]
- McShea, W.J.; Forrester, T.; Costello, R.; He, Z.; Kays, R. Volunteer-run cameras as distributed sensors for macrosystem mammal research. Landsc. Ecol. 2016, 31, 55–66. [Google Scholar] [CrossRef]
- Gong, Y.N.; Zhao, G.J.; Yang, H.X.; Li, Y.; Tan, M.Y.; Wang, N.; Ge, J.P.; Yang, H.T.; Feng, L.M. Prevalence of Varied Coat Coloration in a Yellow-Throated Marten (Martes flavigula) Population. Animals 2021, 11, 2838. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Yang, X.; Jia, G.Q.; Wang, Y.; Zheng, X.A.; Jiang, Y.; Yang, B.; Li, S.Q. Spatio-Temporal Niche Differentiation of White Eared Pheasant (Crossoptilon crossoptilon) and Blood Pheasant (Ithaginis cruentus) in Gongga Mountain National Nature Reserve, Sichuan Province. J. Sichuan For. Sci. Technol. 2022, 43, 47–55. [Google Scholar]
- Xia, W.C.; Shao, F.L.; Jia, G.Q.; Cui, P.; Zhou, H.M.; Yang, C.; Wang, J. Avian diversity, distribution, and fauna of the Gongga Mountains. Chin. J. Appl. Environ. 2020, 26, 1028–1039. [Google Scholar]
- Qiao, J.; Jia, G.Q.; Zhou, H.M.; Gong, L.; Jiang, Y.; Xiao, N.W.; Gao, X.Q.; Wen, A.X.; Wang, J. Mammal and bird diversity recorded with camera-traps in Gongga Mountain National Nature Reserve, Sichuan. China Biodivers. Sci. 2022, 30, 116–123. [Google Scholar] [CrossRef]
- Broadley, K.; Burton, A.C.; Avgar, T.; Boutin, S. Density-dependent space use affects interpretation of camera trap detection rates. Ecol. Evol. 2019, 9, 14031–14041. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, R.; Mohamed, A.; Samejima, H.; Wilting, A. Risky business or simple solution-Relative abundance indices from camera-trapping. Biol. Conserv. 2013, 159, 405–412. [Google Scholar] [CrossRef]
- Tourani, M.; Brøste, E.N.; Bakken, S.; Odden, J.; Bischof, R. Sooner, closer, or longer: Detectability of mesocarnivores at camera traps. J. Zool. 2020, 312, 259–270. [Google Scholar] [CrossRef]

| Classification | Variable | Source |
|---|---|---|
| Topographic variables | Elevation | http://www.gscloud.cn (accessed on 15 April 2022). |
| Slope | ||
| Aspect | ||
| Vegetation variables | Vegetation type | https://data.ess.tsinghua.edu.cn (accessed on 10 March 2022) |
| Normalized difference vegetation index (NDVI) | http://www.nesdc.org.cn (accessed on 13 April 2022) | |
| Climate variables | Isothermality | https://worldclim.org (accessed on 25 February 2022) |
| Mean temperature of the driest quarter | ||
| Total annual precipitation | ||
| Precipitation seasonality | ||
| Water source distance | Distance to river | http://loess.geodata.cn (accessed on 26 March 2022) |
| Human disturbance variable | Distance to human settlement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Z.; Lu, B.; Du, B.; Liu, W.; Jiang, Y.; Ruan, G.; Yang, N. Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China. Animals 2022, 12, 2694. https://doi.org/10.3390/ani12192694
You Z, Lu B, Du B, Liu W, Jiang Y, Ruan G, Yang N. Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China. Animals. 2022; 12(19):2694. https://doi.org/10.3390/ani12192694
Chicago/Turabian StyleYou, Zhiyuan, Bigeng Lu, Beibei Du, Wei Liu, Yong Jiang, Guangfa Ruan, and Nan Yang. 2022. "Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China" Animals 12, no. 19: 2694. https://doi.org/10.3390/ani12192694
APA StyleYou, Z., Lu, B., Du, B., Liu, W., Jiang, Y., Ruan, G., & Yang, N. (2022). Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China. Animals, 12(19), 2694. https://doi.org/10.3390/ani12192694




