Changes in the Oxidative Status of Dual-Purpose Hens Rearing in the Free-Range System during Cold, Thermoneutral and Hot Period
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing
2.2. Ethics Statement
2.3. Oxidative Status Determination—Blood Collection and Biochemical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roger, H. Maritime certified organiz growersorganic profiles. Erişim Tar. 2004, 21, 6. Available online: www.avolonhouse.ca/food/profiles (accessed on 20 August 2022).
- Fossum, O.; Jansson, D.S.; Etterlin, P.E.; Vågsholm, I. Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Vet. Scand. 2009, 51, 3. [Google Scholar] [CrossRef] [PubMed]
- Wuthijaree, K.; Lambertz, C.; Gauly, M. Prevalence of gastrointestinal helminth infections in free-range laying hens under mountain farming production conditions. Br. Poult. Sci. 2017, 58, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Lambton, S.L.; Knowles, T.G.; Yorke, C.; Nicol, C.J. The risk factors affecting the development of vent pecking and cannibalism in free-range and organic laying hens. Anim. Welfare 2015, 24, 101–111. [Google Scholar] [CrossRef]
- Bestman, M.; Bikker-Ouwejan, J. Predation in organic and free-range egg production. Animals 2014, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heate stress induces oxidative stress in broiler chicken. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef]
- Alexandrov, V.; Eitzinger, J.; Cajic, V.; Oberforster, M. Potential impact of climate change on selected agricultural crops in north-eastern Austria. Global Chang. Biol. 2002, 8, 372–389. [Google Scholar] [CrossRef]
- Lamarca, D.S.F.; Pereira, D.F.; Magalhães, M.M.; Salgado, D.D. Climate change in layer poultry farming: Impact of heat waves in region of bastos, Brazil. Braz. J. Poultry Sci. 2018, 20, 657–664. [Google Scholar] [CrossRef]
- Selye, H. Stress without distress. In Psychopathology of Human Adaptation; Serban, G., Ed.; Springer: Boston, MA, USA, 1976; pp. 137–146. [Google Scholar]
- Sies, H. Oxidative Stress: Introductory Remarks. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–507. [Google Scholar]
- Akşit, M.; Altan, Ö.; Karul, A.B.; Balkaya, M.; Özdemir, D. Effects of cold temperature and vitamin E supplementation on oxidative stress, troponin-t level, and other ascites-related traits in broilers. Arch. Geflügelkd. 2008, 72, 221–230. [Google Scholar]
- Ming, X.; Xian-yi, Y.; Jin-long, L.; Yan-hui, H.; Shu, L.; Shi-wen, X. Effects of acute and chronic cold stress on antioxidant function in intestinal tracts of chickens. J. Northeast. Agric. Univ. 2012, 19, 54–61. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Zhang, Z.W.; Qu, J.P.; Li, M.; Li, S.; Xu, S.W. Cold stress induces antioxidants and hsps in chicken immune organs. Cell Stress Chaperones 2014, 19, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, R.P.; Baumgard, L.H.; Suagee, J.K.; Sanders, S.R. Nutritional interventions to alleviate the negative consequences of heat stress. Adv. Nutr. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Council Directive (EU) No 74/1999 of 19 July 1999. Laying Down Minimum Standards for the Protection of Laying Hens. Off. J. Eur. Communities 1999, L203, 53–57. Available online: http://data.europa.eu/eli/dir/1999/74/oj (accessed on 13 June 2021).
- Küçükyılmaz, K.; Bozkurt, M.; Herken, E.; Cınar, M.; Catlı, A.U.; Bintaş, E.; Cöven, F. Effects of rearing systems on performance, egg characteristics and immune response in two layer hen genotype. Asian Austral. J. Anim. Sci. 2012, 25, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Mack, L.A.; Felver-Gant, J.N.; Dennis, R.L.; Cheng, H.W. Genetic variation alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013, 92, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ordinance No 20 of 1 November 2012 on the minimum requirements for protection and welfare of experimental animals and site requirements for use, cultivation and/or their delivery. State Gazette № 87 of 9 November 2012: Sofia, Bulgaria (Bg). Available online: https://sofia.obshtini.bg/doc/583135/0 (accessed on 13 June 2021).
- Meyers, L.; Gamst, G.; Guarino, A. Performing Data Analysis Using IBM SPSS; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 736. Available online: https://www.wiley.com/en-us/Performing+Data+Analysis+Using+IBM+SPSS-p-9781118357019 (accessed on 20 February 2021).
- Hinton, P.; McMurray, I. Presenting Your Data with SPSS Explained, 1st ed.; Routledge: London, UK, 2017; p. 356. [Google Scholar] [CrossRef]
- Farahat, G.S. Phenotypic Variation of Glutathione Peroxidase Activity in Different Genotypes of Chicken and Its Correlation with Some Production Traits. Ph.D. Dissertation, Szent István University, Gödöllő, Hungary, 2003; p. 122. Available online: https://archive2020.szie.hu/file/tti/archivum/Giha_Dissertation.pdf (accessed on 20 August 2022).
- Castellini, C.; Dal Bosco, A.; Mugnai, C.; Pedrazzoli, M. Comparison of two chicken genotypes organically reared: Oxidative stability and other qualitative traits of the meat. Ital. J. Anim. Sci. 2006, 5, 29–42. [Google Scholar] [CrossRef]
- Altan, O.; Pabuçcuoglu, A.; Altan, A.; Konyalioglu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress indicators in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Meng, C.; Sun, Y.; Safdar, A.; Pasha, R.H.; Munir, M.; Ding, C. Oxidative stress in Poultry: Lessons from the viral infections. Oxid. Med. Cell. Longev. 2018, 2018, 5123147. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Surai, P.; Kochish, I.; Fisinin, V. Antioxidant systems in poultry biology: Nutritional modulation of vitagenes. Europ. Poult. Sci. 2017, 81, 1–21. [Google Scholar] [CrossRef]
- Wang, R.R.; Pan, X.J.; Peng, Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis major in broilers. Poult. Sci. 2009, 88, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Kikusato, M.; Maekawa, T.; Shirakawa, H.; Toyomizu, M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Phys. A Mol. Integr. Physiol. 2010, 155, 401–406. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Gradinski-Vrbanac, B.; Stojević, Z.; Milinković-Tur, S.; Balenović, T.; Piršljin, J.; Zdelar-Tuk, M. In vitro susceptibility of duck, chicken, and pig erythrocyte lipids to peroxidation. Vet. Med. 2002, 47, 303–308. [Google Scholar] [CrossRef]
- Ramnath, V.; Rekha, P.S.; Sujatha, K.S. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by Brahma rasayana. Evid. Based Compl. Alt. Med. 2008, 5, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, V.; Rekha, P.S. Brahma rasayana enhances in vivo antioxidant status in cold-stressed chickens (Gallus Gallus Domesticus). Indian J. Pharmacol. 2009, 43, 115–119. [Google Scholar] [CrossRef]
- Surai, P. Antioxidant systems in Poultry biology: Superoxide dismutase. J. Anim. Res. Nutr. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Marigómez, I.; Garmendia, L.; Soto, M.; Orbea, A.; Izagirre, U.; Cajaraville, M.P. Marine ecosystem health status assessment through integrative biomarker indices: A comparative study after the prestige oil spill “Mussel Watch”. Ecotoxicology 2013, 22, 486–505. [Google Scholar] [CrossRef]
- Surai, P.; Kochish, I.; Fisinin, V.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Kucuk, O.; Sahin, N.; Sahin, K.; Gursu, M.F.; Gulcu, F.; Ozcelik, M.; Issi, M. Egg production, egg quality, and lipid peroxidation status in laying hens maintained at a low ambient temperature (6 °C) and fed a vitamin c and vitamin e-supplemented diet. Vet. Med. 2003, 48, 33–40. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lin, H.; Wang, L.F.; Song, J.L.; Xie, Y.M.; Yang, Q.M. Effect of dietary supplemental levels of vitamin A on the egg production and immune responses of heat-stressed laying hens. Poult. Sci. 2002, 81, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Mahrose, K.; Arif, M.; Chaudhry, M.T.; Saadeldin, I.M.; Saeed, M.; Soomro, R.N.; Abbasi, I.H.R.; Rehman, Z.U. Alleviating the environmental heat burden on laying hens by feeding on diets enriched with certain antioxidants (vitamin E and selenium) individually or combined. Environ. Sci. Pollut. Res. 2017, 24, 10708–10717. [Google Scholar] [CrossRef] [PubMed]
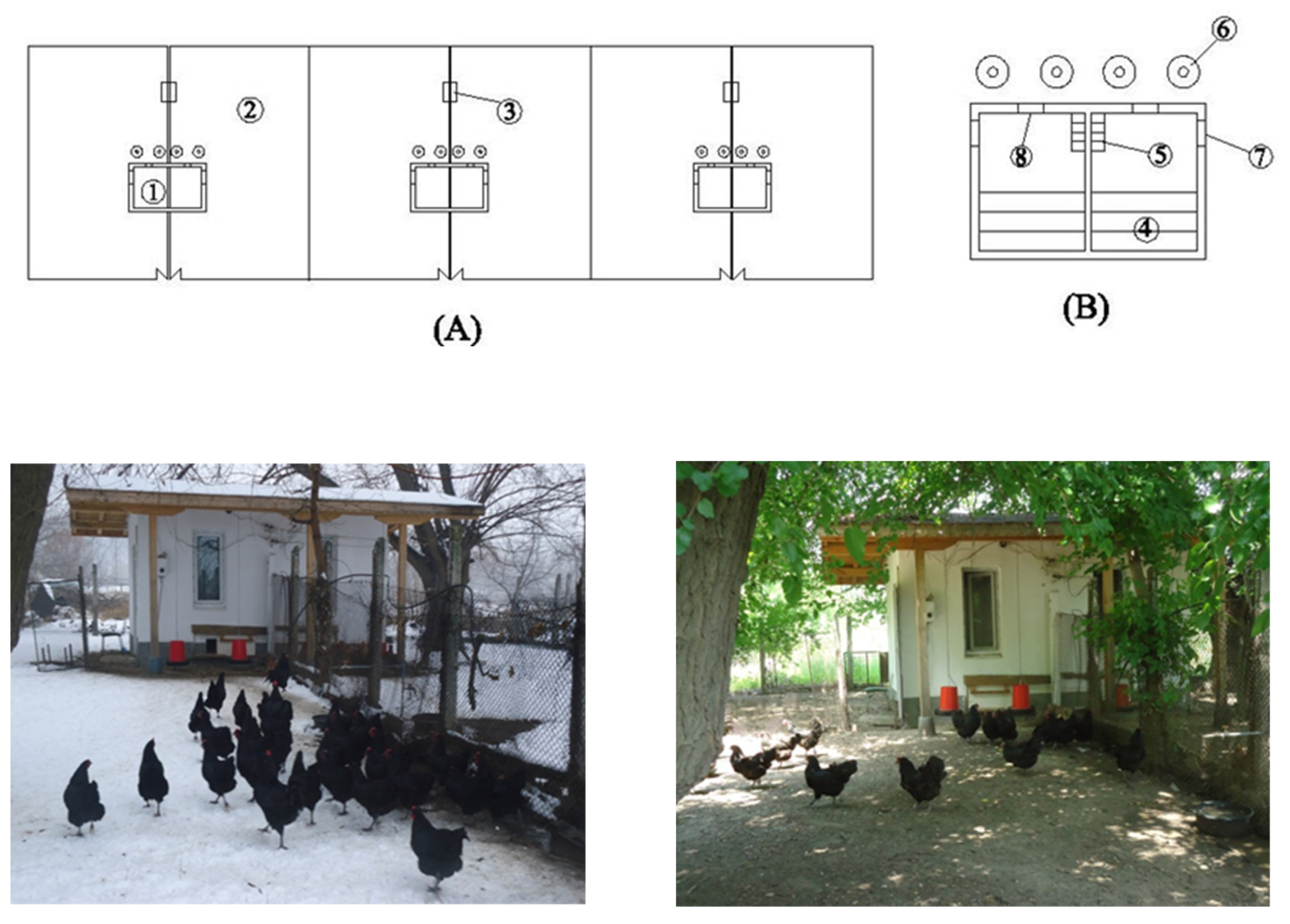
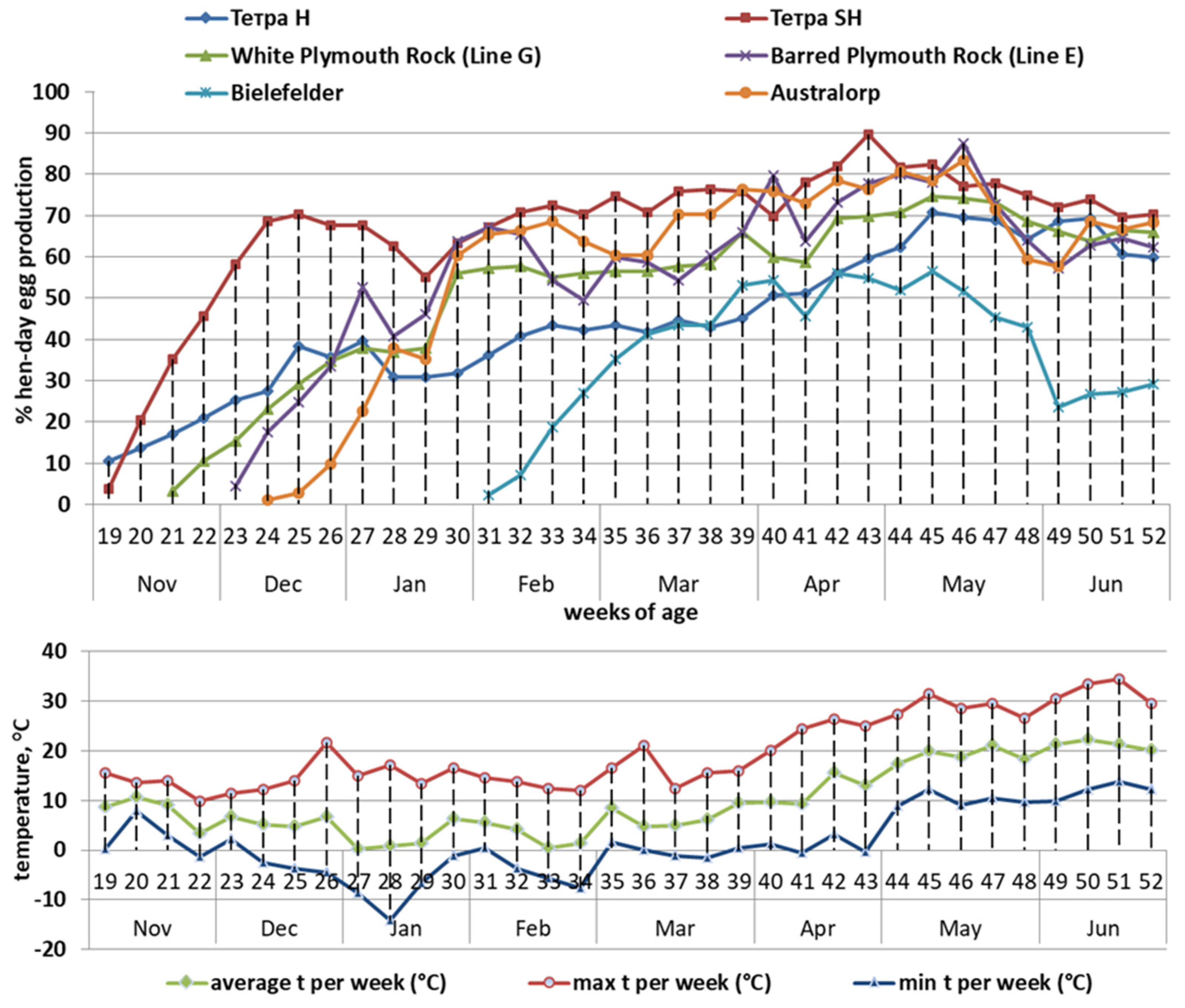
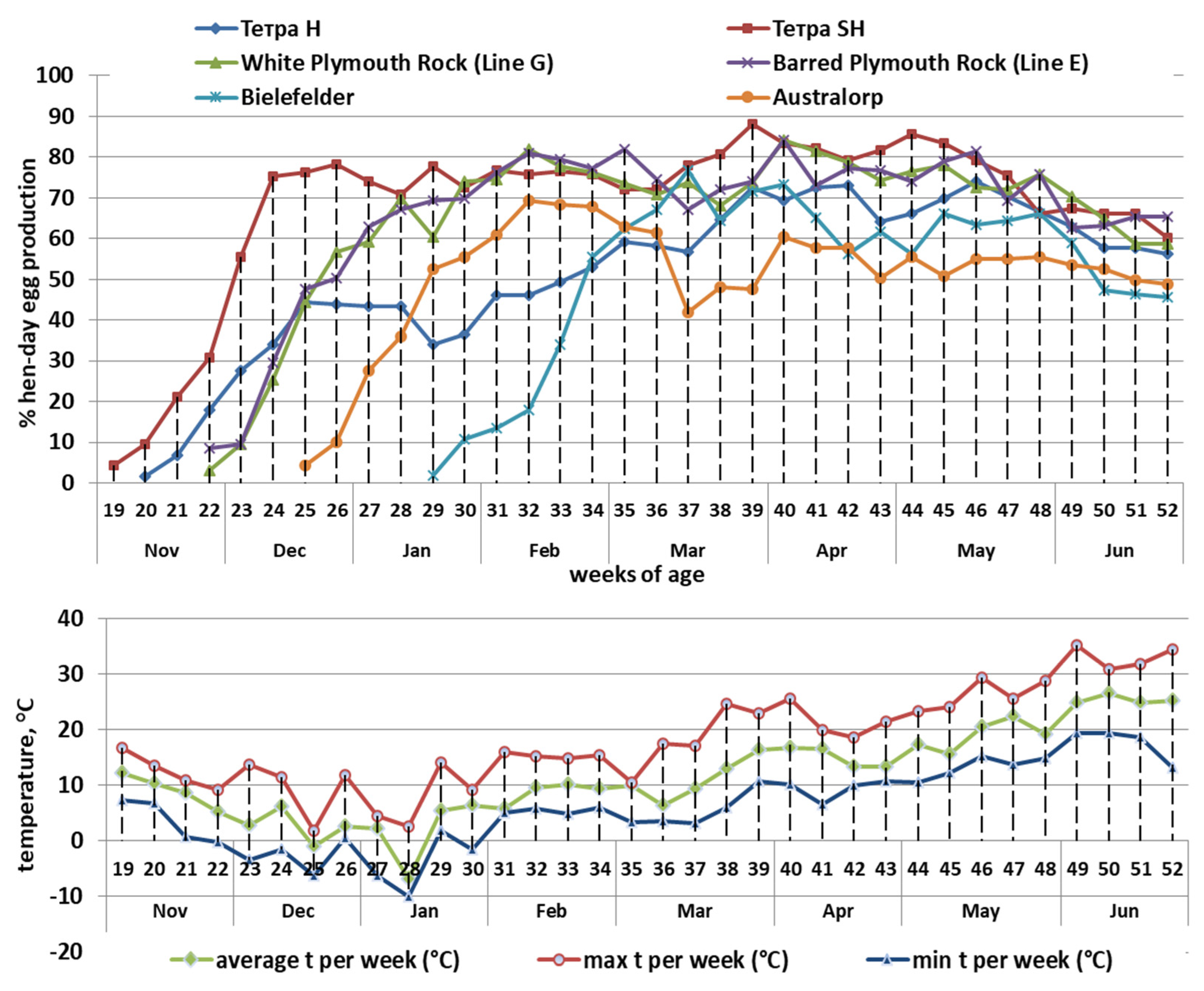
| Components | Laying Period in Week | |||
|---|---|---|---|---|
| 19–20 | 21–28 | 29–40 | 41–52 | |
| Feed ingredients, g/kg | ||||
| Corn yellow | 336.7 | 308.7 | 309.2 | 320.4 |
| Wheat | 336.7 | 308.7 | 309.2 | 320.4 |
| Soybeans meal (440 g crude protein) | 120.0 | 125.0 | 120.0 | 100.0 |
| Sunflower expeller (340 g crude protein) | 150.0 | 150.0 | 150.0 | 150.0 |
| Sunflower oil | - | 12.0 | 5.0 | - |
| L-lysine | 1.1 | 0.7 | 0.1 | 0.5 |
| DL-Methionine | 0.7 | 0.9 | 0.7 | 0.7 |
| Sodium chloride | 2.6 | 2.8 | 2.3 | 2.0 |
| Limestone | 38.0 | 82.0 | 96.0 | 100.0 |
| Dicalcium phosphate | 12.0 | 7.0 | 5.3 | 3.8 |
| Premix TB 301 Layers * | 2.0 | 2.0 | 2.0 | 2.0 |
| Synergen ** | 0.2 | 0.2 | 0.2 | 0.2 |
| Calculated composition (per kg) | ||||
| Metabolizable energy, MJ/kg | 12.1 | 11.8 | 11.5 | 11.5 |
| Crude protein, g | 178.0 | 174.0 | 171.0 | 165.0 |
| Crude fiber, g | 45.0 | 44.0 | 44.0 | 43.0 |
| Crude fats, g | 41.0 | 51.0 | 44.0 | 40.0 |
| Calcium, g | 20.0 | 36.0 | 38.1 | 39.2 |
| Phosphorus available, g | 6.6 | 5.6 | 5.3 | 3.0 |
| Lysine, g | 8.5 | 8.1 | 7.5 | 7.3 |
| Methionine + cysteine, g | 7.3 | 7.3 | 7.0 | 6.9 |
| Period (Month; Week-Old Hens) | Temperature | I Experiment (°C) | II Experiment (°C) |
|---|---|---|---|
| Cold period (March; 36 weeks of age) | Average | 4.82 | 6.39 |
| Min | −1.0 | −2.8 | |
| Max | 21.0 | 18.0 | |
| Thermoneutral period (May; 44 weeks of age) | Average | 17.35 | 17.24 |
| Min | 8.8 | 8.2 | |
| Max | 27.4 | 26.0 | |
| Hot period (July; 52 weeks of age) | Average | 21.2 | 25.6 |
| Min | 13.9 | 16.0 | |
| Max | 34.5 | 35.8 |
| Factors | Oxidative Stress Markers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Period | Genotype | LPO, Nmoles MDA/mg Hb | tGSH, ng/mg Hb | CAT, U/mg Hb | SOD, U/mg Hb | GPx, U/mg Hb | |||||
| I | cold | Tetra H | 1.28 ± 0.04 | abc | 738 ± 27 | cdefghi | 0.29 ± 0.03 | ij | 3.29 ± 0.09 | cdef | 38.45 ± 2.57 | abcdefg |
| Tetra Super Harco | 1.81 ± 0.08 | ab | 601 ± 110 | fghi | 0.37 ± 0.07 | ghij | 2.61 ± 0.52 | defghi | 41.36 ± 2.91 | abcd | ||
| White Plymouth Rock | 1.56 ± 0.03 | ab | 642 ± 5 | efghi | 0.44 ± 0.09 | ghij | 1.92 ± 0.09 | efghi | 42.00 ± 3.67 | abcd | ||
| Barred Plymouth Rock | 1.21 ± 0.04 | abcde | 528 ± 55 | hi | 0.23 ± 0.02 | j | 2.74 ± 0.22 | defghi | 28.37 ± 2.57 | fghijk | ||
| Bielefelder | 1.24 ± 0.01 | abcd | 585 ± 33 | ghi | 0.41 ± 0.06 | ghij | 3.23 ± 0.54 | cdefg | 43.83 ± 3.85 | abc | ||
| Australorp | 1.05 ± 0.01 | abcde | 490 ± 47 | i | 0.46 ± 0.04 | fghij | 2.73 ± 0.31 | defghi | 50.25 ± 3.11 | a | ||
| m ± SEM | 1.36 ± 0.11 | 597 ± 36 | 0.37 ± 0.04 | 2.75 ± 0.20 | 40.71 ± 2.94 | |||||||
| thermoneutral | Tetra H | 0.29 ± 0.03 | cde | 897 ± 26 | abcdefghi | 0.30 ± 0.02 | hij | 1.55 ± 0.22 | i | 31.75 ± 3.71 | cdefghij | |
| Tetra Super Harco | 0.24 ± 0.03 | cde | 793 ± 72 | bcdefghi | 0.45 ± 0.15 | ghij | 1.35 ± 0.03 | i | 16.75 ± 1.09 | kl | ||
| White Plymouth Rock | 0.39 ± 0.01 | cde | 828 ± 23 | bcdefghi | 0.66 ± 0.11 | cdefghij | 1.65 ± 0.18 | hi | 27.50 ± 3.18 | ghijk | ||
| Barred Plymouth Rock | 0.19 ± 0.06 | de | 781 ± 23 | bcdefghi | 0.57 ± 0.03 | defghij | 1.46 ± 0.15 | i | 27.75 ± 2.51 | ghijk | ||
| Bielefelder | 0.25 ± 0.05 | cde | 817 ± 68 | bcdefghi | 0.54 ± 0.04 | defghij | 1.47 ± 0.07 | i | 34.25 ± 3.25 | cdefghi | ||
| Australorp | 0.16 ± 0.01 | e | 804 ± 14 | bcdefghi | 0.57 ± 0.02 | defghij | 1.70 ± 0.47 | ghi | 40.25 ± 3.50 | abcdef | ||
| m ± SEM | 0.26 ± 0.03 | 820 ± 17 | 0.52 ± 0.05 | 1.53 ± 0.05 | 29.71 ± 3.23 | |||||||
| hot | Tetra H | 1.77 ± 0.14 | ab | 847 ± 75 | abcdefghi | 1.32 ± 0.23 | ab | 5.51 ± 0.55 | a | 48.75 ± 3.02 | ab | |
| Tetra Super Harco | 1.30 ± 0.24 | abc | 1082 ± 133 | abcde | 1.54 ± 0.13 | a | 4.87 ± 0.24 | ab | 31.75 ± 2.52 | cdefghij | ||
| White Plymouth Rock | 1.91 ± 0.34 | ab | 853 ± 76 | abcdefghi | 0.78 ± 0.07 | cdefgh | 3.17 ± 0.18 | cdefgh | 34.76 ± 1.84 | cdefghi | ||
| Barred Plymouth Rock | 1.91 ± 0.18 | ab | 1198 ± 24 | ab | 0.95 ± 0.10 | bcde | 2.71 ± 0.45 | defghi | 36.50 ± 3.68 | cdefgh | ||
| Bielefelder | 1.01 ± 0.25 | abcde | 773 ± 32 | bcdefghi | 0.58 ± 0.04 | defghij | 4.67 ± 0.39 | abc | 31.75 ± 2.30 | cdefghij | ||
| Australorp | 0.22 ± 0.02 | de | 1105 ± 41 | abcd | 1.01 ± 0.06 | bcd | 3.37 ± 0.14 | bcde | 28.55 ± 1.99 | fghijk | ||
| m ± SEM | 1.35 ± 0.27 | 976 ± 71 | 1.03 ± 0.14 | 4.05 ± 0.46 | 35.34 ± 2.91 | |||||||
| II | cold | Tetra H | 1.16 ± 0.18 | abcde | 597 ± 53 | fghi | 0.53 ± 0.07 | defghij | 3.17 ± 0.14 | cdefgh | 35.30 ± 1.48 | cdefghi |
| Tetra Super Harco | 1.98 ± 0.21 | a | 704 ± 43 | defghi | 0.39 ± 0.07 | ghij | 2.54 ± 0.26 | defghi | 32.97 ± 0.78 | cdefghij | ||
| White Plymouth Rock | 1.29 ± 0.18 | abc | 1042 ± 61 | abcdef | 0.40 ± 0.06 | ghij | 2.60 ± 0.19 | defghi | 33.67 ± 1.20 | cdefghi | ||
| Barred Plymouth Rock | 1.55 ± 0.28 | ab | 742 ± 61 | cdefghi | 0.26 ± 0.03 | ij | 2.39 ± 0.22 | defghi | 34.58 ± 1,40 | cdefghi | ||
| Bielefelder | 0.44 ± 0.07 | cde | 658 ± 49 | defghi | 0.43 ± 0.07 | ghij | 2.39 ± 0.22 | defghi | 36.96 ± 0.91 | bcdefgh | ||
| Australorp | 1.02 ± 0.11 | abcde | 888 ± 51 | abcdefghi | 0.74 ± 0.09 | cdefghi | 2.45 ± 0.23 | defghi | 40.96 ± 1.00 | abcde | ||
| m ± SEM | 1.24 ± 0.21 | 772 ± 67 | 0.46 ± 0.07 | 2.59 ± 0.12 | 35.74 ± 1.19 | |||||||
| thermoneutral | Tetra H | 0.423 ± 0.09 | cde | 911 ± 88 | abcdefghi | 0.69 ± 0.04 | cdefghij | 1.71 ± 0.18 | ghi | 26.38 ± 1.16 | ghijk | |
| Tetra Super Harco | 0.364 ± 0.06 | cde | 829 ± 30 | bcdefghi | 0.46 ± 0.04 | fghij | 1.75 ± 0.19 | fghi | 13.50 ± 0.45 | l | ||
| White Plymouth Rock | 0.360 ± 0.05 | cde | 1282 ± 113 | a | 0.63 ± 0.03 | cdefghij | 1.84 ± -.13 | efghi | 23.34 ± 1.29 | ijkl | ||
| Barred Plymouth Rock | 0.258 ± 0.05 | cde | 1097 ± 144 | abcd | 0.33 ± 0.02 | ghij | 1.83 ± 0.10 | efghi | 20.63 ± 1.01 | jkl | ||
| Bielefelder | 0.268 ± 0.05 | cde | 1029 ± 86 | abcdefg | 0.52 ± 0.02 | efghij | 2.14 ± 0.15 | efghi | 31.54 ± 1.70 | defghij | ||
| Australorp | 0.320 ± 0.04 | cde | 1158 ± 60 | abc | 0.66 ± 0.02 | cdefghij | 2.19 ± 0.19 | efghi | 35.47 ± 1.27 | cdefghi | ||
| m ± SEM | 0.33 ± 0.03 | 1051 ± 67 | 0.55 ± 0.06 | 1.91 ± 0.08 | 25.14 ± 3.2 | |||||||
| hot | Tetra H | 1.900 ± 0.34 | ab | 1046 ± 76 | abcdef | 0.97 ± 0.06 | bcde | 3.84 ± 0.14 | bcde | 41.36 ± 1.31 | abcd | |
| Tetra Super Harco | 1.181 ± 0.11 | abcde | 831 ± 76 | abcdefghi | 1.08 ± 0.04 | abc | 3.26 ± 0.29 | cdef | 25.18 ± 1.31 | hijkl | ||
| White Plymouth Rock | 1.537 ± 0.19 | ab | 706 ± 111 | cdefghi | 0.67 ± 0.04 | cdefghij | 2.75 ± 0.08 | defghi | 29.13 ± 1.20 | efghij | ||
| Barred Plymouth Rock | 1.540 ± 0.36 | ab | 811 ± 71 | bcdefghi | 0.79 ± 0.06 | cdefg | 2.68 ± 0.14 | defghi | 30.35 ± 2.01 | defghij | ||
| Bielefelder | 1.040 ± 0.16 | abcde | 696 ± 56 | defghi | 0.41 ± 0.02 | ghij | 3.11 ± 0.14 | defghi | 33.05 ± 1.02 | cdefghij | ||
| Australorp | 0.864 ± 0.10 | bcde | 980 ± 96 | abcdegh | 0.95 ± 0.07 | bcdef | 2.64 ± 0.24 | defghi | 32.44 ± 1.24 | cdefghij | ||
| m ± SEM | 1.34 ± 0.16 | 845 ± 58 | 0.81 ± 0.09 | 3.05 ± 0.19 | 31.92 ± 2.21 | |||||||
| m ± SEM (for I and II years) | 0.98 ± 0.10 | 844 ± 33 | 0.62 ± 0.05 | 2.65 ± 0.16 | 33.09 ± 1.32 | |||||||
| Indicator | LPO | tGSH | CAT | SOD | GPx |
|---|---|---|---|---|---|
| LPO | - | −0.262 ** | 0.228 ** | 0.402 ** | 0.200 ** |
| tGSH | - | 0.224 ** | −0.02 n.s. | −0.169 * | |
| CAT | - | 0.477 ** | 0.155 * | ||
| SOD | - | 0.164 * | |||
| GPx | - |
| Factors | Source of Variance | |||||
|---|---|---|---|---|---|---|
| LPO | tGSH | CAT | SOD | GPx | ||
| Year | F | 1.369 | 5.622 | 3.770 | 12.401 | 21.346 |
| probability | n.s. | p < 0.05 | p < 0.05 | p < 0.001 | p < 0.001 | |
| Period | F | 81.285 | 22.343 | 65.846 | 59.648 | 9.889 |
| probability | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Genotype | F | 5.002 | 1.754 | 5.280 | 3.522 | 9.744 |
| probability | p < 0.01 | n.s. | p < 0.001 | p < 0.01 | p < 0.001 | |
| Year × Period | F | 32.408 | 16.326 | 30.651 | 32.605 | 8.585 |
| probability | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Year × Genotype | F | 2.783 | 2.296 | 3.400 | 3.367 | 8.407 |
| probability | p < 0.05 | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Period × Genotype | F | 20.602 | 5.128 | 21.273 | 13.303 | 8.792 |
| probability | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Year × Period × Genotype | F | 10.812 | 5.970 | 14.341 | 10.193 | 7.49 |
| probability | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerzilov, V.; Alexandrova, A.; Petrov, P.; Boncheva, V.; Keranova, N.; Andreeva, M.; Georgieva, A.; Tzvetanova, E. Changes in the Oxidative Status of Dual-Purpose Hens Rearing in the Free-Range System during Cold, Thermoneutral and Hot Period. Animals 2022, 12, 2650. https://doi.org/10.3390/ani12192650
Gerzilov V, Alexandrova A, Petrov P, Boncheva V, Keranova N, Andreeva M, Georgieva A, Tzvetanova E. Changes in the Oxidative Status of Dual-Purpose Hens Rearing in the Free-Range System during Cold, Thermoneutral and Hot Period. Animals. 2022; 12(19):2650. https://doi.org/10.3390/ani12192650
Chicago/Turabian StyleGerzilov, Vasko, Albena Alexandrova, Petar Petrov, Veselina Boncheva, Neli Keranova, Madlena Andreeva, Almira Georgieva, and Elina Tzvetanova. 2022. "Changes in the Oxidative Status of Dual-Purpose Hens Rearing in the Free-Range System during Cold, Thermoneutral and Hot Period" Animals 12, no. 19: 2650. https://doi.org/10.3390/ani12192650
APA StyleGerzilov, V., Alexandrova, A., Petrov, P., Boncheva, V., Keranova, N., Andreeva, M., Georgieva, A., & Tzvetanova, E. (2022). Changes in the Oxidative Status of Dual-Purpose Hens Rearing in the Free-Range System during Cold, Thermoneutral and Hot Period. Animals, 12(19), 2650. https://doi.org/10.3390/ani12192650







