Optimization of the Thawing Protocol for Iberian Boar Sperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Semen Collection and Freezing-Thawing Protocol
2.2. Experimental Design
2.2.1. Experiment 1: Evaluation of Different Thawing Rates
2.2.2. Experiment 2: Evaluation of Different Thawing Extenders
2.3. Post-Thaw Sperm Quality Assessments
2.3.1. Assessment of Sperm Motility
2.3.2. Assessment of Acrosome Status
2.3.3. Assessment of Plasma Membrane Integrity
2.4. Statistical Analysis
3. Results
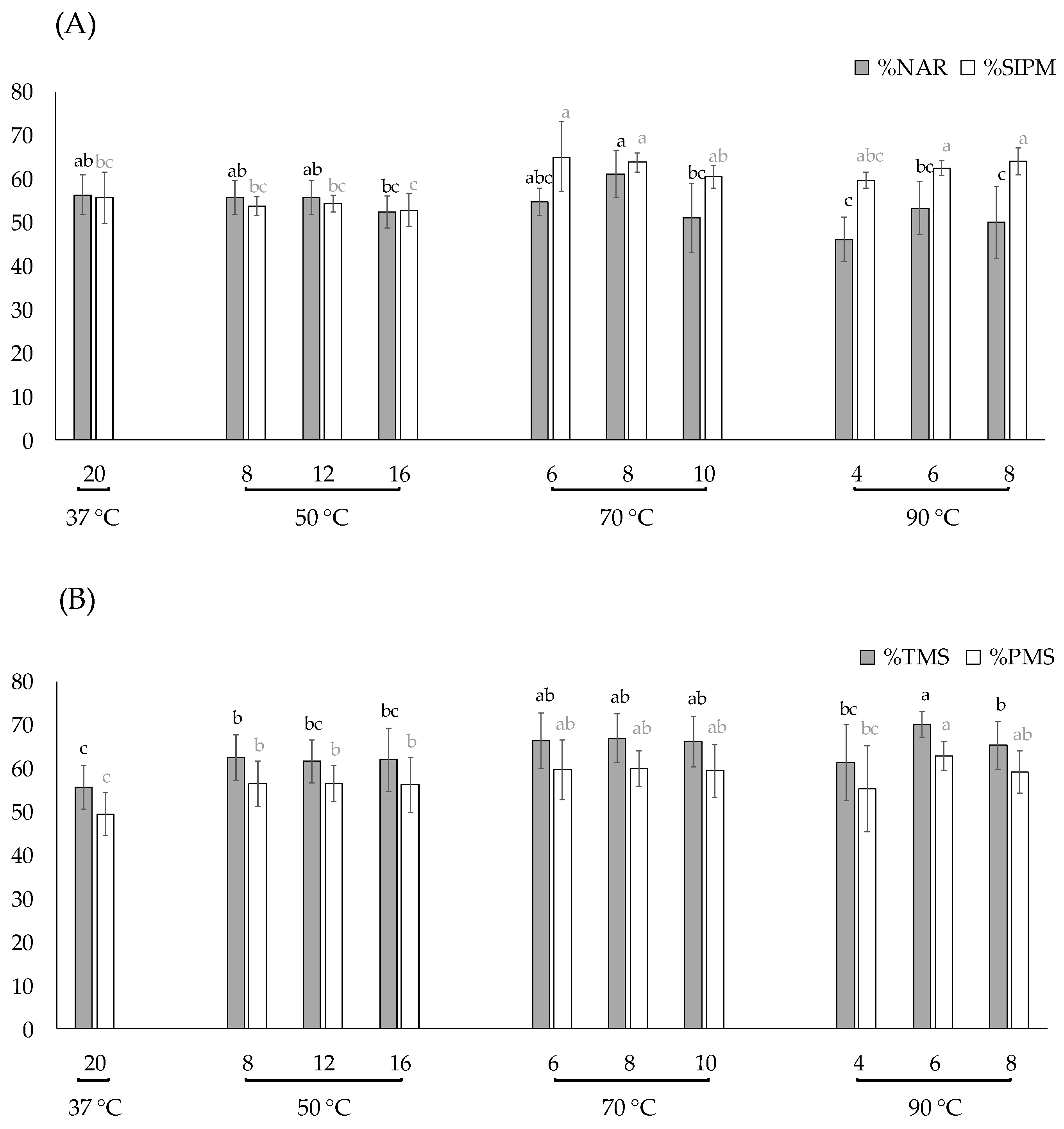
3.1. Experiment 1: Evaluation of Different Thawing Conditions
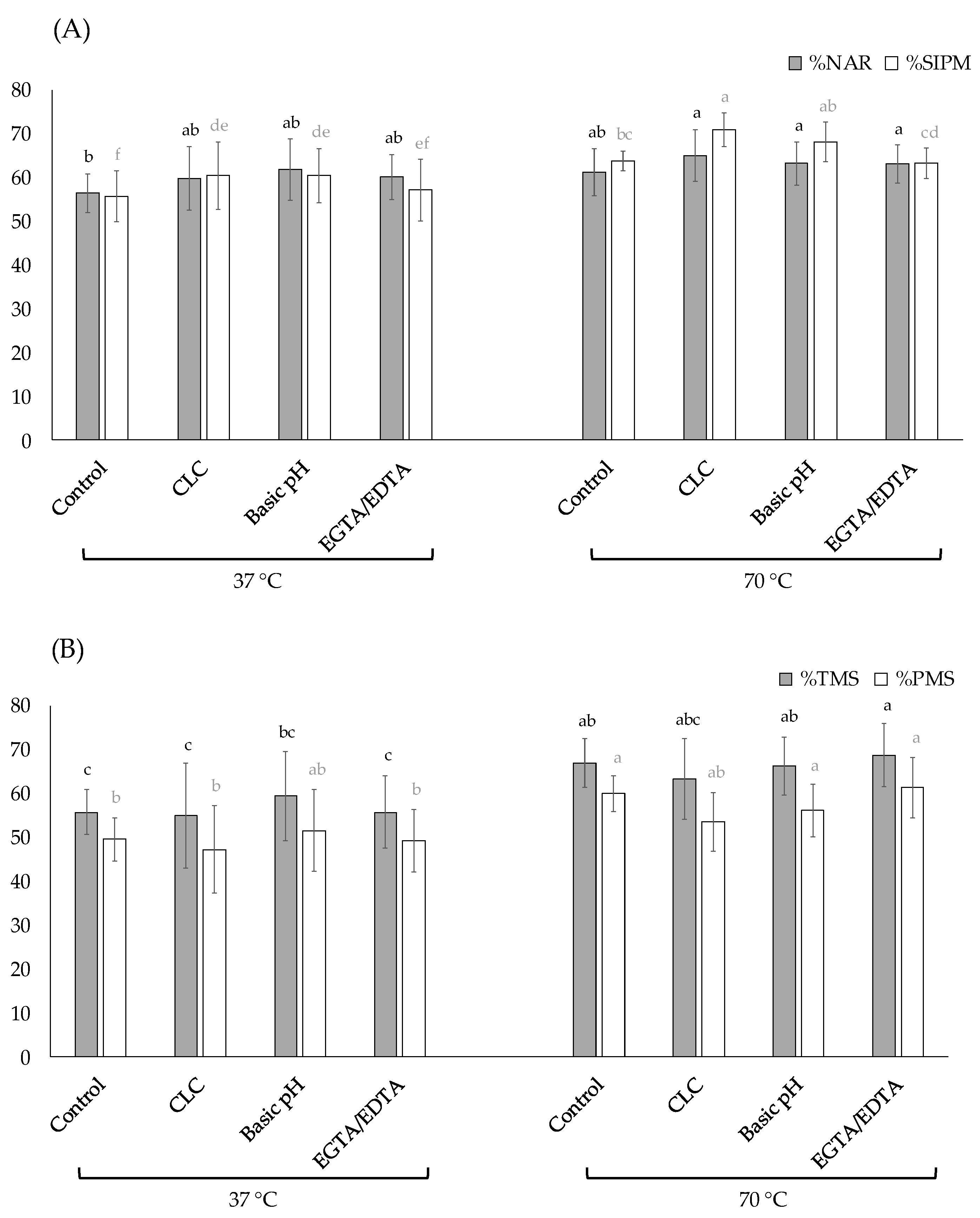
3.2. Experiment 2: Evaluation of Different Modifications in the Thawing Extenders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrero-Medrano, J.M.; Megens, H.; Groenen, M.A.M.; Ramis, G.; Bosse, M.; Pérez-Enciso, M.; Crooijmans, R.P.M.A. Conservation genomic analysis of domestic and wild pig populations from the Iberian Peninsula. BMC Genet. 2013, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cryoconservation of animal genetic resources. In FAO Animal Production and Health Guidelines No. 12; FAO: Rome, Italy, 2012. [Google Scholar]
- Knox, R. The current value of frozen–thawed boar semen for commercial companies. Reprod. Domest. Anim. 2011, 46, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Recent Advances in Boar Sperm Cryopreservation: State of the Art and Current Perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Romero, F.; Zambrano, F.; Sánchez, R.S. Preservation of boar semen: An update. Reprod. Domest. Anim. 2019, 54, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Fiser, P.S.; Fairfull, R.W.; Hansen, C.; Panich, P.L.; Shrestha, J.N.; Underhill, L. The effect of warming velocity on motility and acrosomal integrity of boar sperm as influenced by the rate of freezing and glycerol level. Mol. Reprod. Dev. 1993, 34, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Roca, J.; Gil, M.A.; Vázquez, J.M.; Martínez, E.A. Adjustments on the cryopreservation conditions reduce the incidence of boar ejaculates with poor sperm freezability. Theriogenology 2007, 67, 1436–1445. [Google Scholar] [CrossRef]
- Tomás, C.; Gómez-Fernández, J.; Gómez-Izquierdo, E.; de Mercado, E. Effect of the holding time at 15 °C prior to cryopreservation, the thawing rate and the post-thaw incubation temperature on the boar sperm quality after cryopreservation. Anim. Reprod. Sci. 2014, 144, 115–121. [Google Scholar] [CrossRef]
- Athurupana, R.; Ioki, S.; Funahashi, H. Rapid thawing and stabilizing procedure improve postthaw survival and in vitro penetrability of boar spermatozoa cryopreserved with a glycerol-free trehalose-based extender. Theriogenology 2015, 84, 940–947. [Google Scholar] [CrossRef]
- Nöthling, J.O.; Shuttleworth, R. The effect of straw size, freezing rate and thawing rate upon post-thaw quality of dog semen. Theriogenology 2005, 63, 1469–1480. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Althouse, G.C. Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology 2011, 76, 1508–1516. [Google Scholar] [CrossRef]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; González-Bulnes, A.; Sánchez-Sánchez, R.; de Mercado, E. Inclusion of seminal plasma in sperm cryopreservation of Iberian pig. Anim. Reprod. Sci. 2012, 130, 82–90. [Google Scholar] [CrossRef]
- Tomás, C.; Gómez-Fernández, J.; Gómez-Izquierdo, E.; Mocé, E.; de Mercado, E. Addition of cholesterol-loaded cyclodextrins to the thawing extender: Effects on boar sperm quality. Reprod. Domest. Anim. 2014, 49, 427–432. [Google Scholar] [CrossRef]
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology. 2017, 90, 88–93. [Google Scholar] [CrossRef]
- Weng, X.G.; Cai, M.M.; Zhang, Y.T.; Liu, Y.; Gao, Z.; Song, J.; Liu, Z. Effect of Astragalus polysaccharide addition to thawed boar sperm on in vitro fertilization and embryo development. Theriogenology 2018, 121, 21–26. [Google Scholar] [CrossRef]
- Arranz-Virseda, L.; Tomás-Almenar, C.; Ciruelos, J.J.; Gómez-Izquierdo, E.; de Mercado, E. Use of antioxidants in the freezing and thawing extender in Iberian pig sperm. ITEA-Inf. Tec. 2021, 117, 544–556. [Google Scholar] [CrossRef]
- Okazaki, T.; Yoshida, S.; Teshima, H.; Shimada, M. The addition of calcium ion chelator, EGTA to thawing solution improves fertilizing ability in frozen-thawed boar sperm. Anim. Sci. J. 2011, 82, 412–419. [Google Scholar] [CrossRef]
- de Mercado, E.; Tomás-Almenar, C.; Gómez-Izquierdo, E. Improvement of the motility of boar sperm after cryopreservation. Anim. Reprod. Sci. 2020, 222, 106610. [Google Scholar] [CrossRef]
- Roca, J.; Hernández, M.; Carvajal, G.; Vázquez, J.M.; Martínez, E.A. Factors influencing boar sperm cryosurvival. J. Anim. Sci. 2006, 84, 2692–2699. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Castaño, C.; Toledano-Díaz, A.; Coloma, M.; Lopez, A.; Prieto, M.; Campo, J. Semen cryopreservation for the creation of a Spanish poultry breeds cryobank: Optimization of freezing rate and equilibration time. Poult. Sci. 2011, 90, 2047–2053. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Martín-Hidalgo, D.; Barón, F.J.; Robina, A.; Bragado, M.; Hurtado, A.; Marin, L.; Gil, M. Inter-and intra-breed comparative study of sperm motility and viability in Iberian and Duroc boar semen during long-term storage in MR-A and XCell extenders. Anim. Reprod. Sci. 2013, 139, 109–114. [Google Scholar] [CrossRef]
- 53/2013, R. Real Decreto 53/2013, de 1 de Febrero, Por el Que se Establecen las Normas Básicas Aplicables para la Protección de Los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. 2013. Available online: https://www.boe.es/eli/es/rd/2013/02/01/53 (accessed on 17 November 2021).
- Pursel, V.G.; Johnson, L.A. Freezing of boar spermatozoa: Fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef]
- De Mercado, E.; Rodríguez, A.; Gómez, E.; Sanz, E. Cryopreservation of Iberian pig spermatozoa. Comparison of different freezing extenders based on post-thaw sperm quality. Anim. Reprod. Sci. 2010, 118, 54–61. [Google Scholar] [CrossRef]
- Cremades, T.; Roca, J.; Rodriguez-Martinez, H.; Abaigar, T.; Vazquez, J.M.; Martinez, E.A. Kinematic changes during the cryopreservation of boar spermatozoa. J. Androl. 2005, 26, 610–618. [Google Scholar] [CrossRef]
- Pursel, V.G.; Johnson, L.A.; Schulman, L.L. Interaction of extender composition and incubation period on cold shock susceptibility of boar spermatozoa. J. Anim. Sci. 1972, 35, 580–584. [Google Scholar] [CrossRef]
- Tomás, C.; Gómez-Fernández, J.; Gómez-Izquierdo, E.; de Mercado, E. Combined use of fluorescence and phase contrast microscopy for the determination of sperm viability. Reprod. Domest. Anim. 2014, 49, 103. [Google Scholar] [CrossRef]
- R Core Team. R: AL and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 June 2022).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio; PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 15 June 2022).
- Knox, R.V.; Ringwelski, J.M.; McNamara, K.A.; Aardsma, M.; Bojko, M. The effect of extender, method of thawing, and duration of storage on in vitro fertility measures of frozen-thawed boar sperm. Theriogenology 2015, 84, 407–412. [Google Scholar] [CrossRef]
- Eriksson, B.M.; Rodriguez-Martinez, H. Effect of freezing and thawing rates on the post-thaw viability of boar spermatozoa frozen in FlatPacks and Maxi-straws. Anim. Reprod. Sci. 2000, 63, 205–220. [Google Scholar] [CrossRef]
- Koshimoto, C.; Mazur, P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology 2002, 45, 49–59. [Google Scholar] [CrossRef]
- Lyashenko, A. Effect of different thawing procedures on the quality and fertility of the bull spermatozoa. Asian Pac. J. Reprod. 2015, 4, 17–21. [Google Scholar] [CrossRef]
- Diskin, M.G. Review: Semen handling, time of insemination and insemination technique in cattle. Animals 2018, 12, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bamba, K.; Cran, D.G. Further studies on rapid dilution and warming of boar semen. J. Reprod. Fertil. 1988, 82, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pursel, V.G.; Johnson, L.A.; Schulman, L.L. Effect of dilution, seminal plasma and incubation period on cold shock susceptibility of boar spermatozoa. J. Anim. Sci. 1973, 37, 528–531. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Yi, Y.J.; Im, G.S.; Park, C.S. Lactose-egg yolk diluent supplemented with N-acetyl-D-glucosamine affect acrosome morphology and motility of frozen-thawed boar sperm. Anim. Reprod. Sci. 2002, 74, 187–194. [Google Scholar] [CrossRef]
- Muiño, R.; Rivera, M.M.; Rigau, T.; Rodriguez-Gil, J.E.; Peña, A.I. Effect of different thawing rates on post-thaw sperm viability, kinematic parameters and motile sperm subpopulations structure of bull semen. Anim. Reprod. Sci. 2008, 109, 50–64. [Google Scholar] [CrossRef]
- Rastegarnia, A.; Shahverdi, A.; Rezaei Topraggaleh, T.; Ebrahimi, B.; Shafipour, V. Effect of different thawing rates on post-thaw viability, kinematic parameters and chromatin structure of Buffalo (Bubalus bubalis) spermatozoa. Cell J. 2013, 14, 306–313. [Google Scholar]
- Malo, C.; Elwing, B.; Soederstroem, L.; Lundeheim, N.; Morrell, J.M.; Skidmore, J.A. Effect of different freezing rates and thawing temperatures on cryosurvival of dromedary camel spermatozoa. Theriogenology 2019, 125, 43–48. [Google Scholar] [CrossRef]
- Bezerra, F.S.B.; de Souza Castelo, T.; Araújo dos Santos, E.A.; da Costa Dantas, T.; Rodrigo Simão, B.; Rodrigues Silva, A. Assessment of the interaction between straw size and thawing rate and its impact on in vitro quality of post-thaw goat semen. R. Bras. Zootec. 2012, 41, 592–597. [Google Scholar] [CrossRef]
- Schmidt, H.; Kamp, G. Induced hyperactivity in boar spermatozoa and its evaluation by computer-assisted sperm analysis. Reproduction 2004, 128, 171–179. [Google Scholar] [CrossRef]
- Suarez, S.S. Hyperactivated motility in sperm. J. Androl. 1996, 17, 331–335. [Google Scholar] [CrossRef]
- Basioura, A.; Tsakmakidis, I.A.; Martinez, E.A.; Roca, J.; Li, J.; Molina, M.; Theodoridis, A.; Boscos, C.; Parrilla, I. Effect of astaxanthin in extenders on sperm quality and functional variables of frozen-thawed boar semen. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef]
- Parasassi, T.; Di Stefano, M.; Loiero, M.; Ravagnan, G.; Gratton, E. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: A fluorescence study using Laurdan probe. Biophys. J. 1994, 66, 763–768. [Google Scholar] [CrossRef]
- Kim, J.C.; Li, Y.; Lee, S.; Yi, Y.J.; Park, C.S.; Woo, S.H. Effects of cryopreservation on Ca2+ signals induced by membrane depolarization, caffeine, thapsigargin and progesterone in boar spermatozoa. Mol. Cells 2008, 26, 558–565. [Google Scholar]
- Navarro, B.; Kirichok, Y.; Clapham, D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA 2007, 104, 7688–7692. [Google Scholar] [CrossRef]
- Qi, H.; Moran, M.M.; Navarro, B.; Claphman, D. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef]
- Lee, H.C.; Garbers, D.L. Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J. Biol. Chem. 1986, 261, 16026–16032. [Google Scholar] [CrossRef]
- Granados-Gonzalez, G.; Mendoza-Lujambio, I.; Rodriguez, E.; Galindo, B.E.; Beltrán, C.; Darszon, A. Identification of voltage-dependent Ca2+ channels in sea urchin sperm. FEBS. Lett. 2005, 579, 6667–6672. [Google Scholar] [CrossRef]
- Gatti, J.L.; Chevrier, C.; Paquignon, M.; Dacheux, J.L. External ionic conditions, internal pH and motility of ram and boar spermatozoa. J. Reprod. Fertil. 1993, 98, 439–449. [Google Scholar] [CrossRef]
- Turner, R.M. Moving to the beat: A review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2006, 18, 25–38. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Rates, D.M.; Pugliesi, G.; Ker, P.; Arruda, R.; Moraes, E.; Cravarho, E. Use of cholesterol-loaded cyclodextrin in donkey semen cryopreservation improves sperm viability but results in low fertility in mares. Reprod. Domest. Anim. 2014, 49, 845–850. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]

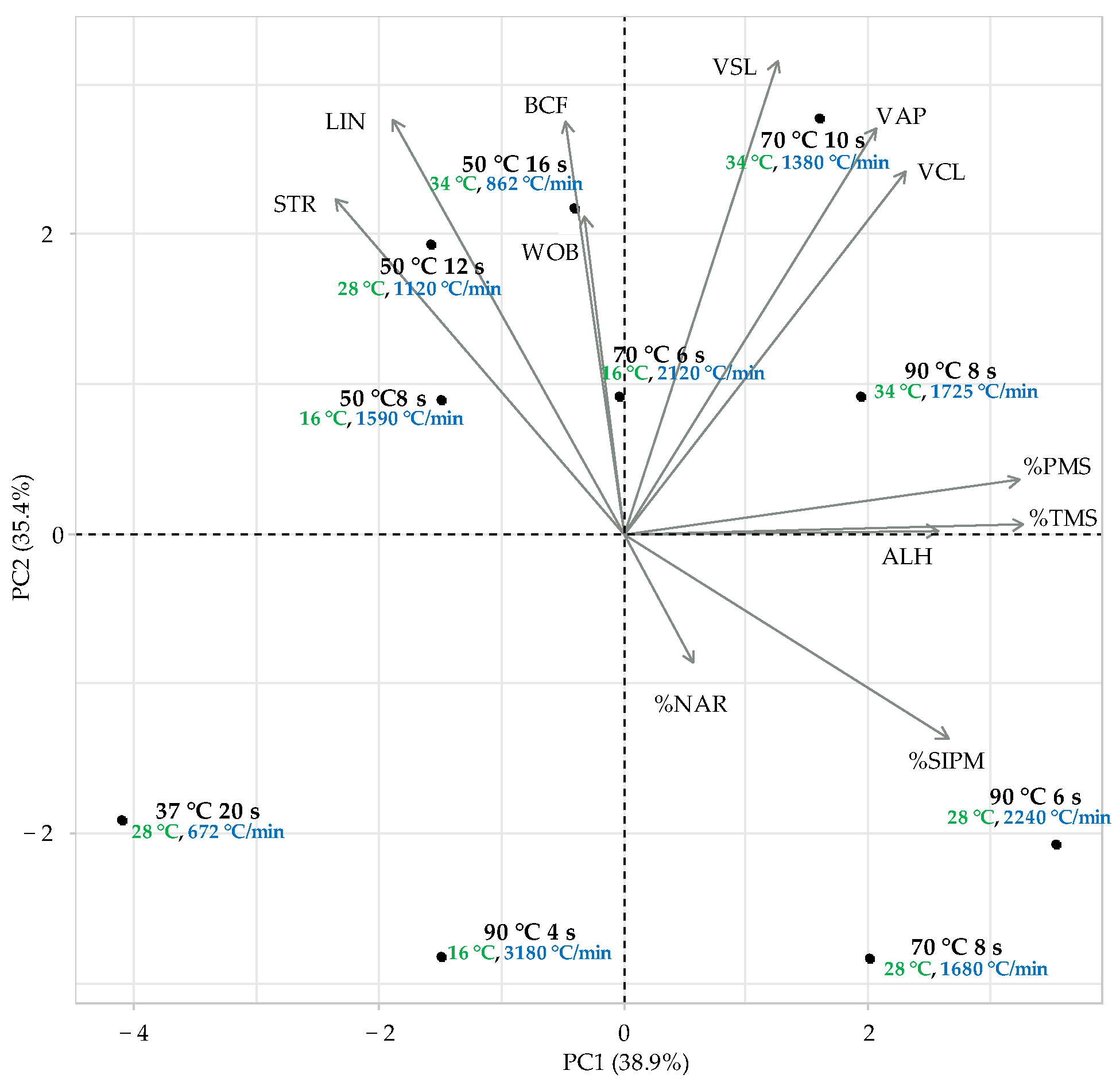
| Thawing Temp. (°C) | Plunge Time (s) | Final Temp. (°C) | Thawing Rate (°C/min) |
|---|---|---|---|
| 37 (control) | 20 | 28 | 672 |
| 50 | 8 | 16 | 1590 |
| 12 | 28 | 1120 | |
| 16 | 34 | 862 | |
| 70 | 6 | 16 | 2120 |
| 8 | 28 | 1680 | |
| 10 | 34 | 1380 | |
| 90 | 4 | 16 | 3180 |
| 6 | 28 | 2240 | |
| 8 | 34 | 1725 |
| Thawing Temp. (°C) | Plunge Time (s) | Final Temp. (°C) | Rate (°C/min) | VCL | VSL | VAP | LIN | STR | WOB | ALH | BCF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | 20 | 28 | 672 | 73.5 ± 4.5 c | 53.3 ± 4 d | 63.6 ± 4 b | 72.5 ± 3.9 b | 83.8 ± 3.5 | 86.5 ± 1.3 ab | 2.28 ± 0.1 de | 8.1 ± 0.6 bcd |
| 50 | 8 | 16 | 1590 | 79.5 ± 8.3 abc | 58 ± 6.4 abcd | 69.1 ± 8.6 ab | 73 ± 4 ab | 84.1 ± 3.3 | 86.7 ± 3.2 a | 2.58 ± 0.16 a | 8.05 ± 0.1 cd |
| 12 | 28 | 1120 | 80.6 ± 5.2 ab | 59.4 ± 3.4 abc | 70.7 ± 4.5 a | 74.2 ± 4.3 a | 84.4 ± 3.4 | 87.9 ± 1.8 a | 2.54 ± 0.21 ab | 8 ± 0.2 d | |
| 16 | 34 | 862 | 84.1 ± 8.1 a | 61 ± 5.9 ab | 72.2 ± 7.7 a | 72.6 ± 5.1 ab | 84.7 ± 4.4 | 85.7 ± 2.7 ab | 2.45 ± 0.21 abc | 7.9 ± 0.3 d | |
| 70 | 6 | 16 | 2120 | 79.3 ± 7.9 abc | 57.7 ± 3.5 bcd | 69.2 ± 7 ab | 73.1 ± 4.7 ab | 83.7 ± 5.4 | 87.2 ± 0.5 a | 2.4 ± 0.19 bcd | 8.7 ± 0.4 a |
| 8 | 28 | 1680 | 79.7 ± 9.5 abc | 55.2 ± 4.6 cd | 67.8 ± 8.1 ab | 69.6 ± 4.5 ab | 81.7 ± 3.9 | 85.1 ± 2.3 ab | 2.32 ± 0.25 cde | 8.2 ± 0.2 bcd | |
| 10 | 34 | 1380 | 86.2 ± 5.2 a | 62.7 ± 3.2 a | 74.5 ± 3.7 a | 72.9 ± 3.5 ab | 84.2 ± 2.8 | 86.5 ± 1.5 ab | 2.23 ± 0.23 e | 8.5 ± 0.4 ab | |
| 90 | 4 | 16 | 3180 | 75.5 ± 3.7 bc | 53.6 ± 5.5 d | 63.9 ± 4.5 b | 70.8 ± 5.1 b | 83.8 ± 4.1 | 84.5 ± 2.8 b | 2.33 ± 0.14 cde | 8.4 ± 0.3 abc |
| 6 | 28 | 2240 | 81.1 ± 7.5 ab | 56.5 ± 4.5 bcd | 70.2 ± 6.8 ab | 69.7 ± 3.1 b | 80.7 ± 2.3 | 86.7 ± 2.1 a | 2.31 ± 0.21 cde | 8.2 ± 0.1 bcd | |
| 8 | 34 | 1725 | 84.4 ± 4.5 a | 60.7 ± 2.4 ab | 73.2 ± 2.6 a | 72 ± 4.1 ab | 83 ± 3.9 | 86.4 ± 1.7 ab | 2.51 ± 0.2 ab | 8.5 ± 0.3 ab |
| Thawing Temp. (°C) | Modifications | VCL | VSL | VAP | LIN | STR | WOB | ALH | BCF |
|---|---|---|---|---|---|---|---|---|---|
| 37 | Control | 73.5 ± 4.5 bcd | 53.3 ± 4 ab | 63.6 ± 4 abc | 72.5 ± 3.9 a | 83.8 ± 3.5 ab | 86.5 ± 1.3 a | 2.28 ± 0.1 cd | 8.1 ± 0.6 ab |
| CLC | 67.5 ± 6.1 d | 48 ± 6.4 bc | 57.9 ± 6 c | 71.1 ± 7.5 ab | 82.8 ± 5 abc | 85.7 ± 4.3 ab | 2.22 ± 0.25 cd | 7.35 ± 0.8 cd | |
| Basic pH | 67.9 ± 2.3 d | 45.5 ± 4.2 c | 57.6 ± 3.4 c | 67 ± 5.9 bc | 78.9 ± 5.3 cd | 84.8 ± 3.2 ab | 2.18 ± 0.13 d | 8.3 ± 0.4 a | |
| EGTA/EDTA | 72.1 ± 5.9 cd | 52.2 ± 5.3 ab | 61.4 ± 5.9 bc | 72.4 ± 3.7 a | 85.2 ± 3 a | 85 ± 1.9 ab | 2.35 ± 0.12 bcd | 7.9 ± 0.6 ab | |
| 70 | Control | 79.7 ± 9.5 ab | 55.2 ± 4.6 a | 67.8 ± 8.1 ab | 69.6 ± 4.5 ab | 81.7 ± 3.9 abc | 85.1 ± 2.3 ab | 2.32 ± 0.25 ab | 8.2 ± 0.2 ab |
| CLC | 72.8 ± 7.1 bcd | 49.4 ± 4.6 bc | 61.2 ± 5.3 bc | 68.1 ± 6.5 abc | 80.7 ± 4.8 bc | 84.2 ± 3.2 ab | 2.4 ± 0.29 bcd | 7.1 ± 0.4 d | |
| Basic pH | 75.9 ± 4.8 abc | 48.5 ± 3.6 bc | 63.9 ± 4.8 abc | 63.9 ± 2.3 c | 75.8 ± 2.6 d | 84.2 ± 1.7 ab | 2.43 ± 0.13 abc | 8.2 ± 0.3 ab | |
| EGTA/EDTA | 82.5 ± 8.3 a | 68.8 ± 7.2 a | 68.5 ± 7.7 a | 68.5 ± 4.5 abc | 82.2 ± 3.4 abc | 83.3 ± 2.5 b | 2.65 ± 0.18 a | 7.8 ± 0.7 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomás-Almenar, C.; de Mercado, E. Optimization of the Thawing Protocol for Iberian Boar Sperm. Animals 2022, 12, 2600. https://doi.org/10.3390/ani12192600
Tomás-Almenar C, de Mercado E. Optimization of the Thawing Protocol for Iberian Boar Sperm. Animals. 2022; 12(19):2600. https://doi.org/10.3390/ani12192600
Chicago/Turabian StyleTomás-Almenar, Cristina, and Eduardo de Mercado. 2022. "Optimization of the Thawing Protocol for Iberian Boar Sperm" Animals 12, no. 19: 2600. https://doi.org/10.3390/ani12192600
APA StyleTomás-Almenar, C., & de Mercado, E. (2022). Optimization of the Thawing Protocol for Iberian Boar Sperm. Animals, 12(19), 2600. https://doi.org/10.3390/ani12192600






