Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Diets
2.2. Carcass Data Collection
2.3. Product Preparation
2.4. Retail Color
2.5. Lipid Oxidation
2.6. Cooking
2.7. Warner–Bratzler Shear Force
2.8. Fatty Acid Analysis
2.9. Statistical Analysis
3. Results
3.1. Dietary Chemical Composition
3.2. Carcass and Meat Quality
3.3. Lipid Oxidation
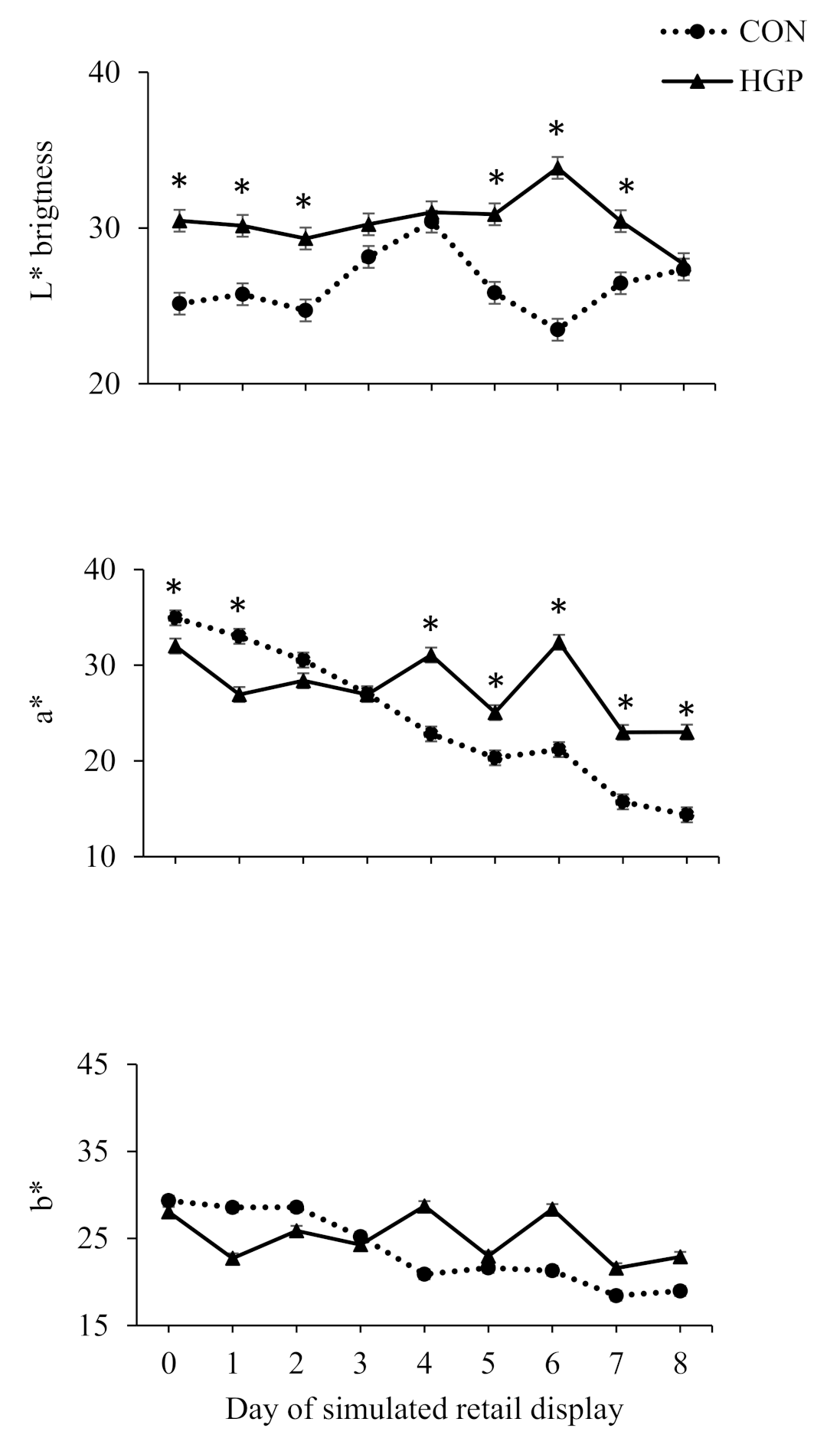
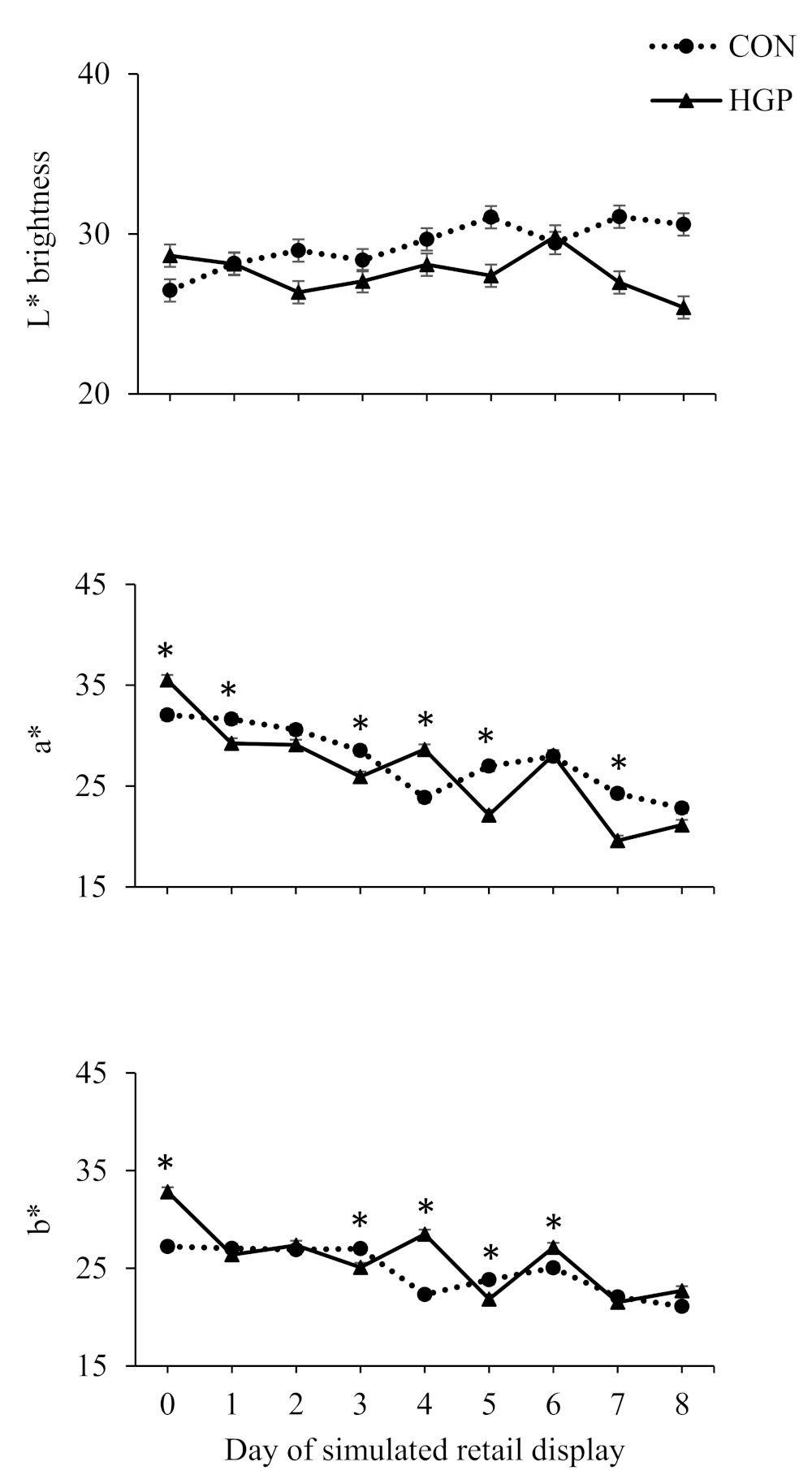
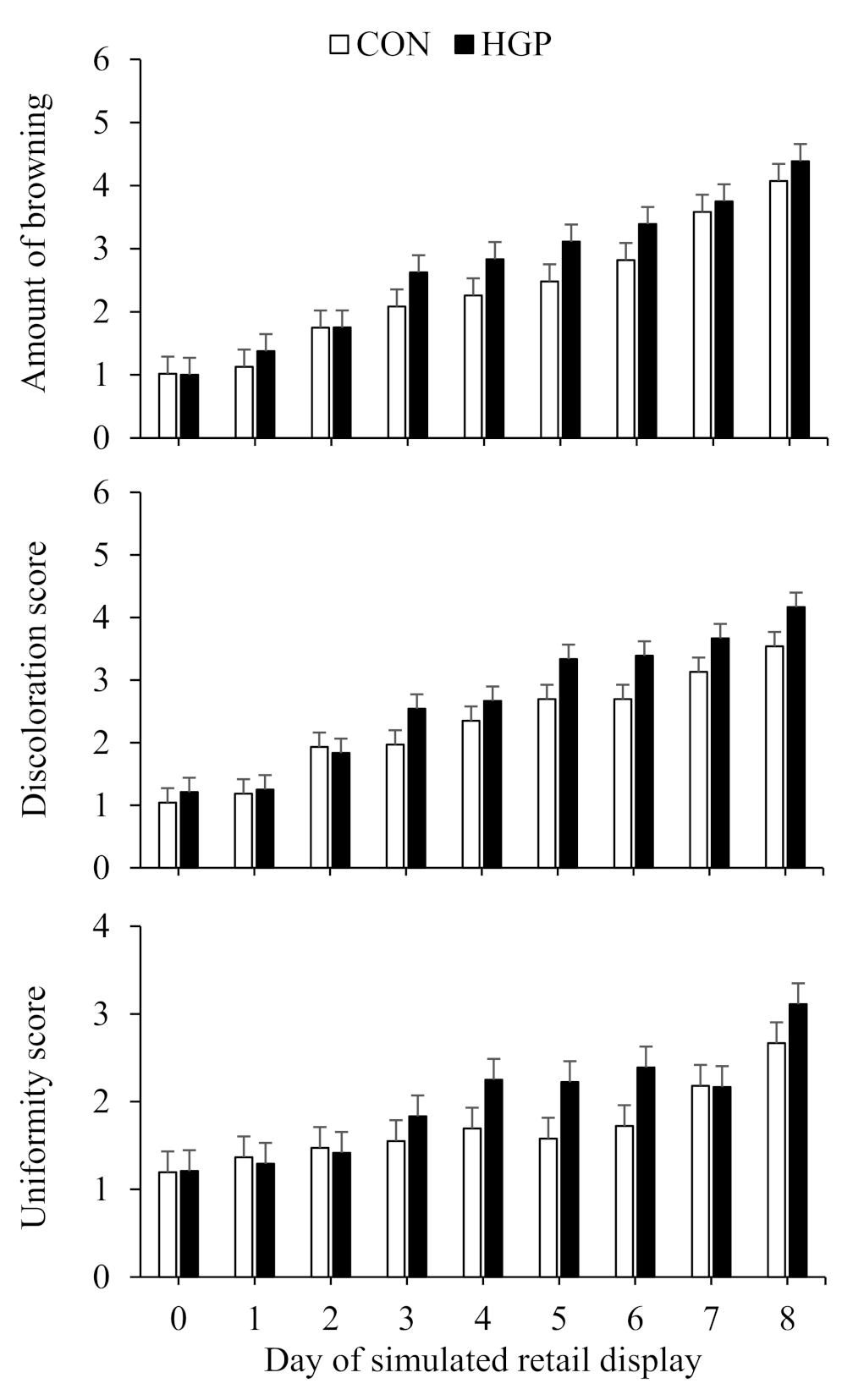
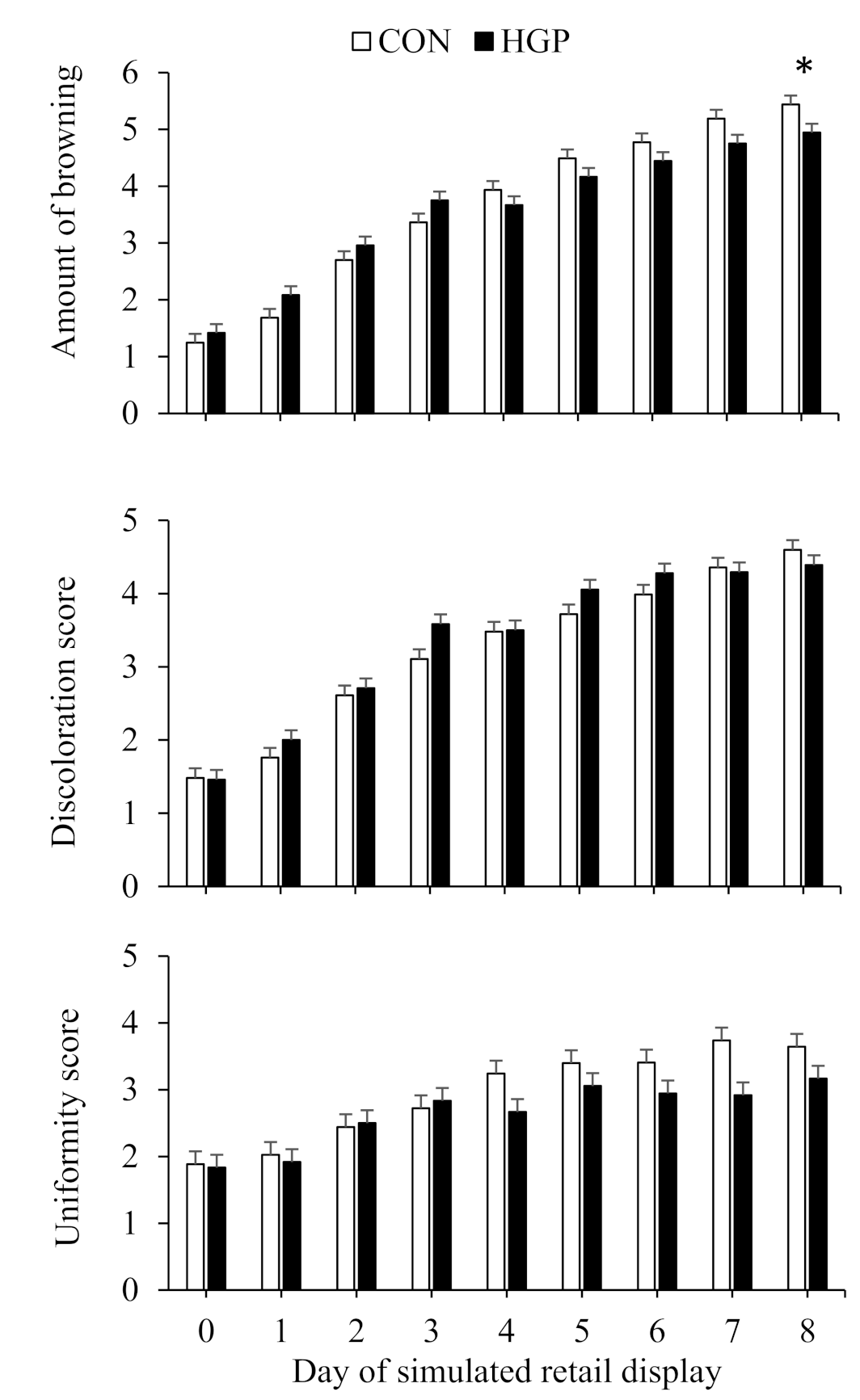
3.4. Color Stability
3.5. Fatty Acid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol. Cancer Ther. 2007, 6, 995–1005. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Apeleo, E.; Muiño, I.; Pérez, C.; Lauzurica, S.; Perez-Santaescolastica, C.; Díaz-Chirón, M.T.D.; Cañeque, V.; de la Fuente, J. Effect of dietary supplementation with either red wine extract or vitamin E on the volatile profile of lamb meat fed with omega-3 sources. Meat Sci. 2013, 93, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Jerónimo, E.; Brogna, D.M.; Dentinho, M.; Biondi, L.; Santos-Silva, J.; Priolo, A.; Bessa, R. The effect of grape seed extract or Cistus ladanifer L. on muscle volatile compounds of lambs fed dehydrated lucerne supplemented with oil. Food Chem. 2010, 119, 1339–1345. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.; Alves, S.P.; Dentinho, M.T.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- Henchion, M.M.; McCarthy, M.; Resconi, V.C. Beef quality attributes: A systematic review of consumer perspectives. Meat Sci. 2017, 128, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.J. A brief history of meat in the human diet and current health implications. Meat Sci. 2018, 144, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Minieri, S.; Rapaccini, S.; Antongiovanni, M.; Mele, M. Effect of chestnut and quebracho tannins on fatty acid profile in rumen liquid- and solid-associated bacteria: An in vitro study. Animal 2011, 5, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Khiaosa-Ard, R.; Bryner, S.F.; Scheeder, M.R.L.; Wettstein, H.-R.; Leiber, F.; Kreuzer, M.; Soliva, C.R. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Gaspa, G.; Pulina, G.; Nudda, A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016, 99, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Gallardo, B.; Salvá, A.; Guerra-Rivas, C.; Mantecón, Á.R.; Lavin, P.; De La Fuente, M.A. Influence of dietary grape pomace combined with linseed oil on fatty acid profile and milk composition. J. Dairy Sci. 2016, 99, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Moate, P.J.; Williams, S.R.O.; Torok, V.A.; Hannah, M.C.; Ribaus, B.E.; Tavendale, M.H.; Eckard, R.J.; Jacobs, J.L.; Auldist, M.J.; Wales, W.D. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; AOAC: Arlington, VA, USA, 2012. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Metzler-Zebeli, B.U.; Ahmed, S.; Muro-Reyes, A.; Deckardt, K.; Chizzola, R.; Böhm, J.; Zebeli, Q. Fortification of dried distillers grains plus solubles with grape seed meal in the diet modulates methane mitigation and rumen microbiota in Rusitec. J. Dairy Sci. 2015, 98, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- NAMP. The Meat Buyer’s Guide; North American Meat Processors Association: Reston, VA, USA, 2011. [Google Scholar]
- AMSA. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Clark, R.M.; Ferris, A.M.; Fey, M.; Brown, P.B.; Hundrieser, K.E.; Jensen, R.G. Changes in the Lipids of Human Milk from 2 to 16 Weeks Postpartum. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 311–316. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesterol esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Allen, M.S. Effects of Diet on Short-Term Regulation of Feed Intake by Lactating Dairy Cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.; Wilkes, M.; Pitchford, W.; Lee, S.; Hynd, P. Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Anim. Feed Sci. Technol. 2019, 247, 166–172. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Guerra-Rivas, C.; Gallardo, B.; Lavín, P.; Mantecón, A.; de la Fuente, M.; Manso, T.; Guerra, C. Grape pomace in ewes diet: Effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 2018, 113, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Chikwanha, O.; Muchenje, V.; Nolte, J.E.; Dugan, M.E.; Mapiye, C. Grape pomace (Vitis vinifera L. cv. Pinotage) supplementation in lamb diets: Effects on growth performance, carcass and meat quality. Meat Sci. 2019, 147, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef]
- Luciano, G.; Vasta, V.; Monahan, F.; López-Andrés, P.; Biondi, L.; Lanza, M.; Priolo, A. Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem. 2011, 124, 1036–1042. [Google Scholar] [CrossRef]
- Banović, M.; Fontes, M.A.; Barreira, M.M.; Grunert, K.G. Impact of Product Familiarity on Beef Quality Perception. Agribusiness 2012, 28, 157–172. [Google Scholar] [CrossRef]
- Neethling, N.E.; Suman, S.P.; Sigge, G.O.; Hoffman, L.C.; Hunt, M.C. Exogenous and Endogenous Factors Influencing Color of Fresh Meat from Ungulates. Meat Muscle Biol. 2017, 1, 253–275. [Google Scholar] [CrossRef]
- Ianni, A.; Di Luca, A.; Martino, C.; Bennato, F.; Marone, E.; Grotta, L.; Cichelli, A.; Martino, G. Dietary Supplementation of Dried Grape Pomace Increases the Amount of Linoleic Acid in Beef, Reduces the Lipid Oxidation and Modifies the Volatile Profile. Animals 2019, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Hannula, T.; Puolanne, E. The effect of the cooling rate on beef tenderness: The significance of pH at 7 °C in relation to tenderness. Meat Sci. 2004, 67, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Jacques, J.; Chouinard, Y.; Gariépy, C.; Cinq-Mars, D. Meat quality, organoleptic characteristics and fatty acid composition of Dorset lambs fed different forage to concentrate ratio or fresh grass. Can. J. Anim. Sci. 2017, 97, 290–301. [Google Scholar] [CrossRef]
- Lopes, L.; Martins, S.; Chizzotti, M.; Busato, K.; Oliveira, I.; Neto, O.M.; Paulino, P.; Lanna, D.; Ladeira, M. Meat quality and fatty acid profile of Brazilian goats subjected to different nutritional treatments. Meat Sci. 2014, 97, 602–608. [Google Scholar] [CrossRef][Green Version]
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the contribution of tenderness, juiciness, and flavor to the overall consumer beef eating experience1. Transl. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef]
- Miller, M.F.; Hoover, L.C.; Cook, K.D.; Guerra, A.L.; Huffman, K.L.; Tinney, K.S.; Ramsey, C.B.; Brittin, H.C.; Huffman, L.M. Consumer Acceptability of Beef Steak Tenderness in the Home and Restaurant. J. Food Sci. 1995, 60, 963–965. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Tenderness Marketing Claims Associated with Meat Cuts Derived from Beef; ASTM Int.: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Martinez, H.A.; Arnold, A.N.; Brooks, J.C.; Carr, C.C.; Gehring, K.B.; Griffin, D.B.; Hale, D.S.; Mafi, G.G.; Johnson, D.D.; Lorenzen, C.L.; et al. National Beef Tenderness Survey—2015: Palatability and Shear Force Assessments of Retail and Foodservice Beef. Meat Muscle Biol. 2017, 1, 138–148. [Google Scholar] [CrossRef]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Nudda, A.; Battacone, G.; Boe, R.; Francesconi, A.; Pulina, G. Effects of grape seed supplementation, alone or associated with linseed, on ruminal metabolism in Sarda dairy sheep. Anim. Feed Sci. Technol. 2015, 199, 61–72. [Google Scholar] [CrossRef]
- Vasta, V.; Mele, M.; Serra, A.; Scerra, M.; Luciano, G.; Lanza, M.; Priolo, A. Metabolic fate of fatty acids involved in ruminal biohydrogenation in sheep fed concentrate or herbage with or without tannins1. J. Anim. Sci. 2009, 87, 2674–2684. [Google Scholar] [CrossRef]
- Wang, Y.; Jacome-Sosa, M.M.; Proctor, S.D. The role of ruminant trans fat as a potential nutraceutical in the prevention of cardiovascular disease. Food Res. Int. 2012, 46, 460–468. [Google Scholar] [CrossRef]
- Scollan, N.D.; Dannenberger, D.; Nuernberg, K.; Richardson, I.; MacKintosh, S.; Hocquette, J.-F.; Moloney, A.P. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans Fatty Acids and Bioactive Lipids in Ruminant Milk. Adv. Exp. Med. Biol. 2008, 606, 3–65. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A.; Scerra, M.; Hallett, K.G.; Wood, J.D.; Doran, O. Δ9 desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins. Meat Sci. 2009, 82, 357–364. [Google Scholar] [CrossRef]
- Dewanckele, L.; Toral, P.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression: An update. J. Dairy Sci. 2020, 103, 7655–7681. [Google Scholar] [CrossRef]


| Diet | ||
|---|---|---|
| Item | Control (CON) | Grape Pomace (HGP) |
| Ingredient, % of dry matter | ||
| Straw | 1.80 | 4.61 |
| Cattle finisher supplement 1 | 1.71 | 2.88 |
| Wheat mill run | 9.02 | 34.7 |
| Grape pomace | 7.54 | 57.8 |
| Apple pomace | 7.58 | _ |
| High moisture corn | 22.5 | _ |
| Wet corn gluten | 21.0 | _ |
| Barley grain, dry rolled | 11.2 | _ |
| Bakery waste | 9.02 | _ |
| Whey | 5.69 | _ |
| Optaflexx | 2.93 | _ |
| Chemical analysis | ||
| Dry matter (DM), % | 50.6 ± 1.79 | 54.7 ± 3.44 |
| Organic matter (OM), % of DM | 93.2 ± 1.30 | 87.0 ± 0.80 |
| Neutral detergent fiber (NDF), % of DM | 20.7 ± 2.52 | 47.9 ± 3.62 |
| Acid detergent fiber (ADF), % of DM | 14.1 ± 3.11 | 35.8 ± 3.21 |
| Crude fat (CF), % of DM | 7.85 ± 0.779 | 7.12 ± 0.600 |
| Crude protein (CP), % of DM | 15.5 ± 1.85 | 16.0 ± 1.15 |
| Net energy of gain, Mcal/kg | 1.34 ± 0.07 | 0.49 ± 0.13 |
| Phenolic compounds, % of DM | ||
| Total phenols | 1.60 ± 0.180 | 3.19 ± 0.404 |
| Free phenols | 0.96 ± 0.067 | 0.43 ± 0.082 |
| Tannins | 0.94 ± 0.271 | 2.77 ± 0.379 |
| Nontannins | 0.66 ± 0.091 | 0.42 ± 0.057 |
| Diet | ||
|---|---|---|
| Fatty Acid | Control (CON) | Grape Pomace (HGP) |
| Myristic (14:0) | 0.962 ± 0.6022 | 0.273 ± 0.0869 |
| Pentadecanoic (15:0) | 0.174 ± 0.0977 | 0.096 ± 0.0308 |
| Palmitic (16:0) | 15.7 ± 4.25 | 12.8 ± 1.03 |
| Palmitoleic (16:1) | 0.862 ± 0.5526 | 0.291 ± 0.0201 |
| Margaric (17:0) | 0.368 ± 0.2366 | 0.150 ± 0.0365 |
| Stearic (18:0) | 6.53 ± 3.671 | 3.22 ± 0.567 |
| Oleic (18:1) | 26.5 ± 5.91 | 17.6 ± 1.14 |
| Linoleic (18:2n-6) | 30.2 ± 6.99 | 54.6 ± 2.62 |
| α-Linolenic (18:3n-3) | 3.06 ± 1.400 | 3.32 ± 1.282 |
| Diet | ||||
|---|---|---|---|---|
| Variable | CON | HGP | SEM | p-Value |
| HCW, kg | 343 | 301 | 5.55 | <0.01 |
| Dressing % | 55.6 | 54.2 | 0.93 | 0.30 |
| Backfat thickness, cm | 0.836 | 0.323 | 0.0401 | <0.01 |
| Preliminary yield grade | 2.85 | 2.26 | 0.044 | <0.01 |
| Ribeye area, cm2 | 69.0 | 65.8 | 1.66 | 0.14 |
| Kidney, Pelvic, and Heart, % | 4.63 | 4.25 | 0.177 | 0.15 |
| Finally, yield grade | 3.32 | 2.94 | 0.094 | 0.04 |
| Marbling | 529 | 500 | 18.1 | 0.27 |
| Diet | ||||
|---|---|---|---|---|
| Variable | CON | HGP | SEM | p-Value |
| Striploin (longissimus lumborum) | ||||
| Fluid loss, % | 1.829 | 1.327 | 0.1767 | 0.057 |
| Cook loss, % | 25.3 | 25.3 | 1.32 | 0.98 |
| Warner-Bratzler shear force, kg | 3.31 | 3.62 | 0.226 | 0.35 |
| Top round (semimembranosus) | ||||
| Fluid loss, % | 1.235 | 1.738 | 0.1033 | <0.01 |
| Cook loss, % | 26.8 | 35.5 | 1.82 | <0.01 |
| Warner-Bratzler shear force, kg/cm3 | 4.39 | 4.74 | 0.239 | 0.30 |
| Diet | ||||
|---|---|---|---|---|
| Variable | CON | HGP | SEM | p-Value |
| 10:0 | 0.041 | 0.039 | 0.0045 | 0.77 |
| 12:0 | 0.063 | 0.059 | 0.0063 | 0.59 |
| 13:0 | 0.029 | 0.029 | 0.0078 | 0.99 |
| 14:0 | 2.79 | 2.23 | 0.239 | 0.11 |
| 14:1 c9 | 0.726 | 0.546 | 0.0643 | 0.06 |
| 15:0 | 0.397 | 0.283 | 0.0361 | 0.04 |
| 15:1 | 0.0570 | 0.0463 | 0.0110 | 0.27 |
| 16:0 | 22.0 | 20.1 | 1.66 | 0.44 |
| 16:1 c9 | 3.14 | 2.36 | 0.238 | 0.03 |
| 17:0 | 1.04 | 0.68 | 0.072 | <0.01 |
| 17:1 | 0.661 | 0.396 | 0.0719 | 0.02 |
| 18:0 | 12.2 | 15.6 | 1.17 | 0.052 |
| 18:1 t | 4.35 | 1.36 | 0.437 | <0.01 |
| 18:1 c9 | 33.4 | 32.5 | 2.35 | 0.80 |
| 18:1 t12 | 1.82 | 1.21 | 0.117 | <0.01 |
| 18:2 n-6 | 3.86 | 6.10 | 0.461 | <0.01 |
| 18:2 n-4 | 0.116 | 0.115 | 0.0111 | 0.95 |
| 18:3n-3 | 0.366 | 0.365 | 0.0262 | 0.96 |
| 18:2 c9t11 | 0.213 | 0.419 | 0.0312 | <0.01 |
| 18:2 t9t12 | 0.273 | 0.340 | 0.0249 | 0.07 |
| 18:2 t10c12 | 0.0531 | 0.0144 | 0.00577 | 0.04 |
| 20:2 c11c14 | 0.0542 | 0.0747 | 0.00699 | 0.049 |
| 20:0 | 0.0060 | 0.0197 | 0.00610 | 0.93 |
| 20:1 | 0.0540 | 0.0056 | 0.00534 | 0.03 |
| 21:0 | 0.041 | 0.088 | 0.00820 | 0.03 |
| 23:0 | 0.0040 | 0.0147 | 0.00566 | 0.11 |
| 24:1 | 0.0240 | 0.0865 | 0.01230 | 0.06 |
| 20:3 n-6 | 0.160 | 0.227 | 0.0215 | 0.04 |
| 20:4 n-6 | 0.540 | 0.742 | 0.0986 | 0.16 |
| 22:5 n-3 | 0.0479 | 0.0669 | 0.01114 | 0.24 |
| SFA 1 | 38.6 | 39.2 | 3.06 | 0.89 |
| MUFA 2 | 44.2 | 38.5 | 2.94 | 0.19 |
| PUFA 3 | 5.68 | 8.46 | 0.623 | <0.01 |
| UFA 4 | 49.9 | 47.0 | 3.37 | 0.55 |
| SFA:UFA | 0.77 | 0.83 | 0.029 | 0.17 |
| Total CLA 5 | 0.539 | 0.772 | 0.0581 | <0.01 |
| Δ9—desaturase 16 6 | 12.5 | 10.5 | 0.48 | 0.01 |
| Δ9—desaturase 18 7 | 73.2 | 67.6 | 1.23 | 0.03 |
| Elongase 8 | 64.6 | 68.4 | 0.54 | <0.01 |
| Diet | ||||
|---|---|---|---|---|
| Variable | CON | HGP | SEM | p-Value |
| 10:0 | 0.124 | 0.064 | 0.0141 | 0.01 |
| 12:0 | 0.0334 | 0.0319 | 0.00478 | 0.82 |
| 13:0 | _ | _ | _ | _ |
| 14:0 | 2.07 | 1.82 | 0.213 | 0.47 |
| 14:1 c9 | 0.517 | 0.479 | 0.0621 | 0.67 |
| 15:0 | 0.297 | 0.218 | 0.0284 | 0.06 |
| 15:1 | 0.062 | 0.139 | 0.0390 | 0.27 |
| 16:0 | 20.3 | 19.2 | 1.86 | 0.68 |
| 16:1 c9 | 2.69 | 2.30 | 0.263 | 0.35 |
| 17:0 | 0.976 | 0.627 | 0.0793 | <0.01 |
| 17:1 | 0.677 | 0.379 | 0.0531 | <0.01 |
| 18:0 | 11.9 | 12.8 | 1.39 | 0.66 |
| 18:1 t | 3.99 | 1.78 | 0.354 | <0.01 |
| 18:1 c9 | 32.9 | 30.9 | 2.73 | 0.62 |
| 18:1 t12 | 1.99 | 1.45 | 0.161 | 0.03 |
| 18:2 n-6 | 4.29 | 7.74 | 0.737 | <0.01 |
| 18:2 n-4 | 0.083 | 0.106 | 0.0097 | 0.11 |
| 18:3 n-3 | 0.332 | 0.288 | 0.0307 | 0.32 |
| 18:2 c9t11 | 0.178 | 0.350 | 0.0294 | <0.01 |
| 18:2 t9t12 | 0.268 | 0.317 | 0.0282 | 0.23 |
| 18:2 t10c12 | 0.0227 | 0.0000 | 0.00515 | <0.01 |
| 20:2 c11c14 | 0.0208 | 0.0633 | 0.00767 | 0.28 |
| 20:0 | 0.0000 | 0.0118 | 0.00446 | 0.07 |
| 20:1 | 0.0121 | 0.000 | 0.00469 | 0.08 |
| 21:0 | 0.0211 | 0.0900 | 0.01065 | 0.67 |
| 23:0 | 0.0054 | 0.0277 | 0.00994 | 0.76 |
| 24:1 | 0.0079 | 0.0645 | 0.01400 | 0.86 |
| 20:3 n-6 | 0.141 | 0.279 | 0.0343 | <0.01 |
| 20:4 n-6 | 0.52 | 1.12 | 0.139 | <0.01 |
| 22:5 n-3 | 0.0110 | 0.0655 | 0.01086 | <0.01 |
| SFA1 | 35.8 | 35.0 | 3.17 | 0.85 |
| MUFA2 | 42.8 | 37.5 | 3.47 | 0.29 |
| PUFA3 | 5.87 | 10.3 | 0.980 | <0.01 |
| UFA4 | 48.7 | 47.9 | 4.25 | 0.89 |
| SFA:UFA | 0.735 | 0.735 | 0.0243 | 0.99 |
| Total CLA5 | 0.468 | 0.666 | 0.0576 | 0.02 |
| Δ9—desaturase 16 6 | 11.8 | 10.5 | 0.48 | 0.07 |
| Δ9—desaturase 18 7 | 73.6 | 70.7 | 1.87 | 0.29 |
| Elongase 8 | 66.0 | 76.7 | 0.92 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arend, F.A.; Murdoch, G.K.; Doumit, M.E.; Chibisa, G.E. Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition. Animals 2022, 12, 2597. https://doi.org/10.3390/ani12192597
Arend FA, Murdoch GK, Doumit ME, Chibisa GE. Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition. Animals. 2022; 12(19):2597. https://doi.org/10.3390/ani12192597
Chicago/Turabian StyleArend, Frances A., Gordon K. Murdoch, Matt E. Doumit, and Gwinyai E. Chibisa. 2022. "Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition" Animals 12, no. 19: 2597. https://doi.org/10.3390/ani12192597
APA StyleArend, F. A., Murdoch, G. K., Doumit, M. E., & Chibisa, G. E. (2022). Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition. Animals, 12(19), 2597. https://doi.org/10.3390/ani12192597





