Distribution of Breeding Population and Predicting Future Habitat under Climate Change of Black-Necked Crane (Grus nigricollis Przevalski, 1876) in Shaluli Mountains
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Survey
2.2. Habitat Predicting
2.2.1. Species Occurrence Records
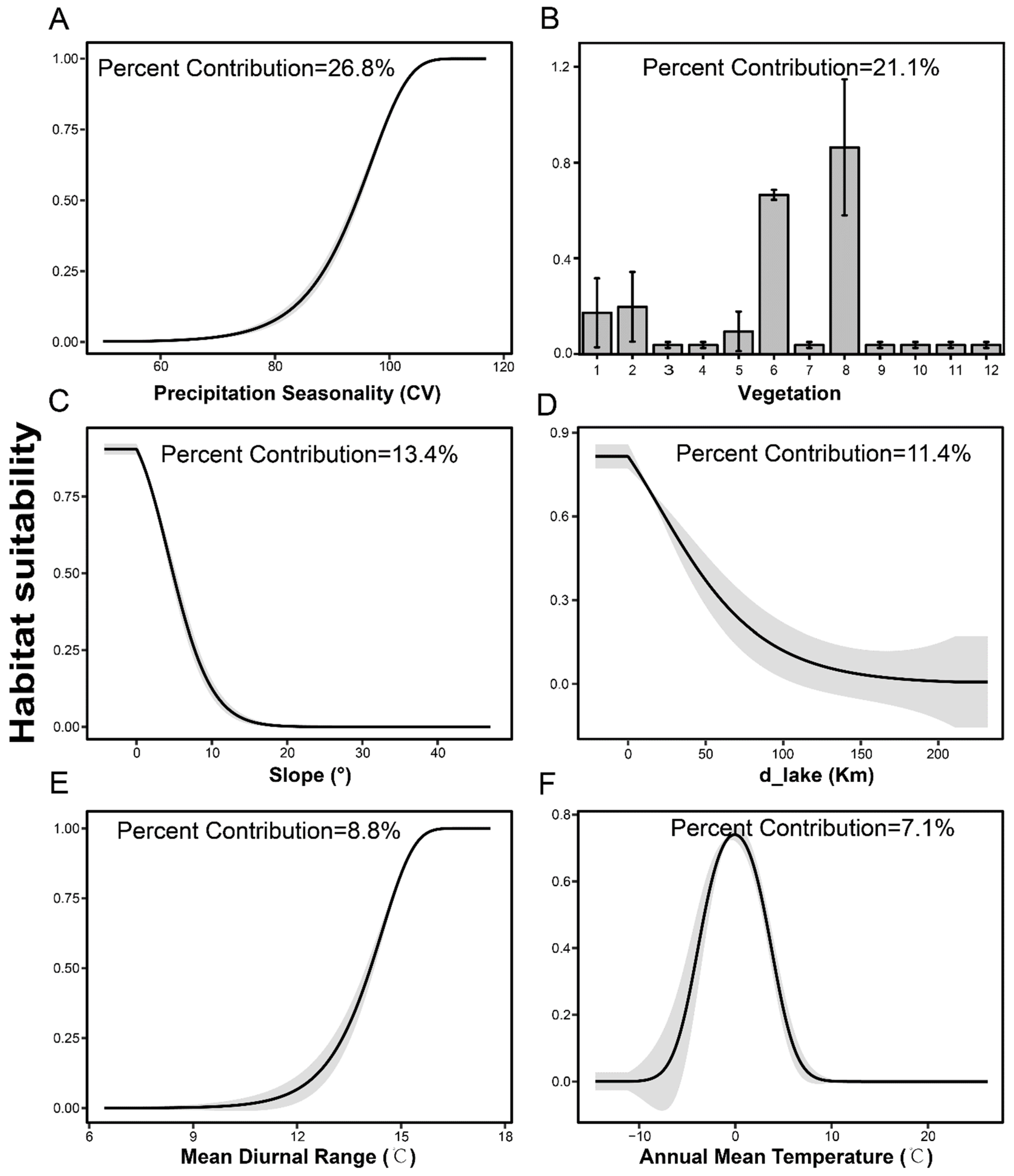
2.2.2. Environmental Variables
2.2.3. Model Procedure
2.3. Spatial Analysis
3. Results
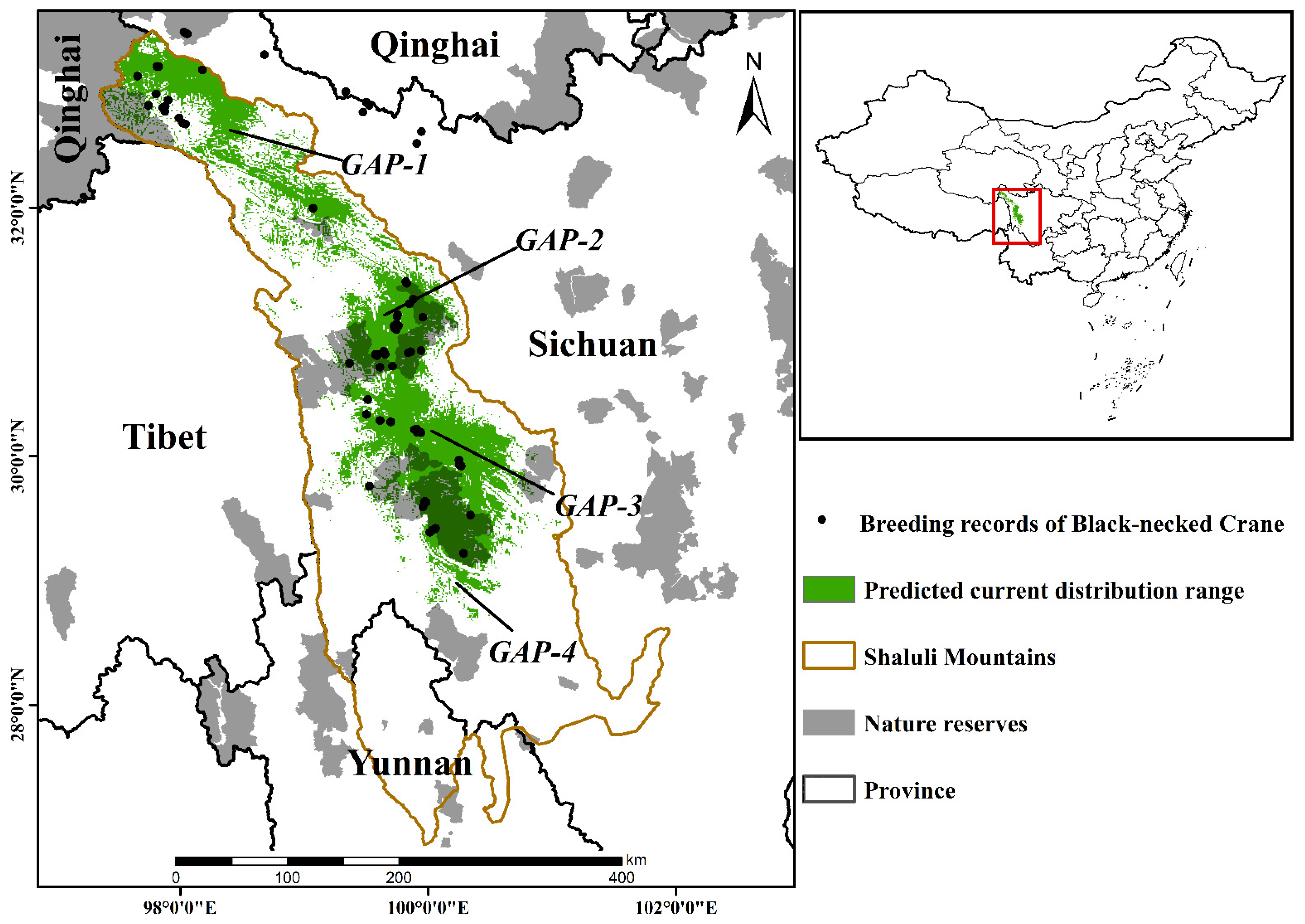
3.1. Current Breeding Numbers and Habitat Ranges
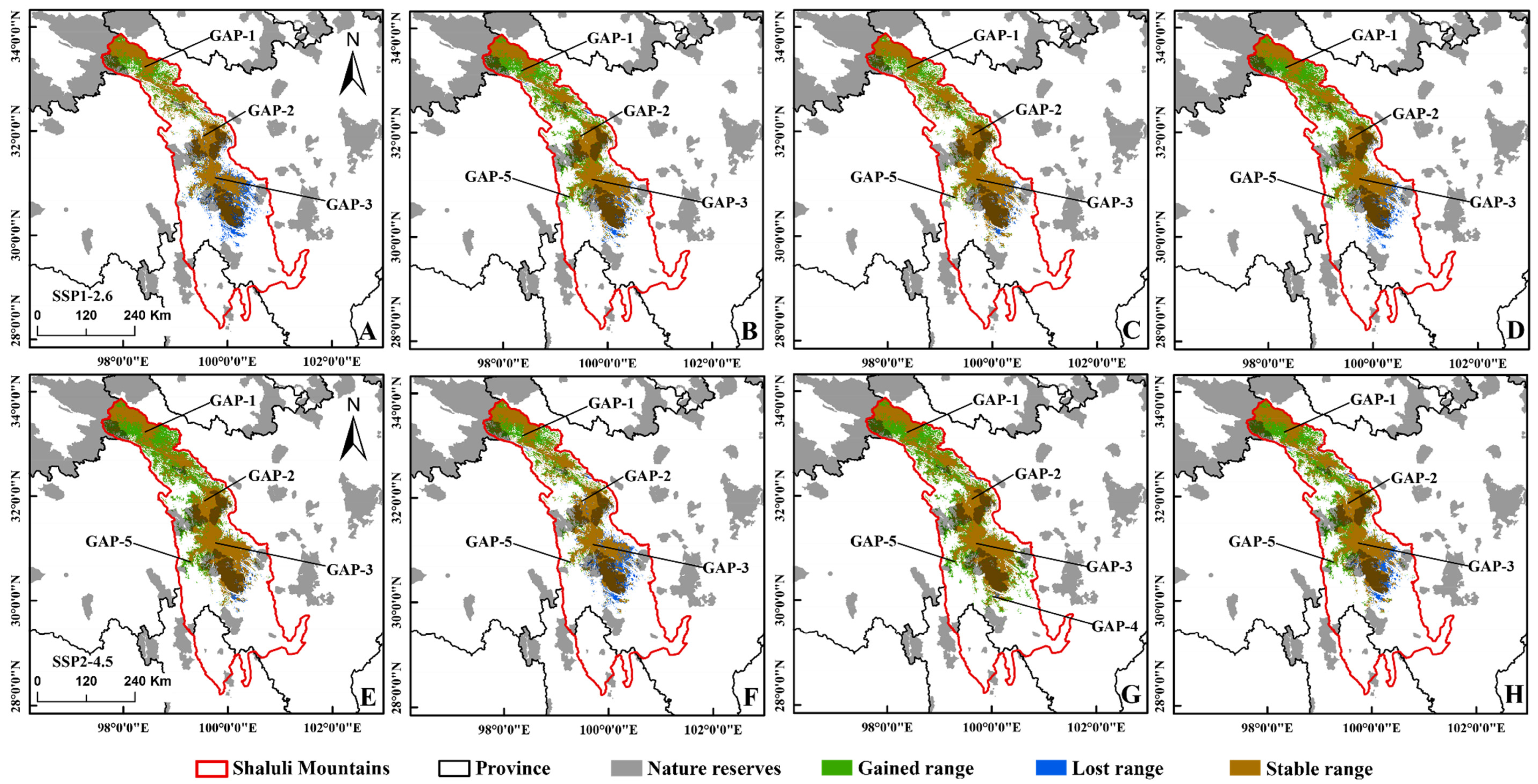
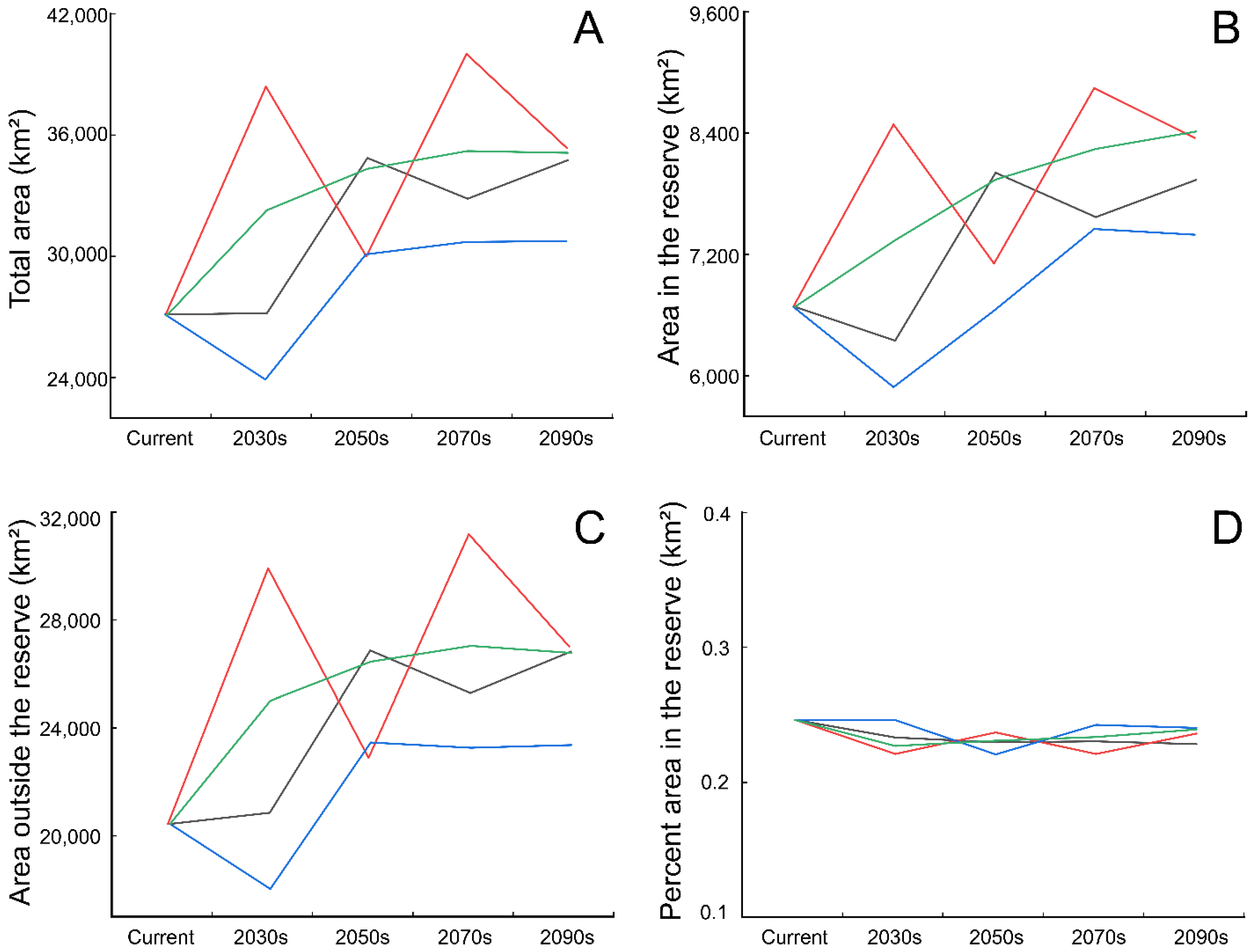
3.2. Predicting Suitable Habitats in Future
3.3. Conservation Gaps
4. Discussion
4.1. Current Breeding Numbers and Habitat Ranges
4.2. Projected Breeding Ranges under Climate Change Scenarios
4.3. Conservation Implications and Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araujo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.E.A.; Grooten, M.; Petersen, T. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; WWF: Gland, Switzerland, 2020; p. 13. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Urban, M.C. Climate change. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Zhong, X.; Yang, B.; Zhang, J.D.; Zhao, C.; He, X.C.; Li, J.G.; Ran, J.H.; Zhou, C.Q. Predicting range shifts of the Chinese monal (Lophophorus lhuysii) under climate change: Implications for long-term conservation. Glob. Ecol. Conserv. 2020, 22, e01018. [Google Scholar] [CrossRef]
- Gill, D.A.; Mascia, M.B.; Ahmadia, G.N.; Glew, L.; Lester, S.E.; Barnes, M.; Craigie, I.; Darling, E.S.; Free, C.M.; Geldmann, J.; et al. Capacity shortfalls hinder the performance of marine protected areas globally. Nature 2017, 543, 665–669. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single-species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Caro, T.M.; O’Doherty, G. On the Use of Surrogate Species in Conservation Biology. Conserv. Biol. 2010, 13, 805–814. [Google Scholar] [CrossRef]
- McGowan, J.; Beaumont, L.J.; Smith, R.J.; Chauvenet, A.L.M.; Harcourt, R.; Atkinson, S.C.; Mittermeier, J.C.; Esperon-Rodriguez, M.; Baumgartner, J.B.; Beattie, A.; et al. Conservation prioritization can resolve the flagship species conundrum. Nat. Commun. 2020, 11, 994. [Google Scholar] [CrossRef]
- Dietz, J.M.; Dietz, L.A.; Nagagata, E.Y. The effective use of flagship species for conservation of biodiversity: The example of lion tamarins in Brazil. In Creative Conservation; Springer: Dordrecht, The Netherlands, 1994; pp. 32–49. [Google Scholar]
- Qian, J.; Zhuang, H.; Yang, W.; Chen, Y.; Chen, S.; Qu, Y.; Zhang, Y.; Yang, Y.; Wang, Y. Selecting flagship species to solve a biodiversity conservation conundrum. Plant Divers. 2020, 42, 488–491. [Google Scholar] [CrossRef]
- Verissimo, D.; Pongiluppi, T.; Santos, M.C.; Develey, P.F.; Fraser, I.; Smith, R.J.; MacMilan, D.C. Using a systematic approach to select flagship species for bird conservation. Conserv. Biol. 2014, 28, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, J. Species richness and adaptive capacity in animal communities: Lessons from China. Integr. Zool. 2008, 3, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Li, B.V.; Pimm, S.L. China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv. Biol. 2016, 30, 329–339. [Google Scholar] [CrossRef]
- Wei, F.; Costanza, R.; Dai, Q.; Stoeckl, N.; Gu, X.; Farber, S.; Nie, Y.; Kubiszewski, I.; Hu, Y.; Swaisgood, R.; et al. The Value of Ecosystem Services from Giant Panda Reserves. Curr. Biol. 2018, 28, 2174–2180. [Google Scholar] [CrossRef]
- Verissimo, D.; MacMillan, D.C.; Smith, R.J. Toward a systematic approach for identifying conservation flagships. Conserv. Lett. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Mallinson, J. Flagship species aiding the conservation of animals and associated habitat. In Proceedings of the 46th annual conference of International Union of Directors of Zoologocal Gardens, Singapore, 3 October 1991. [Google Scholar]
- Smith, A.M.; Sutton, S.G. The role of a flagship species in the formation of conservation intentions. Hum. Dimens. Wildl. 2008, 13, 127–140. [Google Scholar] [CrossRef]
- Senzaki, M.; Yamaura, Y.; Shoji, Y.; Kubo, T.; Nakamura, F. Citizens promote the conservation of flagship species more than ecosystem services in wetland restoration. Biol. Conserv. 2017, 214, 1–5. [Google Scholar] [CrossRef]
- Cianfrani, C.; Le Lay, G.; Maiorano, L.; Satizabal, H.F.; Loy, A.; Guisan, A. Adapting global conservation strategies to climate change at the European scale: The otter as a flagship species. Biol. Conserv. 2011, 144, 2068–2080. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.S.; Buzzard, P.; Qian, F.W.; Zhang, F.; Zhao, J.L.; Yang, J.X.; Yang, X.J. Migration Routes and New Breeding Areas of Black-Necked Cranes. Wilson J. Ornithol. 2012, 124, 704–712. [Google Scholar] [CrossRef]
- Jia, R.; Ma, T.; Zhang, F.J.; Zhang, G.G.; Liu, D.P.; Lu, J. Population dynamics and habitat use of the Black-necked Crane (Grus nigricollis) in the Yarlung Tsangpo River basin, Tibet, China. Avian Res. 2019, 10, 366–373. [Google Scholar] [CrossRef]
- BirdLife International. Grus nigricollis. The IUCN Red List of Threatened Species 2020: e.T22692162A180030167. Available online: https://www.iucnredlist.org/species/22692162/180030167 (accessed on 2 September 2020).
- LI, F. IUCN Black-necked Crane (Grus nigricollis) conservation plan. Zool. Res. 2014, 35, 3–9. [Google Scholar] [CrossRef]
- Niemi, G.J.; Hanowski, J.M.; Lima, A.R.; Nicholls, T.; Weiland, N. A critical analysis on the use of indicator species in management. J. Wildl. Manage 1997, 61, 1240–1252. [Google Scholar] [CrossRef]
- Hou, M.J.; Bao, X.K.; Ge, J.; Liang, T.G. Land cover pattern and habitat suitability on the global largest breeding sites for Black-necked Cranes. J. Clean. Prod. 2021, 322, 128968. [Google Scholar] [CrossRef]
- Scott, D.A. The Black-Necked Cranes Grus nigricollis of Ruoergai Marshes, Sichuan, China. Bird Conserv. Int. 1993, 3, 245–259. [Google Scholar]
- Li, Z.M.; Li, F. Research on the Black-Necked Crane; Shanghai Scientific and Technological Education Publishing House: Shanghai, China, 2005. [Google Scholar]
- Wang, N.; Zhu, P.; Wan, M.; Ye, Y.; Qu, S. Size and Distribution of the Breeding Population of Black-necked Crane in Haizhishan, Sichuan Province. J. Ecol. Rural Environ. 2013, 29, 265–268. [Google Scholar]
- Lei, G.L.; Sang, J.; Zheng, Z. Site and behavior of sleep of Grus nigricollis in Kazi water reserve of Linzhou, Tibet. J. West China For. Sci. 2012, 4, 93–97. [Google Scholar] [CrossRef]
- Zhao, J.L.; Han, L.X.; Feng, L.; Wu, Z.R. Wintering behaviors and habitat using of black-necked crane in Napahai nature reserve, Yunnan province. Sichuan J. Zool. 2008, 27, 78–91. [Google Scholar]
- Yu, Y.Q.; Liu, W.L.; Sang, J. A preminary study on the overwintering ecology of black-necked crane (Grus nigricollis) in The Upper Lhasa River. Zool. Res. 1993, 14, 250–251. [Google Scholar]
- Liu, Q.; Yang, X.J.; Zhu, J.G.; Zhao, J.L. Flock of black-necked crane wintering at Napahai nature reserve, China. Zool. Res. 2008, 29, 553–560. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, W.; Zhong, L.; Hu, Z.; Li, M.; Zhu, Z.; Han, C.; Wang, B.; Liu, X. Monitoring and Modeling the Tibetan Plateau’s climate system and its impact on East Asia. Sci. Rep. 2017, 7, 44574. [Google Scholar] [CrossRef]
- Tang, Q.H.; Lan, C.; Su, F.G.; Liu, X.C.; Sun, H.; Ding, J.; Wang, L.; Leng, G.Y.; Zhang, Y.Q.; Sang, Y.F.; et al. Streamflow change on the Qinghai-Tibet Plateau and its impacts. Chin. Sci. Bull. 2019, 64, 2807–2821. [Google Scholar] [CrossRef]
- Gong, C.Q.; Dong, X.H.; Wei, C.; Ouyang, X.J. Precipitation division of the Qinghai-Tibet Plateau from 1978 to 2018 and spatiotemporal evolution characteristics of each zone. J. Water Resour. Water Eng. 2022, 33, 1–13. [Google Scholar]
- Song, H.T.; Zhang, Y.S.; Gao, H.F.; Guo, Y.H.; Li, S.N. Plateau Wetlands, an Indispensible Habitat for the Black-Necked Crane (Grus nigricollis)—A Review. Wetlands 2014, 34, 629–639. [Google Scholar] [CrossRef]
- Wu, Z.K.; Li, Z.M.; Wang, Y.H.; Jiang, Y.M.; Li, R.X.; Li, D.H.; Zhou, Z.J.; Li, L.X. Migration of black-necked crane in China. Curr. Zool. 1993, 1, 105–106. [Google Scholar]
- Qian, F.W.; Wu, H.Q.; Gao, L.B.; Zhang, H.G.; Li, F.S.; Zhong, X.Y.; Yang, X.J.; Zheng, G.M. Migration routes and stopover sites of Black-necked Cranes determined by satellite tracking. J. Field Ornithol. 2009, 80, 19–26. [Google Scholar] [CrossRef]
- Kong, D.J.; Li, F.S.; Yang, X.J. Using bird banding and recovery to study the migration of Blacknecked Cranes (Grus nigricollis) in China. Zool. Res. 2014, 35, 20–38. [Google Scholar] [CrossRef]
- Dong, H.Y.; Lu, G.Y.; Zhong, X.Y.; Yang, X.J. Winter diet and food selection of the Black-necked Crane Grus nigricollis in Dashanbao, Yunnan, China. PeerJ 2016, 4, e1968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.; Yang, X.; Zhao, J.; Yu, H. Foraging habitats and utilization distributions of Black-necked Cranes wintering at the Napahai Wetland, China. J. Field Ornithol. 2010, 81, 21–30. [Google Scholar] [CrossRef]
- Dou, L.; Li, H.; Li, F.; Zhang, M.; Zheng, Z.; Ran, J. Survey on the Black-necked Crane during the Breeding Period at Sichuan Ruoergai Wetland National Nature Reserve. Sichuan J. Zool. 2013, 32, 770–773. [Google Scholar]
- Farrington, J.D.; Xiulei, Z. The Black-necked Cranes of the Longbao National Nature Reserve, Qinghai, China. Mt. Res. Dev. 2013, 33, 305–313. [Google Scholar] [CrossRef]
- Bishop, M.A.; Tsamchu, D.; Li, F. Number and distribution of Black-necked Cranes wintering in Zhigatse Prefecture, Tibet. Chin. Birds 2012, 3, 191–198. [Google Scholar] [CrossRef]
- Wong, H. Rendezvous with the Black-Necked Crane: Over a Decade of Exploration and Conservation of an Auspicious Bird; Tianxia Yuanjian Publishing House: Taipei, Taiwan, 2002. [Google Scholar]
- Wei, L.; Ran, J.H.; Zhang, B.; Zhao, C.H.; Zhang, M. Distribution and protection status of Black-necked Crane (Grus nigricollis) in Sichuan Province. Zool. Res. 2014, 35, 72–75. [Google Scholar]
- Lai, Y.; Liu, Y.; Liu, X. Elevational Diversity Patterns of Green Lacewings (Neuroptera: Chrysopidae) Uncovered with DNA Barcoding in a Biodiversity Hotspot of Southwest China. Front. Ecol. Evol. 2021. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Gu, F.; Li, Y.; Shao, H.; Liu, S. Local residents’ perceptions of climate and ecological changes in the eastern Tibetan Plateau. Reg. Environ. Chang. 2020, 20, 56. [Google Scholar] [CrossRef]
- Gu, H.J.; Guo, P.; He, X.J.; Hu, J.; Jiang, C. China Wetlands Resources: Sichuan Volume; China Forestry Publishing House: Beijing, China, 2015; pp. 23–43. [Google Scholar]
- Cui, X.F.; Graf, H.F. Recent land cover changes on the Tibetan Plateau: A review. Clim. Chang. 2009, 94, 47–61. [Google Scholar] [CrossRef]
- Noss, R.F.; Platt, W.J.; Sorrie, B.A.; Weakley, A.S.; Means, D.B.; Costanza, J.; Peet, R.K. How global biodiversity hotspots may go unrecognized: Lessons from the North American Coastal Plain. Divers. Distrib. 2015, 21, 236–244. [Google Scholar] [CrossRef]
- He, X.C.; Wang, X.Y.; DuBay, S.; Reeve, A.H.; Alstrom, P.; Ran, J.H.; Liu, Q.; Wu, Y.J. Elevational patterns of bird species richness on the eastern slope of Mt. Gongga, Sichuan Province, China. Avian Res. 2019. [Google Scholar] [CrossRef]
- Zhang, T.X.; Chen, X.; Wu, Y.J.; Ran, J.H. Diversity and structure of bird communities in contrasting forests of the Hengduan Mountains, China. Biodivers. Conserv. 2020, 29, 3739–3755. [Google Scholar] [CrossRef]
- He, X.C.; DuBay, S.; Zhangshang, M.; Cheng, Y.W.; Liu, Z.W.; Li, D.R.; Ran, J.H.; Wu, Y.J. Seasonal elevational patterns and the underlying mechanisms of avian diversity and community structure on the eastern slope of Mt. Gongga. Divers. Distrib. 2022. [Google Scholar] [CrossRef]
- Yao, Y.F.; Song, X.Y.; Wortley, A.H.; Blackmore, S.; Li, C.S. A 22,570-year record of vegetational and climatic change from Wenhai Lake in the Hengduan Mountains biodiversity hotspot, Yunnan, Southwest China. Biogeosciences 2015, 12, 1525–1535. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, J. Number and distribution of black-necked crane on Ruoergai Plateau Marsh. Sichuan J. Zool. 1991, 10, 37–38. [Google Scholar]
- Liu, W.; Jin, Y.Y.; Wu, Y.J.; Zhao, C.H.; He, X.C.; Wang, B.; Ran, J.H. Home Range and Habitat Use of Breeding Black-Necked Cranes. Animals 2020, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Chang. 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Zhu, B.W.; Wang, B.; Zou, B.Y.; Xu, Y.; Yang, B.; Yang, N.; Ran, J.H. Assessment of habitat suitability of a high-mountain Galliform species, buff-throated partridge (Tetraophasis szechenyii). Glob. Ecol. Conserv. 2020, 24, e01230. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). 2019. Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 23 April 2022).
- Wang, B.; Xu, Y.; Ran, J.H. Predicting suitable habitat of the Chinese monal (Lophophorus lhuysii) using ecological niche modeling in the Qionglai Mountains, China. PeerJ 2017, 5, e3477. [Google Scholar] [CrossRef]
- Ma, D.; Lun, X.; Li, C.; Zhou, R.; Zhao, Z.; Wang, J.; Zhang, Q.; Liu, Q. Predicting the Potential Global Distribution of Amblyomma americanum (Acari: Ixodidae) under Near Current and Future Climatic Conditions, Using the Maximum Entropy Model. Biology 2021, 10, 1057. [Google Scholar] [CrossRef]
- Ji, W.; Gao, G.; Wei, J. Potential Global Distribution of Daktulosphaira vitifoliae under Climate Change Based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. WALLACE: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Dan, L.W.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar]
- Ceylan, Y.; Gul, S. Potential habitats of an alien species (Asterias rubens Linnaeus, 1758) in the Black Sea: Its current and future distribution patterns. Environ. Sci. Pollut. Res. Int. 2022, 29, 19563–19571. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Freer, J.J.; Daase, M.; Tarling, G.A. Modelling the biogeographic boundary shift of Calanus finmarchicus reveals drivers of Arctic Atlantification by subarctic zooplankton. Glob. Chang. Biol. 2022, 28, 429–440. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, H.; Peng, S. The development evaluation of species distribution models. Acta Ecol. Sin. 2015, 35, 557–567. [Google Scholar]
- Freeman, E.A.; Moisen, G.G. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Stanton, J.C.; Pearson, R.G.; Horning, N.; Ersts, P.; Akcakaya, H.R. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evol. 2012, 3, 349–357. [Google Scholar] [CrossRef]
- Liu, C.R.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Han, X.; Huettmann, F.; Guo, Y.; Mi, C.; Wen, L. Conservation prioritization with machine learning predictions for the black-necked crane Grus nigricollis, a flagship species on the Tibetan Plateau for 2070. Reg. Environ. Chang. 2018, 18, 2173–2182. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Ji, S.Y.; Jeong, J.C.; Lee, P.S.H. Landscape Analysis to Assess the Impact of Development Projects on Forests. Sustainability 2016, 8, 1012. [Google Scholar] [CrossRef]
- Rosa, I.M.D.; Gabriel, C.; Carreiras, J.M.B. Spatial and temporal dimensions of landscape fragmentation across the Brazilian Amazon. Reg. Environ. Chang. 2017, 17, 1687–1699. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. Fragstats V4.2: Spatial Pattern Analysis Program for Categorical and Continuous Maps. 2012. Available online: https://www.umass.edu/landeco/research/fragstats/fragstats (accessed on 25 April 2022).
- Jones, A.G.; Small, C.M.; Paczolt, K.A.; Ratterman, N.L. A practical guide to methods of parentage analysis. Mol. Ecol. Resour. 2010, 10, 6–30. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Coetzee, B.W.T.; Robertson, M.P.; Erasmus, B.F.N.; van Rensburg, B.J.; Thuiller, W. Ensemble models predict Important Bird Areas in southern Africa will become less effective for conserving endemic birds under climate change. Glob. Ecol. Biogeogr. 2009, 18, 701–710. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, K.; Li, F.; Kong, D.; Yang, X. Numbers and distribution of Black-necked Cranes (Grus nigricollis) at Ruoergai Wetland on the Eastern Qinghai-Tibet Plateau. Zool. Res. 2014, 35, 134–138. [Google Scholar]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1; IUCN Species Survival Commission: Gland, Switzerland, 2001. [Google Scholar]
- Rumpf, S.B.; Gravey, M.; Bronnimann, O.; Luoto, M.; Cianfrani, C.; Mariethoz, G.; Guisan, A. From white to green: Snow cover loss and increased vegetation productivity in the European Alps. Science 2022, 376, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Chen, Y.; Felton, A.J.; Hopping, K.A.; Wang, R.W.; Niu, S.; Cheng, X.; Zhang, Y.; Cao, J.; et al. Plants with lengthened phenophases increase their dominance under warming in an alpine plant community. Sci. Total Environ 2020, 728, 138891. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Cao, G.; Ma, Z.; Li, Y.; Zhang, F.; Zhao, X.; Zhao, X.; Jiang, L.; Sanders, N.J.; et al. Alpine grassland plants grow earlier and faster but biomass remains unchanged over 35 years of climate change. Ecol. Lett. 2020, 23, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Zhang, Y.; Mathewson, P.D.; Zhang, Q.; Porter, W.P.; Ran, J. An ecophysiological perspective on likely giant panda habitat responses to climate change. Glob. Chang. Biol. 2018, 24, 1804–1816. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Bai, X.; Ma, J. Speciation of Black-necked Crane. J. Northeast Agric. Univ. 2002, 9, 32–37. [Google Scholar]
- Johnsgard, P. Cranes of the World; Indiana University Press: Bloomington, IN, USA, 1983; pp. 35–43. [Google Scholar]
- Ye, T.; Liu, W.; Chen, S.; Chen, D.; Shi, P.; Wang, A.; Li, Y. Reducing livestock snow disaster risk in the Qinghai-Tibetan Plateau due to warming and socioeconomic development. Sci. Total Environ. 2022, 813, 151869. [Google Scholar] [CrossRef]
- Tang, L. Geographic information system and climate big data applied in the research of bird migration. In Proceedings of the 2022 IEEE International Conference on Electrical Engineering, Big Data and Algorithms (EEBDA), Changchun, China, 25–27 February 2022; pp. 338–341. [Google Scholar]
- Volkov, S.V.; Grinchenko, O.S.; Sviridova, T.V. Changes in Climate and Weather Parameters and Their Correlation with Spring Arrival of the Common Crane (Grus grus) in Northern Moscow Region. Zool. Zhurnal 2013, 92, 834–840. [Google Scholar] [CrossRef]
- Thorup, K.; Tottrup, A.P.; Willemoes, M.; Klaassen, R.H.; Strandberg, R.; Vega, M.L.; Dasari, H.P.; Araujo, M.B.; Wikelski, M.; Rahbek, C. Resource tracking within and across continents in long-distance bird migrants. Sci. Adv. 2017, 3, e1601360. [Google Scholar] [CrossRef]
- Bai, J.; Hou, P.; Jin, D.; Zhai, J.; Ma, Y.; Zhao, J. Habitat Suitability Assessment of Black-Necked Crane (Grus nigricollis) in the Zoige Grassland Wetland Ecological Function Zone on the Eastern Tibetan Plateau. Diversity 2022, 14, 579. [Google Scholar] [CrossRef]
- Li, F.; Ma, J. A Study on the Black-Necked Crane’s Behavior in Incubation Period at Longboatan, China; International Crane Foundation: Baraboo, WI, USA, 1989. [Google Scholar]
- Chandan, P.; Ahmed, T.; Khan, A. Breeding behaviour and productivity of Black-necked crane (Grus nigricolis) in Ladakh, Indian Trans-Himalaya. bioRxiv 2019. [Google Scholar] [CrossRef]
- Kattel, G.R. Climate warming in the Himalayas threatens biodiversity, ecosystem functioning and ecosystem services in the 21st century: Is there a better solution? Biodivers. Conserv. 2022, 31, 2017–2044. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Vokou, D.; Almpanidou, V.; Turkozan, O.; Sgardelis, S.P. Low conservatism of the climatic niche of sea turtles and implications for predicting future distributions. Ecosphere 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Lu, B.G.; Luo, L.L.; Xie, F.; Ye, S.; Yang, N. Quality assessment of the breeding habitat of black-necked cranes in Baiyu County, Sichuan Province. J. Southwest Minzu Univ. (Nat. Sci. Ed.) 2022, 48, 237–244. [Google Scholar]
- Li, F. IUCN SSC Crane Specialist Group—Crane Conservation Strategy. In SPECIES REVIEW:BLACK-NECKED CRANE (Grus nigricollis); Mirande, C.M., Harris, J.T., Eds.; International Crane Foundation: Baraboo, WI, USA, 2019. [Google Scholar]


| Distribution Area | Field Survey | Percentage (%) | Visiting Survey | |
|---|---|---|---|---|
| Adults | Subadult | |||
| Daocheng county | 36 | 0 | 23.69 | 23–29 |
| Litang county | 37 | 4 | 26.97 | 17–18 |
| Batang county | 4 | 0 | 2.63 | |
| Baiyu county | 14 | 1 | 9.87 | |
| Xinlong county | 14 | 1 | 9.87 | 2 |
| Dege county | 2 | 1 | 1.97 | |
| Shiqu county | 34 | 4 | 25.00 | 4 |
| Total | 141 | 11 | 46–53 | |
| 152 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhou, H.; Bai, J.; Zhang, T.; Liu, Y.; Ran, J. Distribution of Breeding Population and Predicting Future Habitat under Climate Change of Black-Necked Crane (Grus nigricollis Przevalski, 1876) in Shaluli Mountains. Animals 2022, 12, 2594. https://doi.org/10.3390/ani12192594
Li M, Zhou H, Bai J, Zhang T, Liu Y, Ran J. Distribution of Breeding Population and Predicting Future Habitat under Climate Change of Black-Necked Crane (Grus nigricollis Przevalski, 1876) in Shaluli Mountains. Animals. 2022; 12(19):2594. https://doi.org/10.3390/ani12192594
Chicago/Turabian StyleLi, Mingming, Huaming Zhou, Jun Bai, Taxing Zhang, Yuxin Liu, and Jianghong Ran. 2022. "Distribution of Breeding Population and Predicting Future Habitat under Climate Change of Black-Necked Crane (Grus nigricollis Przevalski, 1876) in Shaluli Mountains" Animals 12, no. 19: 2594. https://doi.org/10.3390/ani12192594
APA StyleLi, M., Zhou, H., Bai, J., Zhang, T., Liu, Y., & Ran, J. (2022). Distribution of Breeding Population and Predicting Future Habitat under Climate Change of Black-Necked Crane (Grus nigricollis Przevalski, 1876) in Shaluli Mountains. Animals, 12(19), 2594. https://doi.org/10.3390/ani12192594




