Thoracic and Abdominal Mesothelioma in an Older Horse in Lazio Region
Abstract
Simple Summary
Abstract
1. Introduction
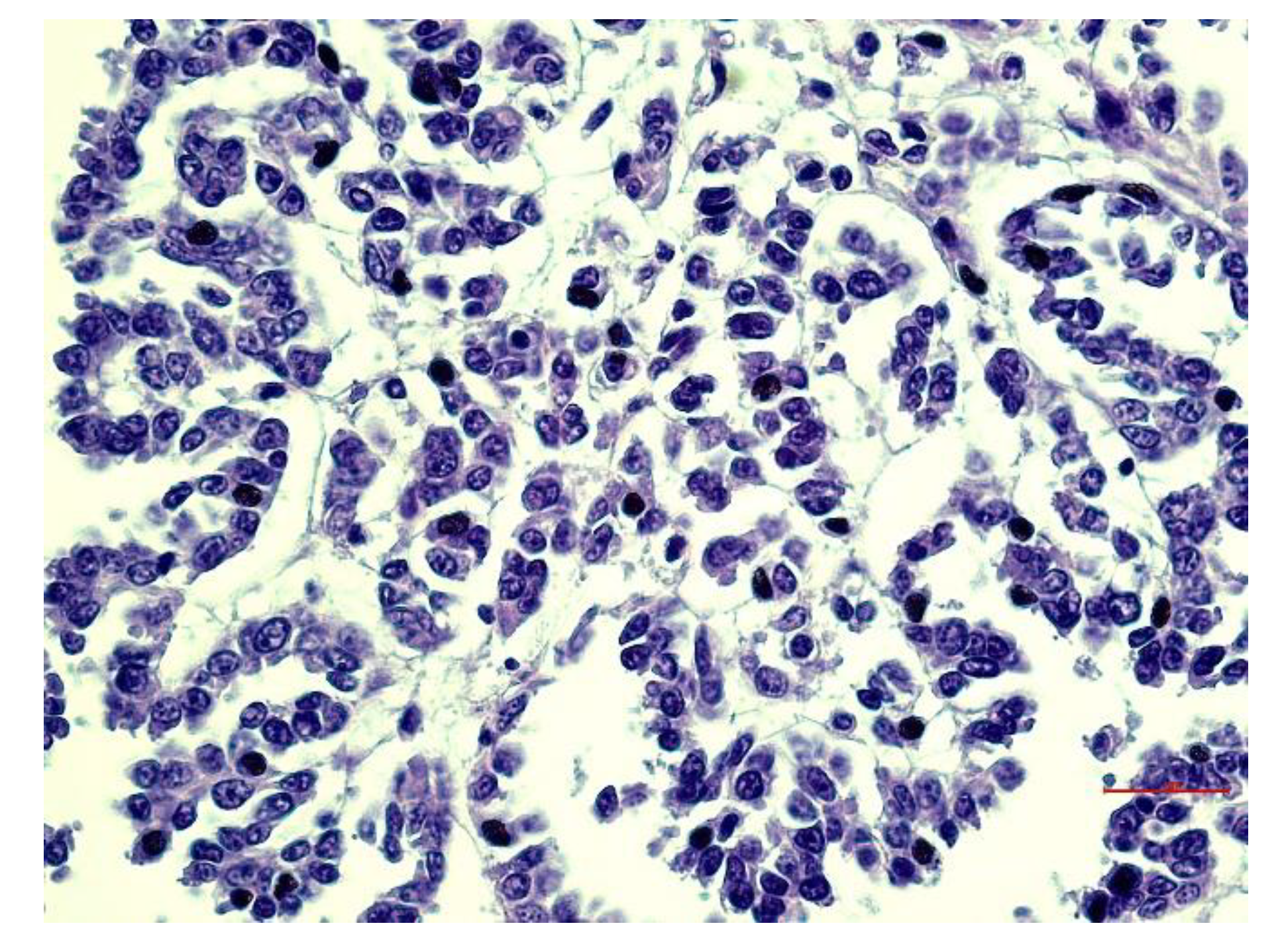
- Epithelioid Mesothelioma—the most common, with the best prognosis. It consists of small cells of an oval to cuboidal shape that connect each other, often forming small tubular or papillary structures;
- Sarcomatoid or fibrous mesothelioma—this is made up of elongated cells (fusiform). Contrary to the epithelioid-type mesothelioma, these cells do not stick together to form structures and spread widely into surrounding tissue;
- Mixed or biphasic mesothelioma—called biphasic, at least 10% of each cell type must be present in the tissue samples examined (epithelioid and sarcomatoid cells) [7].
2. Materials and Methods
2.1. Clinical History
2.2. Cytological Examination
2.3. Histological and Immunohistochemical Examination
3. Results
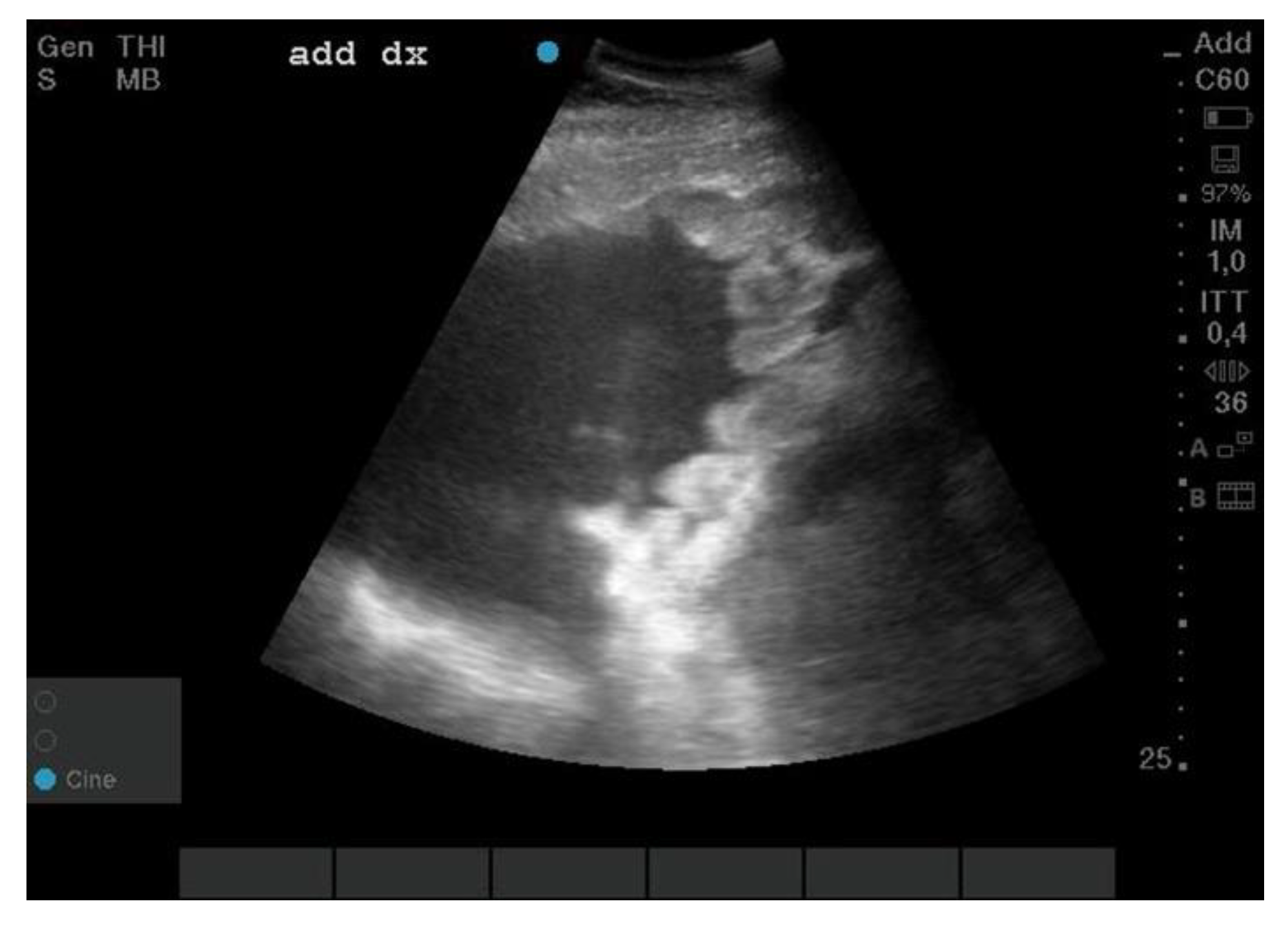
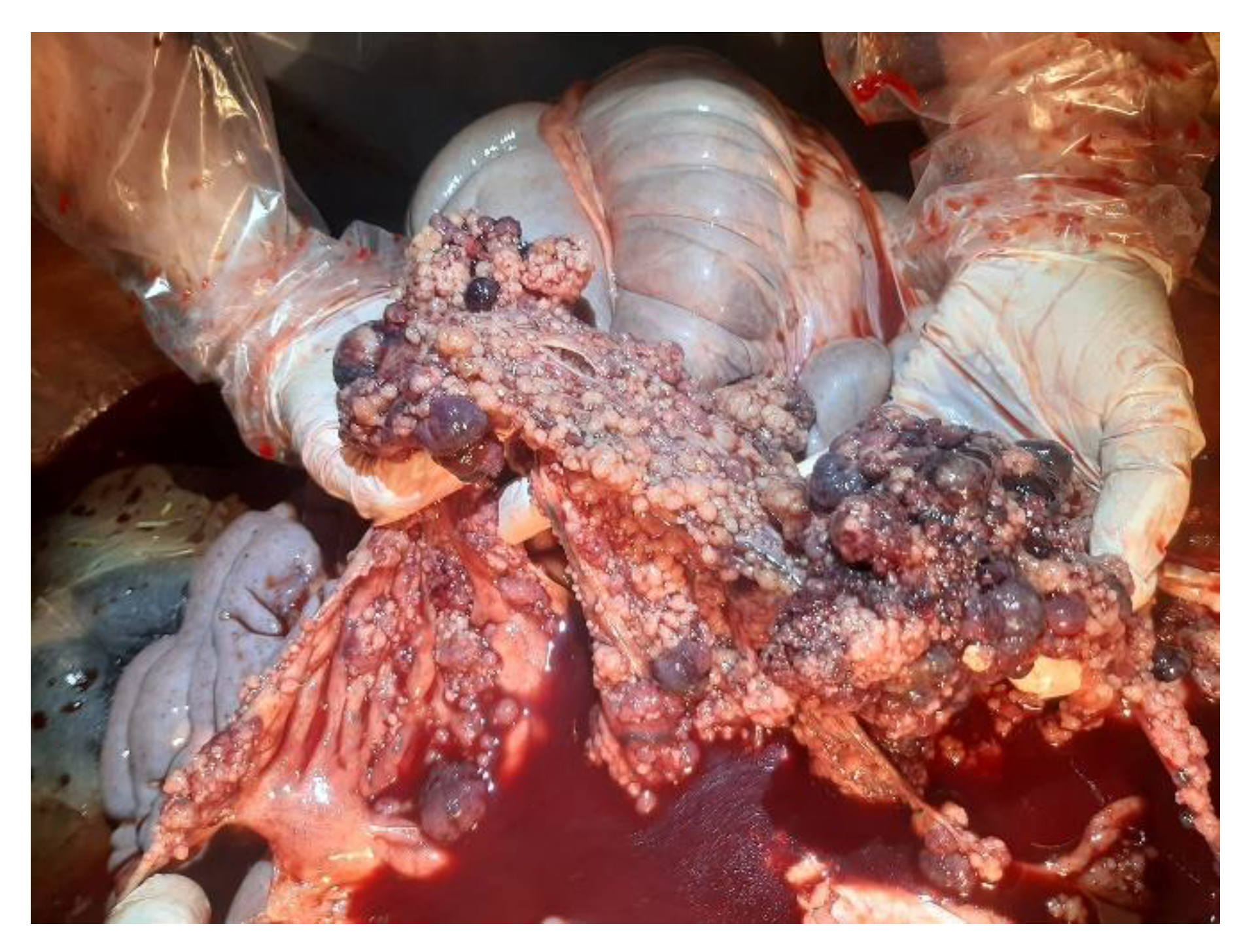
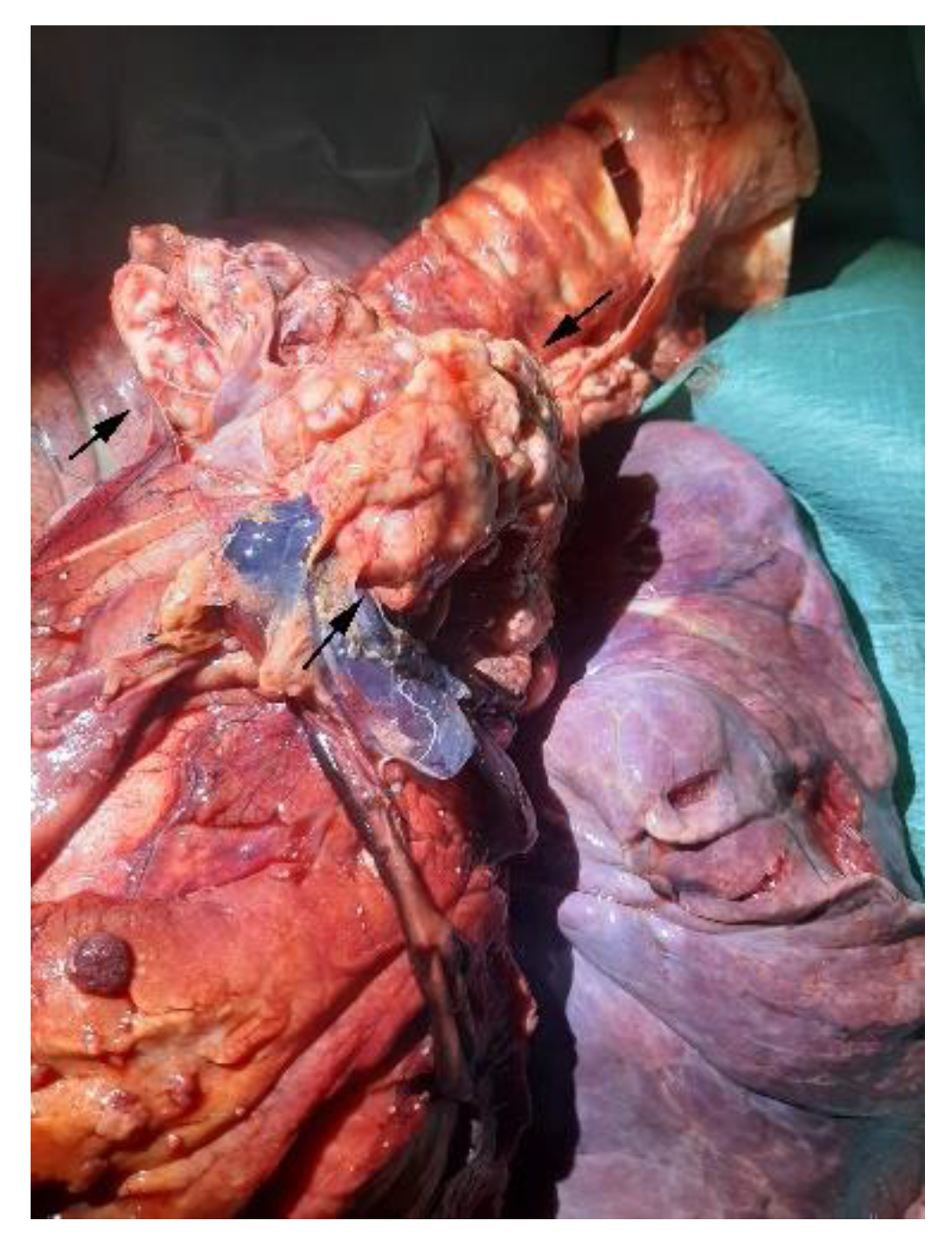
3.1. Necroscopic Examination
3.2. Cytological Aspects
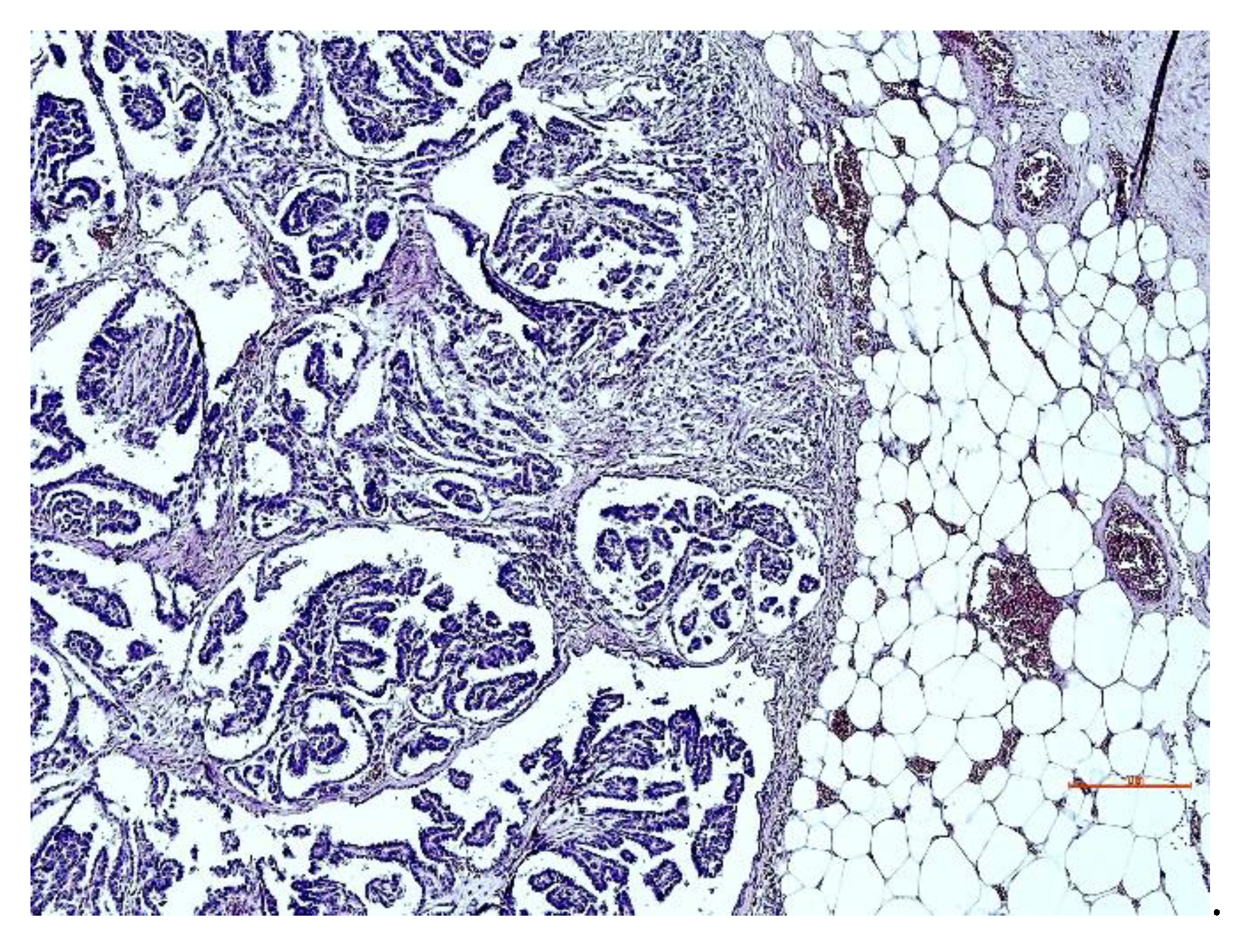
3.3. Histopathological Aspects
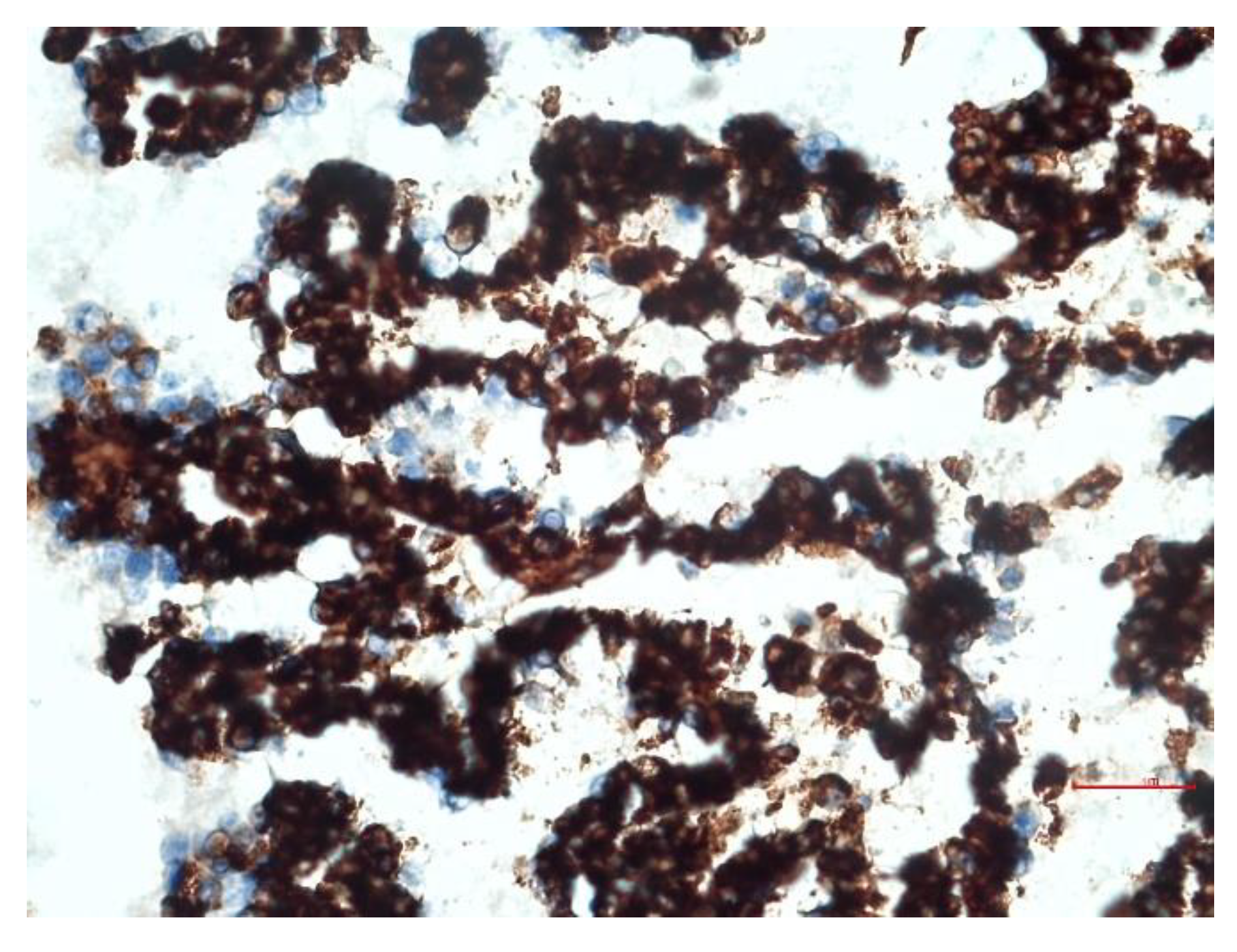
3.4. Immunohistochemical Aspects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, N. Disease Conditions in Geriatric Horses. Equine Pract. 2000, 22, 32. [Google Scholar]
- Fortin, J.S.; Royal, A.B.; Kuroki, K. Concurrent thoracic mesothelioma and thyroid C-cell adenoma with amyloid deposition in an aged horse. Vet. Med. Sci. 2017, 4, 63–70. [Google Scholar] [CrossRef]
- Paciello, O.; Passantino, G.; Costagliola, A.; Papparella, S.; Perillo, A. Histiocytic sarcoma of the nasal cavity in a horse. Res. Vet. Sci. 2013, 94, 648–650. [Google Scholar] [CrossRef]
- De Cock, H.E.V.; MacLachlan, N.J. Simultaneous Occurrence of Multiple Neoplasms and Hyperplasias in the Adrenal and Thyroid Gland of the Horse Resembling Multiple Endocrine Neoplasia Syndrome: Case Report and Retrospective Identification of Additional Cases. Vet. Pathol. 1999, 36, 633–636. [Google Scholar] [CrossRef]
- Ranieri, G.; Ruggieri, E.; Falco, G.; Zizzo, N.; Mattioli, E.; Zito, A.F.; Patruno, R.; Gasparini, G. Drug targets to pro-angiogenetic factors with special reference to primary peritoneal mesothelioma. EMIDDT 2006, 6, 271–277. [Google Scholar] [CrossRef]
- Meuten, D.J. (Ed.) Tumors in Domestic Animals, 5th ed.; Wiley/Blackwell: Ames, IA, USA, 2017; ISBN 978-0-8138-2179-5. [Google Scholar]
- Husain, A.N.; Colby, T.; Ordonez, N.; Krausz, T.; Attanoos, R.; Beasley, M.B.; Borczuk, A.C.; Butnor, K.; Cagle, P.T.; Chirieac, L.R.; et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2012 Update of the Consensus Statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2013, 137, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Moberg, H.L.; Gramer, I.; Schofield, I.; Blackwood, L.; Killick, D.; Priestnall, S.L.; Guillén, A. Clinical presentation, treatment and outcome of canine malignant mesothelioma: A retrospective study of 34 cases. Vet. Comp. Oncol. 2021, 20, 304–312. [Google Scholar] [CrossRef]
- Schlueter, A.H.; Dehghanpir, S.D.; Boudreaux, B.; Robinson, C.; Lima, J.C.M.P.; Langohr, I.M. Feline mesothelioma: Case report and review of cytologic, immunocytochemical, histopathologic, and immunohistochemical findings. J. Vet. Diagn. Investig. 2021, 33, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Misdorp, W. Tumours in Calves: Comparative Aspects. J. Comp. Pathol. 2002, 127, 96–105. [Google Scholar] [CrossRef]
- Movassaghi, A.R.; Maleki, M.; Khanzadi, S. Primary pericardial mesothelioma in a sheep. Comp. Clin. Pathol. 2009, 18, 459–461. [Google Scholar] [CrossRef]
- Krametter, R.; Bagó, Z.; Floeck, M.; Baumgärtner, W. Abdominal mesothelioma in a goat. N. Z. Vet. J. 2004, 52, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Eydner, M.; Grün, A.; Haydn, J.; Baumgärtner, W. A Biphasic Malignant Mesothelioma of the Peritoneum and Pleura in a Horse. Dtsch. Tierarztl. Wochenschr. 2009, 116, 186–191. [Google Scholar] [PubMed]
- Colbourne, C.; Bolton, J.; Mills, J.; Whitaker, D.; Yovich, J.; Howell, J. Mesothelioma in horses. Aust. Vet. J. 1992, 69, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Dobromylskyj, M.; Copas, V.; Durham, A.; Hughes, T.K.; Patterson-Kane, J.C. Disseminated lipid-rich peritoneal mesothelioma in a horse. J. Vet. Diagn. Investig. 2011, 23, 615–618. [Google Scholar] [CrossRef]
- Ricketts, S.W.; Peace, C.K. A Case of Peritoneal Mesothelioma in a Thoroughbred Mare. Equine Vet. J. 1976, 8, 78–80. [Google Scholar] [CrossRef]
- Stoica, G.; Cohen, N.; Mendes, O.; Kim, H.-T. Use of Immunohistochemical Marker Calretinin in the Diagnosis of a Diffuse Malignant Metastatic Mesothelioma in an Equine. J. Vet. Diagn. Investig. 2004, 16, 240–243. [Google Scholar] [CrossRef]
- May, J.; Fews, D.; Tennant, K.; Mair, T. Diagnostic dichotomy: A question of thoracic mesothelioma. Equine Vet. Educ. 2016, 29, 131–137. [Google Scholar] [CrossRef]
- Knottenbelt, D.C.; Snalune, K.; Patterson Kane, J.C. Clinical Equine Oncology; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-7020-4266-9. [Google Scholar]
- National Research Council (U.S.) (Ed.) Animals as Sentinels of Environmental Health Hazards: Committee on Animals as Monitors of Environmental Hazards, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council; National Academy Press: Washington, DC, USA, 1991; ISBN 978-0-309-04046-4. [Google Scholar]
- Rey, F.; Boutin, C.; Viallat, J.R.; Steinbauer, J.; Alessandroni, P.; Jutisz, P.; Di Giambattista, D.; Billon-Galland, M.A.; Hereng, P.; Dumortier, P.; et al. Environmental Asbestotic Pleural Plaques in Northeast Corsica: Correlations with Airborne and Pleural Mineralogic Analysis. Environ. Health Perspect. 1994, 102, 251–252. [Google Scholar] [CrossRef]
- Dumortier, P.; Rey, F.; Viallat, J.R.; Broucke, I.; Boutin, C.; De Vuyst, P. Chrysotile and tremolite asbestos fibres in the lungs and parietal pleura of Corsican goats. Occup. Environ. Med. 2002, 59, 643–646. [Google Scholar] [CrossRef]
- Colombino, E.; Capella, S.; Casalinuovo, F.; Racco, R.; Pruiti, F.; Volante, M.; Presti, V.D.M.L.; Belluso, E.; Capucchio, M.T. Malignant peritoneal mesothelioma in a boar who lived in Calabria (Italy): Wild animal as sentinel system of human health. Sci. Total Environ. 2019, 683, 267–274. [Google Scholar] [CrossRef]
- Filho, S.G.; Magalhaes, G.; Conforti, V.; Santilli, J.; Calazans, S. Biphasic pericardial and pleural mesothelioma in a cat: A case report. Vet. Med. 2016, 60, 105–108. [Google Scholar] [CrossRef]
- Vitellozzi, G.; Rueca, F.; Mariotti, F.; Porciello, F.; Mandara, M.T.; Spaterna, A. Equine Peritoneal Mesothelioma: Clinical, Anatomohistopathological and Ultrastructural Studies. Eur. J. Vet. Pathol. 1998, 4, 29–36. [Google Scholar]
- Fry, M.; Magdesian, K.G.; Judy, C.E.; Pusterla, N.; Vidal, J.D.; Pesavento, P.A.; Zinkl, J.G. Antemortem diagnosis of equine mesothelioma by pleural biopsy. Equine Vet. J. 2010, 35, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Raskin, R.E.; Meyer, D.J. Canine and Feline Cytology: A Color Atlas and Interpretation Guide, 3rd ed.; Elsevier: St. Louis, MO, USA, 2016; ISBN 978-1-4557-4083-3. [Google Scholar]
- Schappa, J.T.; Foutz, C.A.; Olson, E.J.; Armien, A.G.; Ward, C.; Sharkey, L.C. What is your diagnosis? Bicavitary effusion in a horse. Vet. Clin. Pathol. 2017, 46, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Santos, M.; Marrinhas, C.; Caniatti, M. Cell tube block: A newS technique to produce cell blocks from fluid cytology samples. Vet. Clin. Pathol. 2017, 46, 195–201. [Google Scholar] [CrossRef]
- Zizzo, N.; Passantino, G.; D’Alessio, R.M.; Tinelli, A.; Lopresti, G.; Patruno, R.; Tricarico, D.; Maqoud, F.; Scala, R.; Zito, F.A.; et al. Thymidine Phosphorylase Expression and Microvascular Density Correlation Analysis in Canine Mammary Tumor: Possible Prognostic Factor in Breast Cancer. Front. Vet. Sci. 2019, 6, 368. [Google Scholar] [CrossRef]
- Glickman, L.T.; Domanski, L.M.; Maguire, T.G.; Dubielzig, R.R.; Churg, A. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res. 1983, 32, 305–313. [Google Scholar] [CrossRef]
- Monso, E.; Carreres, A.; Tura, J.M.; Ruiz, J.; Fiz, J.; Xaus, C.; Llatjos, M.; Morera, J.; Pairon, J.C.; Orlowski, E.; et al. Electron microscopic microanalysis of bronchoalveolar lavage: A way to identify exposure to silica and silicate dust. Occup. Environ. Med. 1997, 54, 560–565. [Google Scholar] [CrossRef]
- Kozlova, I.; Vanthanouvong, V.; Marie, J.; Roomans, G.M. Composition of Airway Surface Liquid Determined by X-ray Microanalysis. Upsala J. Med. Sci. 2006, 111, 137–153. [Google Scholar] [CrossRef]



| Antibody | Clone | Dilution | Company |
|---|---|---|---|
| CK 8/18 m | EP17/EP30 | 1/100 | Dako, Glostrup, Denmark |
| CK AE1/AE3 m | AE1/AE3 | 1/100 | Dako, Glostrup, Denmark |
| CK 5/6 m | D5/16 B4 | 1/100 | Dako, Glostrup, Denmark |
| Vimentin m | V9 | 1/100 | Dako, Glostrup, Denmark |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passantino, G.; Sassi, E.; Filippi, I.; Serata, V.; Tinelli, A.; Zizzo, N. Thoracic and Abdominal Mesothelioma in an Older Horse in Lazio Region. Animals 2022, 12, 2560. https://doi.org/10.3390/ani12192560
Passantino G, Sassi E, Filippi I, Serata V, Tinelli A, Zizzo N. Thoracic and Abdominal Mesothelioma in an Older Horse in Lazio Region. Animals. 2022; 12(19):2560. https://doi.org/10.3390/ani12192560
Chicago/Turabian StylePassantino, Giuseppe, Emilio Sassi, Ilaria Filippi, Valerio Serata, Antonella Tinelli, and Nicola Zizzo. 2022. "Thoracic and Abdominal Mesothelioma in an Older Horse in Lazio Region" Animals 12, no. 19: 2560. https://doi.org/10.3390/ani12192560
APA StylePassantino, G., Sassi, E., Filippi, I., Serata, V., Tinelli, A., & Zizzo, N. (2022). Thoracic and Abdominal Mesothelioma in an Older Horse in Lazio Region. Animals, 12(19), 2560. https://doi.org/10.3390/ani12192560






