Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animal and Experiment Design
2.3. Sample Collection
2.4. Measurement
2.4.1. Environmental Parameters
2.4.2. Growth Performance
2.4.3. Determination of Antioxidative and Immune Parameters in Serum
2.4.4. Total RNA Extraction and Quantitative RT-PCR Analysis
2.5. Statistical Analysis
3. Results
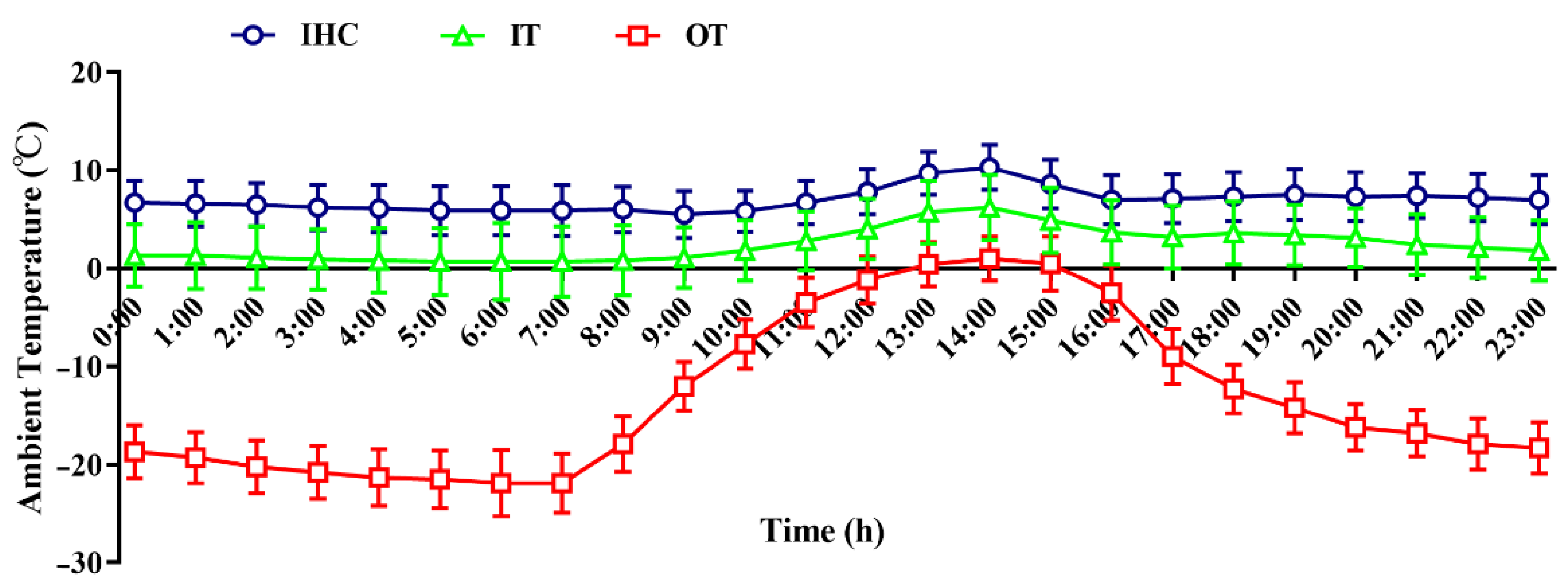
3.1. Environmental Conditions
3.2. Growth Performance
3.3. Immune Indexes and Relevant Genes Expression
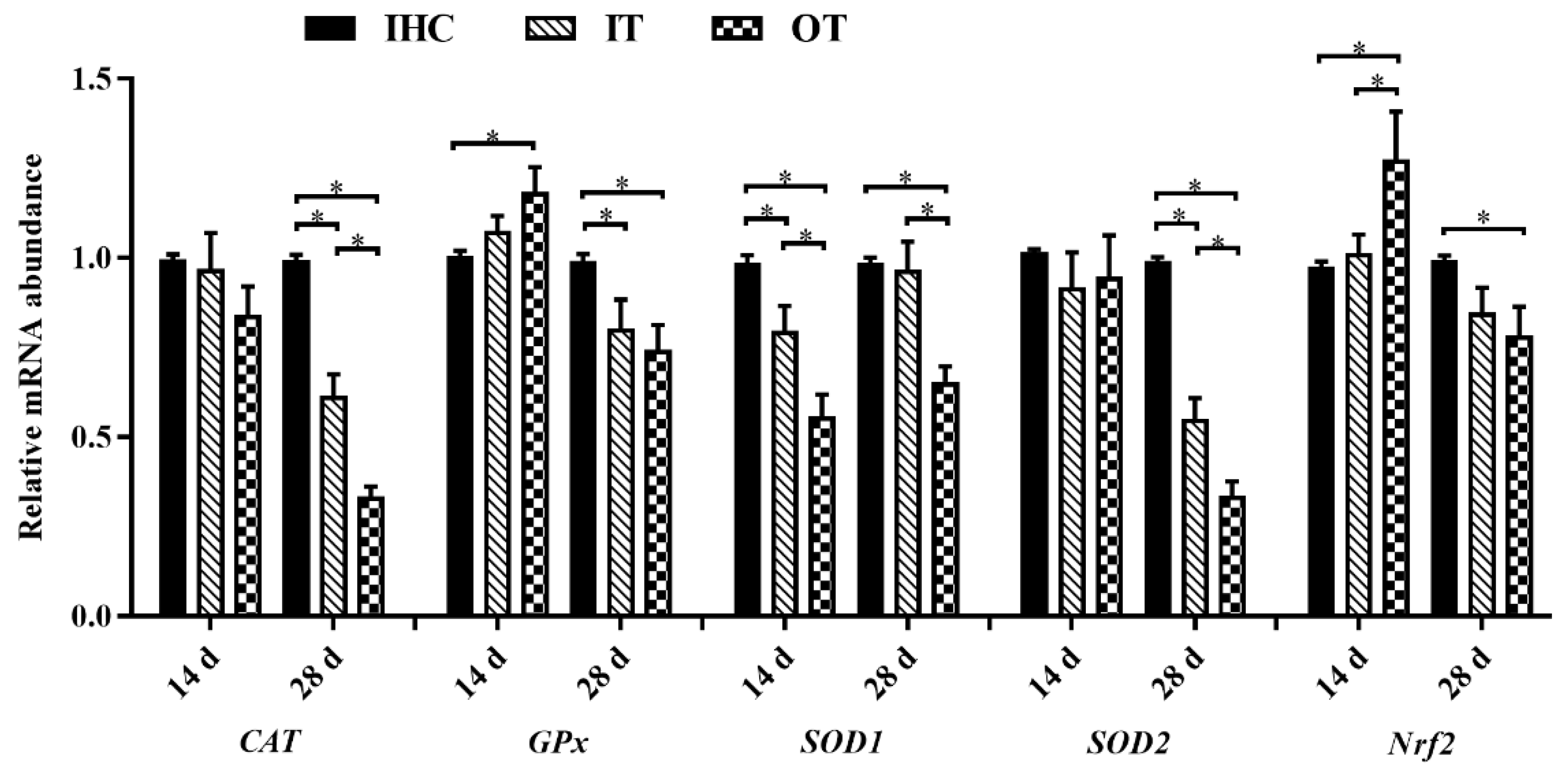
3.4. Antioxidative Properties and Relevant Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kampena, M.V.; Mitchella, B.W.; Siegela, H.S. Thermoneutral zone of chickens as determined by measuring heat production, respiration rate, and electromyographic and electroencephalographic activity in light and dark environments and changing ambient temperatures. J. Agric. Sci. 1979, 92, 219–226. [Google Scholar] [CrossRef]
- Meltzer, A. Thermoneutral zone and resting metabolic rate of broilers. Br. Poult. Sci. 1983, 24, 471–476. [Google Scholar] [CrossRef]
- Liang, X.; Jin, J.; Bi, X.; Kamruzzaman, M.; Kudo, T.; Sano, H. Effects of Chinese herbal medicine and cold exposure on plasma glucose, leucine and energy metabolism in sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, e534–e541. [Google Scholar] [CrossRef]
- Thorn, S.R.; Sekar, S.M.; Lavezzi, J.R. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. J. Anim. Physiol. Anim. Nutr. 2012, 303, R861–R869. [Google Scholar] [CrossRef][Green Version]
- Radostits, O.; Gay, C.; Hinchcliff, K.; Constable, P. Veterinary Medicine: A textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2007. [Google Scholar]
- Sahin, E.; Gumuslu, S. Cold-stress-induced modulation of antioxidant defence: Role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int. J. Biometeorol. 2004, 48, 165–171. [Google Scholar] [CrossRef]
- Trevisan, M. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; He, L.Q.; Zuo, S.N.; Zhang, Y.M.; Wan, D.; Long, C.M.; Huang, P.; Wu, X.; Wu, C.R.; Liu, G. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. BBA Mol. Basis Dis. 2018, 1864, 488–498. [Google Scholar] [CrossRef]
- Rathwa, S.D.; Vasava, A.A.; Pathan, M.M.; Madhira, S.P.; Patel, Y.G.; Pande, A.M. Effect of season on physiological, biochemical, hormonal, and oxidative stress parameters of indigenous sheep. Veter. World 2017, 10, 650–654. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; De, K.; Gowane, G.R. Sheep production system in arid and semi-arid regions of India. Ann. Arid Zone 2013, 52, 265–274. [Google Scholar]
- Marino, R.; Atzori, A.S.; D’Andrea, M.; Iovane, G.; Trabalza-Marinucci, M.; Rinaldi, L. Climate change: Production performance, health issues, greenhouse gas emissions and mitigation strategies in sheep and goat farming. Small Rumin. Res. 2016, 135, 50–59. [Google Scholar] [CrossRef]
- Slee, J.; Sykes, A.R. Acclimatisation of scottish blackface sheep to cold. 1. rectal temperature responses. Anim. Sci. 1967, 9, 333–347. [Google Scholar] [CrossRef]
- Doubek, J.; Slosarkova, S.; Fleischer, P.; Mala, G.; Skrivanek, M. Metabolic and hormonal profiles of potentiated cold stress in lambs during early postnatal period. Czech J. Anim. Sci. 2003, 48, 403–411. [Google Scholar]
- Young, B.A. Ruminant cold stress: Effect on Production. J. Anim. Sci. 1983, 57, 1601–1607. [Google Scholar] [CrossRef]
- Curtis, S.E. Environmental Management in Animal Agriculture; Iowa State Press: Ames, IA, USA, 1983. [Google Scholar]
- Freer, M.; Dove, H.; Nolan, J.V. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Melbourne, Australia, 2007. [Google Scholar]
- Zhang, X.Q.; Kemp, D.; Ma, Y.; Jiang, C. Effect of warm-shed feeding on liveweight loss and lambing performance of ewes during the winter-spring period. Acta Pratacult. Sin. 2017, 26, 203–209. [Google Scholar] [CrossRef]
- Swarnkar, C.P.; Prince, L.; Sonawane, G. Wind chill index and neonatal lamb mortality at an organized farm in semi-arid Rajasthan. Biol. Rhythm Res. 2018, 49, 862. [Google Scholar] [CrossRef]
- Geenty, K.G.; Brien, F.D.; Hinch, G.N.; Dobos, R.C.; McCaskill, M.; Ball, A.J.; Behrendt, R.; Gore, K.P.; Harden, S.; Hocking-Edwards, J.E.; et al. Reproductive performance in the sheep CRC information nucleus across different sheep production environments in southern Australia. Anim. Prod. Sci. 2014, 54, 715–726. [Google Scholar] [CrossRef]
- Barnett, M.C.; McFarlane, J.R.; Hegarty, R.S. Low ambient temperature elevates plasma triiodothyronine concentrations while reducing digesta mean retention time and methane yield in sheep. J. Anim. Physiol. Anim. Nutr. 2015, 99, 483–491. [Google Scholar] [CrossRef]
- Verbeek, E.; Oliver, M.H.; Waas, J.R.; McLeay, L.M.; Blache, D.; Matthews, L.R. Reduced cortisol and metabolic responses of thin ewes to an acute cold challenge in mid-pregnancy: Implications for animal physiology and welfare. PLoS ONE 2012, 7, e37315. [Google Scholar] [CrossRef]
- Peana, I.; Dimauro, C.; Carta, M.; Gaspa, M.; Fois, G.; Cannas, A. Cold markedly influences milk yield of Sardinian dairy sheep farms. In Proceedings of the 17th ASPA Congress, Alghero, Italy, 29 May–1 June 2007. [Google Scholar]
- Ramon, M.; Diaz, C.; Perez-Guzman, M.D.; Carabao, M.J. Effect of exposure to adverse climatic conditions on production in Manchega dairy sheep. J. Dairy Sci. 2016, 99, 5764–5779. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, D.S.V.; Glick, B. Effect of cold exposure on the immune response of chickens. Poult. Sci. 1977, 56, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, G.; Tian, G.; Liu, Y.; Dong, N.; Li, L.; Zhang, S.; Chai, H.; Chen, Y.; Yang, Y. Changes in rumen microbiota affect metabolites, immune responses and antioxidant enzyme activities of sheep under cold stimulation. Animals 2021, 11, 712. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Mao, C.; Wang, Z.; Guo, S.; Jin, X. Effects of heat stress on antioxidant status and immune function and expression of related genes in lambs. Int. J. Biometeorol. 2020, 64, 2039–2104. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Takahashi, H. Insulin response to secretogogues in sheep exposed to cold. J. Physiol. 1983, 334, 155–167. [Google Scholar] [CrossRef]
- Delbro, D.; Lisander, B.; Andersson, S.A. Atropine-sensitive gastric excitation by local heating-the possibility of avisceral axon reflex arrangement. Acta Physiol. Scand. 1982, 114, 319–320. [Google Scholar] [CrossRef]
- Kennedy, P.M.; Christopherson, R.J.; Milligan, L.P. Effects of cold exposure on feed protein degradation, microbial protein synthesis and transfer of plasma urea to the rumen of sheep. Br. J. Nutr. 1982, 47, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W.G.; Mcqueen, R.E.; Burgess, P.L. Effect of cold on digestibility of chopped or pelleted hay by sheep. Can. J. Anim. Sci. 1980, 60, 571–578. [Google Scholar]
- Liu, L. Effect of Cold Stress on Physiological, Biochemical and Endocrinological Values in Fasting Weaned Lambs. Master’s Dissertation, Xinjiang Agricultural University, Ürümqi, China, 2014. [Google Scholar] [CrossRef]
- Zhang, S. Effects of Windy Velocity on Nutrient Metabolism and Serum Physiological and Biochemical Indexes of Sheep. Master’s Dissertation, Northwest Agriculture & Forestry University, Xianyang, China, 2019. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201902&filename=1019901745.nh (accessed on 15 August 2020).
- Nonnecke, B.J.; Foote, M.R.; Miller, B.L.; Fowler, M.; Johnson, T.E.; Horst, R.L. Effects of chronic environmental cold on growth, health, and select metabolic and immunologic responses of preruminant calves. J. Dairy Sci. 2009, 92, 6134–6143. [Google Scholar] [CrossRef]
- Wang, J. The Influence of Low Ambient Temperature and Nutrient Level on Blood Indicators and Muscular Quality of Fattening pig. Master’s Dissertation, Heilongjiang Bayi Agricultural University, Daqing, China, 2015. [Google Scholar]
- Lefaucheur, L.; Dividich, J.L.; Mourot, J.; Monin, G.; Ecolan, P.; Krauss, D. Influence of environmental temperature on growth, muscle and adipose tissue metabolism, and meat quality in swine. J. Anim. Sci. 1991, 69, 2844–2854. [Google Scholar] [CrossRef]
- Christopherson, R.J.; Kennedy, P.M. Effect of the thermal environment on digestion in ruminants. Can. J. Anim. Sci. 1985, 63, 477–496. [Google Scholar] [CrossRef]
- Kozyreva, T.V.; Eliseeva, L.S. Immune response in cold exposures of different types. J. Therm. Biol. 2000, 25, 401–404. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Qin, Z.; Yan, S.; Shi, B. Effects of chitosan addition on growth performance, diarrhoea, anti-oxidative function and serum immune parameters of weaned piglets. S. Afr. J. Anim. Sci. 2018, 48, 142–150. [Google Scholar] [CrossRef]
- Chen, H.; Ao, R.G.L.; Wang, C.; Si, M.J.D.; A, R.C.; Zhang, J.; Liu, B. Effects of chronic cold stress on immune function, antioxidant function and serum hormone secretion of grazing Simmental cows. Chin. J. Vet. Sci. 2020, 40, 1378–1383. [Google Scholar] [CrossRef]
- Kandasamy, M.; Jayasurya, A.S.; Moochhala, S.; Huat, B.B.; Kun, L.Y.; Mahendran, R. Lactobacillus rhamnosus GG secreting an antigen and interleukin-2 translocates across the gastrointestinal tract and induces an antigen specific immune response. Microbiol. Immunol. 2011, 55, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Z.; Xu, Y.; Mao, C.; Guo, S.; Jin, X.; Shi, B. Effects of chronic cold stress on body weight gain, serum immune and antioxidative functions of sheep. Chin. J. Anim. Nutr. 2021, 33, 397–408. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Qi, Y.; Shen, W.; Li, Y. Effect of cold stress on serum factor and Hsp70 mRNA in Hu sheep. China Anim. Husb. Vet. Med. 2015, 42, 890–895. [Google Scholar] [CrossRef]
- Shini, S.; Huff, G.R.; Shini, A.; Kaiser, P. Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010, 89, 841–851. [Google Scholar] [CrossRef]
- Zhai, X.T.; Zhang, Z.Y.; Jiang, C.H.; Chen, J.Q.; Ye, J.Q.; Jia, X.B.; Yang, Y.; Ni, Q.; Wang, S.X.; Song, J. Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway. J. Ethnopharmacol. 2016, 183, 159–165. [Google Scholar] [CrossRef]
- Indrikis, K.; Dina, C.; Tatjana, K.; Jolanta, V. Extremely low ambient temperature affects hematological parameters and body condition in wintering Great Tits (Parus major). J. Ornithol. 2011, 152, 889–895. [Google Scholar] [CrossRef]
- Cong, P.F.; Liu, Y.N.; Liu, N.N.; Zhang, Y.B.; Tong, C.C.; Shi, L.; Liu, X.L.; Shi, X.Y.; Liu, Y.; Tong, Z. Cold exposure induced oxidative stress and apoptosis in the myocardium by inhibiting the Nrf2-Keap1 signaling pathway. BMC Cardiovasc. Disord. 2018, 18, 36. [Google Scholar] [CrossRef]
- Fuente, M.D.L. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mori, A. Stress, aging, and brain oxidative damage. Neurochem. Res. 1999, 24, 1479–1497. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Ahmad, H.; Tian, J.; Wang, J.; Khan, M.A.; Wang, Y.; Zhang, L.; Wang, T. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J. Agric. Food Chem. 2012, 60, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.A.S.; Abdallah, H.M.; Ali, S.S. Mentha longifolia protects against acetic-acid induced colitis in rats. J. Ethnopharmacol. 2016, 90, 354–361. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, R.; Meng, Z.; Duan, Y.; An, X.; Qi, J. Dietary supplementation of ferulic acid improves performance and alleviates oxidative stress of lambs in a cold environment. Can. J. Anim. Sci. 2019, 99, 705–712. [Google Scholar] [CrossRef]
- Wei, H.D.; Zhang, R.X.; Su, Y.Y.; Bi, Y.J.; Li, X.; Zhang, X.; Li, J.H.; Bao, J. Effects of acute cold stress after long-term cold stimulation on antioxidant status, heat shock proteins, inflammation and immune cytokines in broiler heart. Front. Physiol. 2018, 9, 1589–1601. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, X.; Yang, H.; Peng, L.; Ci, X. Isoliquiritigenin exerts antioxidative and anti-inflammatory effects via activating the KEAP-1/Nrf2 pathway and inhibiting the NF-κB and NLRP3 pathways in carrageenan-induced pleurisy. Food Funct. 2020, 11, 2522–2534. [Google Scholar] [CrossRef]
- Li, H. Research on NF-κB Pathway Negative Regulation Keap1-Nrf2 Pathway and Its Molecular Mechanism. Master’s Dissertation, Tianjin University, Tianjin, China, 2009. [Google Scholar] [CrossRef]

| Target | GenBank Accession | Sequence of Nucleotide (5′ to 3′) | Annealing Temp (°C) | Fragment Size (bp) |
|---|---|---|---|---|
| SOD1 | NM_001145185 | For: GGAGACCTGGGCAATGTGAA Rev: CCTCCAGCGTTTCCAGTCTT | 60 | 182 |
| SOD2 | NM_001280703.1 | For: AAACCGTCAGCCTTACACC Rev: ACAAGCCACGCTCAGAAAC | 60 | 116 |
| GPx | XM_004018462.1 | For: TGGTCGTACTCGGCTTCCC Rev: AGCGGATGCGCCTTCTCG | 60 | 163 |
| CAT | XM_004016396 | For: GAGCCCACCTGCAAAGTTCT Rev: CTCCTACTGGATTACCGGCG | 60 | 148 |
| Nrf2 | XM_004004557.1 | For: TGTGGAGGAGTTCAACGAGC Rev: CGCCGCCATCTTGTTCTTG | 61 | 88 |
| IL-1β | NM_001009465 | For: CGATGAGCTTCTGTGTGATG Rev: CTGTGAGAGGAGGTGGAGAG | 59 | 161 |
| IL-2 | AF215687.1 | For: AATTGAGCTTAGGCGTATCTACAGG Rev: TACTCGTCTTGGCTTCATTCACA | 61 | 80 |
| TNF-α | NM_001024860 | For: AGTCTGGGCAGGTCTACTTTG Rev: GGTAACTGAGGTGGGAGAGG | 60 | 127 |
| NF-κB p50 | XM_004009667.3 | For: AGCACCACTTATGACGGAACTACA | 60 | 168 |
| Rev: GACCCCTTCATCCTCTCCATC | ||||
| NF-κB p65 | XM_004020143.3 | For: GGAGGCCAAGGAACTGAAGA | 60 | 101 |
| Rev: TCAGGGGCAGAGAGAAGGAG | ||||
| β-actin | NM_001009784.1 | For: GAGCGCAAGTACTCCGTGTG Rev: CATTTGCGGTGGACGATG | 58 | 122 |
| Items | Groups | Items | Groups | ||||
|---|---|---|---|---|---|---|---|
| IHC | IT | OT | IHC | IT | OT | ||
| Day 1 | 4.98 (3.12, 8.21) | 2.10 (−0.21, 8.04) | −8.33 (−18.51, 7.74) | Day 15 | 7.11 (4.43, 13.91) | 5.43 (0.04, 15.18) | −17.19 (−27.08, −1.07) |
| Day 2 | 6.09 (3.37, 12.74) | 2.66 (0.27, 8.85) | −9.68 (−18.52, 5.22) | Day 16 | 6.64 (3.70, 9.91) | 0.46 (−3.22, 3.76) | −15.70 (−28.06, 3.25) |
| Day 3 | 6.30 (4.01, 12.13) | 2.13 (0.16, 7.82) | −10.18 (−18.09, 5.01) | Day 17 | 7.62 (5.97, 13.04) | 1.55 (−0.53, 5.10) | −13.53 (−23.86, 2.21) |
| Day 4 | 6.99 (4.75, 13.21) | 2.47 (−0.25, 8.01) | −11.77 (−21.65, 2.64) | Day 18 | 6.72 (3.33, 10.21) | 2.97 (0.48, 6.90) | −14.20 (−24.29, −0.67) |
| Day 5 | 7.33 (5.02, 12.16) | 3.10 (1.34, 8.89) | −10.70 (−20.09, 4.93) | Day 19 | 6.34 (3.96, 11.87) | 2.63 (0.68, 7.39) | −17.09 (−28.80, −1.52) |
| Day 6 | 7.96 (6.13, 12.73) | 4.69 (2.03, 10.94) | −6.83 (−18.19, 10.93) | Day 20 | 6.38 (4.41, 9.53) | 2.18 (−0.43, 4.31) | −15.03 (−28.04, −1.01) |
| Day 7 | 9.53 (8.06, 11.35) | 4.26 (1.97, 7.54) | −2.06 (−11.22, 12.10) | Day 21 | 2.58 (−1.38, 5.52) | −0.05 (−2.47, 2.18) | −15.53 (−26.31, −7.68) |
| Day 8 | 10.79 (6.91, 12.55) | 3.70 (1.80, 5.31) | −1.33 (−14.00, 2.07) | Day 22 | 4.62 (2.55, 9.03) | 0.28 (−1.11, 2.63) | −19.11 (−27.82, −5.01) |
| Day 9 | 6.27 (2.93, 8.21) | 3.69 (1.10, 8.29) | −12.98 (−21.67, −1.05) | Day 23 | 8.87 (6.68, 11.02) | 1.21 (−0.33, 4.28) | −15.87 (−26.51, −3.05) |
| Day 10 | 6.47 (3.20, 13.32) | 2.89 (0.13, 12.91) | −13.80 (−23.20, −2.31) | Day 24 | 8.28 (2.77, 11.11) | 0.79 (−2.52, 2.76) | −17.61 (28.20, 0.02) |
| Day 11 | 6.95 (2.15, 14.37) | 2.76 (−0.65, 12.32) | −15.22 (−26.69, −3.24) | Day 25 | 11.20 (8.04, 17.93) | 2.40 (−0.60, 7.20) | −13.59 (−26.10, 6.05) |
| Day 12 | 4.68 (0.63, 6.97) | −1.09 (−6.10, 4.52) | −17.38 (−27.85, −3.94) | Day 26 | 11.55 (9.21, 15.02) | 2.89 (−0.18, 6.84) | −10.13 (−23.53, 10.54) |
| Day 13 | 4.07 (0.82, 5.78) | 3.90 (0.03, 9.01) | −17.77 (−27.71, −3.84) | Day 27 | 9.04 (6.09, 12.67) | 1.50 (−0.43, 4.71) | −15.78 (−28.35, −1.09) |
| Day 14 | 5.51 (2.16, 7.68) | 3.93 (−0.53, 6.08) | −16.81 (−26.84, −1.82) | Day 28 | 9.05 (6.27, 12.80) | 0.75 (−1.53, 2.75) | −15.42 (−24.76, −4.70) |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| IHC | IT | OT | ||
| 08:00 | 0.03 ± 0.01 b | 0.04 ± 0.02 b | 0.67 ± 0.65 a | <0.001 |
| 14:00 | 0.04 ± 0.01 b | 0.04 ± 0.02 b | 1.08 ± 0.89 a | <0.001 |
| 20:00 | 0.03 ± 0.01 b | 0.04 ± 0.01 b | 0.80 ± 1.11 a | <0.001 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| IHC | IT | OT | |||
| BW (kg) | |||||
| 1 d | 33.04 ± 2.07 | 33.07 ± 2.43 | 33.10 ± 2.40 | 0.88 | 0.999 |
| 14 d | 35.16 ± 2.43 | 35.09 ± 2.46 | 35.11 ± 2.33 | 0.92 | 0.999 |
| 28 d | 38.17 ± 2.64 | 37.49 ± 2.42 | 37.29 ± 1.95 | 0.92 | 0.797 |
| ADG (g/d) | |||||
| 1–14 d | 151.90 ± 27.40 | 144.29 ± 45.61 | 144.05 ± 32.03 | 13.83 | 0.912 |
| 15–28 d | 215.00 ± 28.82 a | 171.19 ± 33.70 b | 155.71 ± 34.72 b | 16.33 | 0.017 |
| 1–28 d | 183.45 ± 22.99 a | 157.74 ± 19.06 ab | 149.88 ± 28.04 b | 10.89 | 0.065 |
| ADFI (g/d) | |||||
| 1–14 d | 1380.2 ± 49.4 b | 1383.8 ± 33.1 b | 1436.7 ± 15.5 a | 32.67 | 0.025 |
| 15–28 d | 1549.1 ± 48.5 | 1586.3 ± 51.6 | 1597.1 ± 21.0 | 37.04 | 0.159 |
| 1–28 d | 1464.6 ± 39.7 b | 1486.9 ± 39.2 ab | 1516.9 ± 15.3 a | 24.1 | 0.050 |
| F:G | |||||
| 1–14 d | 9.31 ± 1.49 | 10.47 ± 3.36 | 10.36 ± 2.10 | 2.13 | 0.669 |
| 15–28 d | 7.33 ± 1.19 b | 9.60 ± 2.05 ab | 10.75 ± 2.67 a | 1.79 | 0.034 |
| 1–28 d | 8.09 ± 1.04 b | 9.53 ± 1.08 ab | 10.45 ± 2.14 a | 1.32 | 0.048 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| IHC | IT | OT | |||
| 14 d | |||||
| IgA (μg/mL) | 51.38 ± 6.57 | 44.85 ± 10.09 | 44.95 ± 9.73 | 3.63 | 0.443 |
| IgG (μg/mL) | 100.14± 14.17 | 119.10 ± 24.69 | 117.33 ± 11.62 | 7.70 | 0.159 |
| IgM (μg/mL) | 253.76 ± 38.64 | 274.78 ± 27.86 | 252.79 ± 28.51 | 13.07 | 0.445 |
| IL-1β (pg/mL) | 219.71 ± 20.10 | 255.59 ± 62.19 | 203.90 ± 19.57 | 18.19 | 0.162 |
| IL-2 (pg/mL) | 271.87 ± 20.74 | 260.74 ± 65.76 | 240.66 ± 29.66 | 17.83 | 0.511 |
| TNF-α (pg/mL) | 33.18 ± 4.04 | 32.95 ± 6.75 | 35.44 ± 6.60 | 2.29 | 0.752 |
| 28 d | |||||
| IgA (μg/mL) | 59.26 ± 6.31 a | 45.87 ± 3.83 b | 39.37 ± 4.42 b | 3.85 | <0.001 |
| IgG (μg/mL) | 114.46 ± 34.41 | 124.43 ± 20.87 | 114.70 ± 21.90 | 10.41 | 0.791 |
| IgM (μg/mL) | 240.63 ± 42.93 | 282.86 ± 35.93 | 274.12 ± 29.08 | 15.77 | 0.196 |
| IL-1β (pg/mL) | 233.11 ± 35.61 | 282.55 ± 63.32 | 251.00 ± 49.67 | 21.35 | 0.268 |
| IL-2 (pg/mL) | 305.53 ± 52.64 a | 237.00 ± 35.52 b | 216.06 ± 47.07 b | 22.90 | 0.042 |
| TNF-α (pg/mL) | 37.05 ± 10.00 b | 39.57 ± 5.60 b | 53.27 ± 9.74 a | 4.48 | 0.022 |
| Items | Groups | SEM | p-Value | ||
|---|---|---|---|---|---|
| IHC | IT | OT | |||
| 14 d | |||||
| CAT (U/mL) | 3.01 ± 0.47 | 2.50 ± 0.42 | 2.42 ± 0.41 | 0.43 | 0.119 |
| GPx (U/mL) | 186.24 ± 34.38 a | 166.18 ± 27.35 a | 93.90 ± 14.08 b | 20.45 | 0.002 |
| T-SOD (U/mL) | 14.29 ± 1.28 a | 12.23 ± 1.74 a | 6.84 ± 0.79 b | 1.44 | 0.001 |
| T-AOC (U/mL) | 0.60 ± 0.08 a | 0.44 ± 0.05 b | 0.37 ± 0.05 b | 0.04 | 0.002 |
| MDA (nmol/mL) | 1.08 ± 0.11 | 1.05 ± 0.15 | 1.18 ± 0.04 | 0.10 | 0.394 |
| 28 d | |||||
| CAT (U/mL) | 2.57 ± 0.27 a | 1.87 ± 0.41 b | 1.63 ± 0.33 b | 0.34 | 0.002 |
| GPx (U/mL) | 80.16 ± 10.81 a | 65.20 ± 3.86 b | 30.40 ± 4.60 c | 6.81 | <0.001 |
| T-SOD (U/mL) | 12.65 ± 0.81 a | 10.49 ± 0.49 b | 7.75 ± 0.66 c | 0.90 | <0.001 |
| T-AOC (U/mL) | 0.61 ± 0.05 a | 0.58 ± 0.02 a | 0.39 ± 0.06 b | 0.04 | <0.001 |
| MDA (nmol/mL) | 1.03 ± 0.04 b | 1.15 ± 0.09 ab | 1.25 ± 0.04 a | 0.05 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Xu, Y.; Jin, X.; Wang, Z.; Mao, C.; Guo, S.; Yan, S.; Shi, B. Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals 2022, 12, 2535. https://doi.org/10.3390/ani12192535
Shi L, Xu Y, Jin X, Wang Z, Mao C, Guo S, Yan S, Shi B. Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals. 2022; 12(19):2535. https://doi.org/10.3390/ani12192535
Chicago/Turabian StyleShi, Lulu, Yuanqing Xu, Xiao Jin, Zheqi Wang, Chenyu Mao, Shiwei Guo, Sumei Yan, and Binlin Shi. 2022. "Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs" Animals 12, no. 19: 2535. https://doi.org/10.3390/ani12192535
APA StyleShi, L., Xu, Y., Jin, X., Wang, Z., Mao, C., Guo, S., Yan, S., & Shi, B. (2022). Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals, 12(19), 2535. https://doi.org/10.3390/ani12192535





