A Comparison of Aerodynamic Parameters in Two Subspecies of the American Barn Owl (Tyto furcata)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Differences between the Two Subspecies
3.2. Differencees between the Sexes
4. Discussion
4.1. Methodological Considerations
4.2. Comparison with Other Studies
4.3. Speculations on the Evolutionary Basis of the Lower Wing Loading in T.f.punctatissima
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols and Abbreviations
| bm | Body mass |
| l | Wing length |
| c | Wing chord |
| S | Wing area |
| WL | Wing loading |
| AR | Aspect ratio |
References
- Wagner, H.; Weger, M.; Klaas, M.; Schröder, W. Features of owl wings that promote silent flight. Interface Focus 2017, 7, 20160078. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; LePiane, K.; Liu, L. Evolution and ecology of silent flight in owls and other flying vertebrates. Integr. Org. Biol. 2020, 2, obaa001. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.W.; Peake, N. Aeroacoustics of silent owl flight. Ann. Rev. Fluid Mech. 2020, 52, 395–420. [Google Scholar] [CrossRef]
- Cheney, J.A.; Stevenson, J.P.J.; Durston, N.E.; Maeda, M.; Song, J.; Megson-Smith, D.A.; Windsor, S.P.; Usherwood, J.R.; Bomphrey, R.J. Raptor wing morphing with flight speed. J. R. Soc. Interface 2021, 18, 20210349. [Google Scholar] [CrossRef] [PubMed]
- Winzen, A.; Roidl, B.; Schröder, W. Particle-image velocimetry of the fluid-structure interaction of a natural owl wing. Bioinspiration Biomim. 2015, 10, 056009. [Google Scholar] [CrossRef] [PubMed]
- Winzen, A.; Roidl, B.; Schröder, W. Combined particle-image velocimetry and force analysis of the three-dimensional fluid-structure interaction of a natural owl wing. Bioinspiration Biomim. 2016, 11, 026005. [Google Scholar] [CrossRef]
- Nafi, A.; Ben-Gida, H.; Guglielmo, C.G.; Gurka, R. Aerodynmaic forces acting on birds during flight: A comparative study of a shorebird, songbird and a strigiform. Exp. Therm. Fluid Sci. 2020, 113, 110018. [Google Scholar] [CrossRef]
- Graham, R.R. The silent flight of owls. J. Roy. Aero Soc. 1934, 38, 837–843. [Google Scholar] [CrossRef]
- Lilley, G.M. A study of the silent flight of the owl. In Proceedings of the 4th AIAA/CEAS Aeroacoustics Conference, Toulouse, France, 2–4 June 1998. [Google Scholar] [CrossRef]
- Geyer, T.; Sarradj, E.; Fritzsche, C. Silent Owl Flight: Comparative Acoustic Wind Tunnel Measurements on Prepared Wings. Acta Acust. United Acust. 2013, 99, 139–153. [Google Scholar] [CrossRef]
- Rayner, J.M.V. Form and function in avian flight. In Current Ornithology; Johnston, R.F., Ed.; Springer: Boston, MA, USA, 1988; Volume 5, pp. 1–66. [Google Scholar] [CrossRef]
- Marti, C.D. Sex and age dimorphism in the barn owl and a test of mate choice. Auk 1990, 107, 246–254. [Google Scholar] [CrossRef]
- Bachmann, T.; Mühlenbruch, G.; Wagner, H. The barn owl wing: An inspiration for silent flight in the aviation industry? In Bioinspiration, Biomimetics and Bioreplication; Martin-Palma, J., Lakhtakia, A., Eds.; SPIE: Washington, DC, USA, 2011; Volume 7975, pp. N1–N14. [Google Scholar] [CrossRef]
- Johnson, D. Wing loading in 15 species of North American owls. In Biology and Conservation of Owls of the Northern Hemisphere: 2nd International Symposium, Gen Tech Rep NC-190; Duncan, J.R., Johnson, D.H., Nicholls, T.H., Eds.; US Dept. of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1997; pp. 553–561. [Google Scholar]
- Norberg, U.M. Structure, form, and function of flight engineering and the living world. J. Morphol. 2002, 252, 52–81. [Google Scholar] [CrossRef] [PubMed]
- Durston, N.E.; Wan, X.; Liu, J.G.; Windsor, S.P. Avian surface reconstruction in free flight with application to flight stability analysis of a barn owl and peregrine falcon. J. Exp. Biol. 2019, 222, jeb185488. [Google Scholar] [CrossRef] [PubMed]
- Muthuramalingam, M.; Talboys, E.; Wagner, H.; Bruecker, C. Flow turning effect and laminar control by the 3D curvature of leading edge serrations from owl wing. Bioinspiration Biomim. 2021, 16, 026010. [Google Scholar] [CrossRef] [PubMed]
- König, C.; Weick, F. Owls of the World, 2nd ed.; Yale University Press: New Haven, CT, USA; London, UK, 2008. [Google Scholar]
- Uva, V.; Päckert, M.; Cibois, A.; Fumagalli, L.; Roulin, A. Comprehensive molecular phylogeny of barn owls and relatives (Family: Tytonidae) and their six major Pleistocene radiations. Mol. Phylog. Evol. 2018, 125, 127–137. [Google Scholar] [CrossRef]
- Gill, F.; Donsker, D.; Rasmussen, P. IOC World Bird List (v10.2). 2020. Available online: https://www.worldbirdnames.org/ioc-lists/crossref/ (accessed on 1 May 2022).
- Groot de, R.S. Origin, status and ecology of the owls in Galápagos. Ardea 1983, 71, 167–182. [Google Scholar]
- Marti, C.D. A long-term study of food-niche dynamics in the Common Barn Owl: Comparison within and between populations. Can. J. Zool. 1988, 66, 1803–1812. [Google Scholar] [CrossRef]
- Piedrahita, P.; Krings, M.; Nikolay, P.; Mundt, N.; Quezada, G.; Masaquiza Chango, E.; Wagner, H. Integrity of and damage to wings, feather vanes and serrations in barn owls. Zoology 2021, 147, 125930. [Google Scholar] [CrossRef]
- Norberg, U.M. Flight and scaling of flyers in nature. In WIT Transactions on State of the Art in Science and Engineering; WIT Press: Southampton, UK, 2006; Volume 3, pp. 120–154. [Google Scholar] [CrossRef]
- Stiles, F.G.; Altshuler, D.L. Conflicting terminology for wing measurements in ornithology and aerodynamics. Auk 2004, 121, 973–976. [Google Scholar] [CrossRef]
- Krüger, O. The evolution of reversed sexual size dimorphism in hawks, falcons and owls: A comparative study. Evol. Biol. 2005, 19, 467–486. [Google Scholar] [CrossRef]
- Sullivan, T.N.; Meyers, M.A.; Arzt, E. Scaling of bird wings and feathers for efficient flight. Sci. Adv. 2019, 5, eaat4269. [Google Scholar] [CrossRef]
- Tennekes, H. The Simple Science of Flight, Revised and Expanded Edition: From Insects to Jumbo Jets; MIT Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Mikkola, H. Owls of Europe; T & AD Poyser Ltd.: Calton, UK, 1983. [Google Scholar]
- Abs, M.E.; Curio, E.; Kramer, P.; Niethammer, J. Zur Ernährungsweise der Eulen auf Galápagos. Ergebnisse der Deutschen Galápagos Expedition 1962/1963. J. Ornithol. 1965, 106, 49–57. [Google Scholar] [CrossRef]
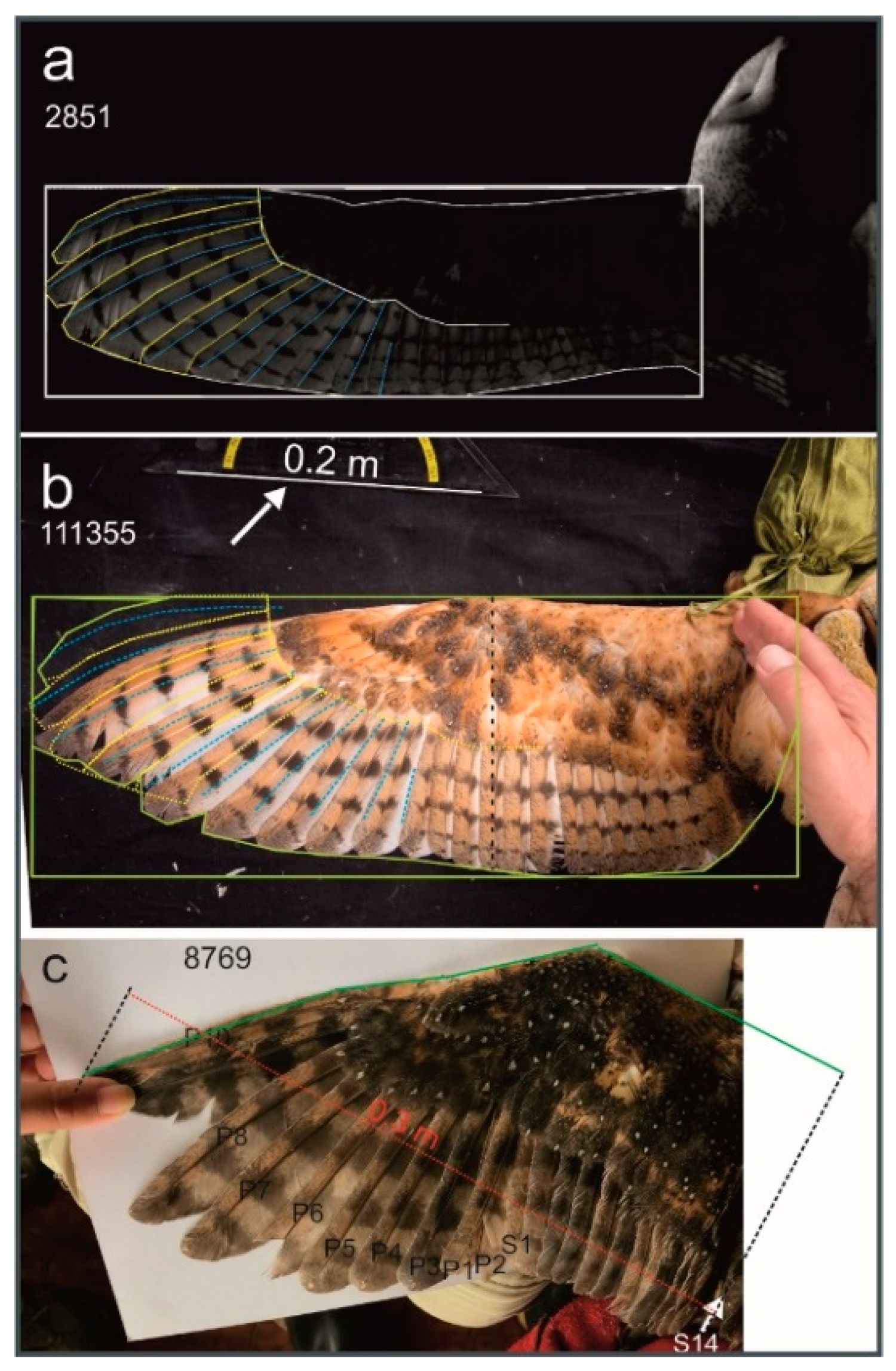
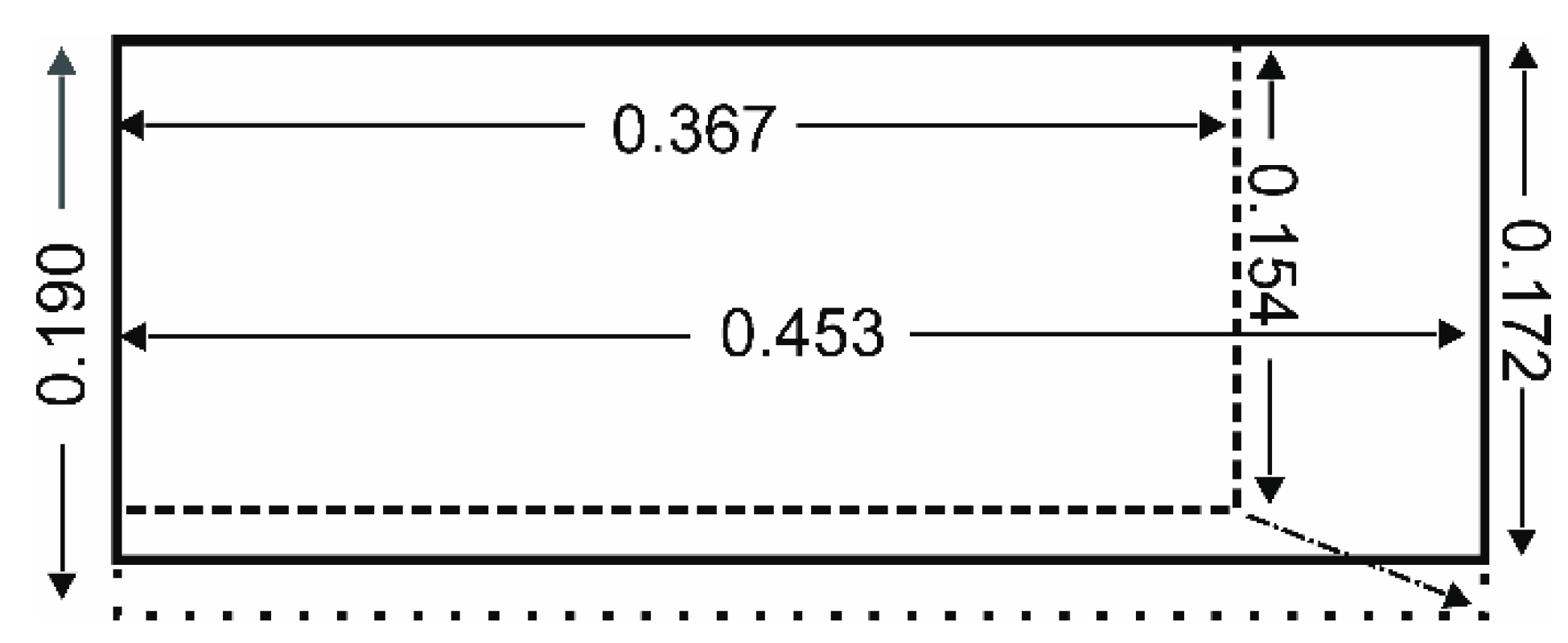
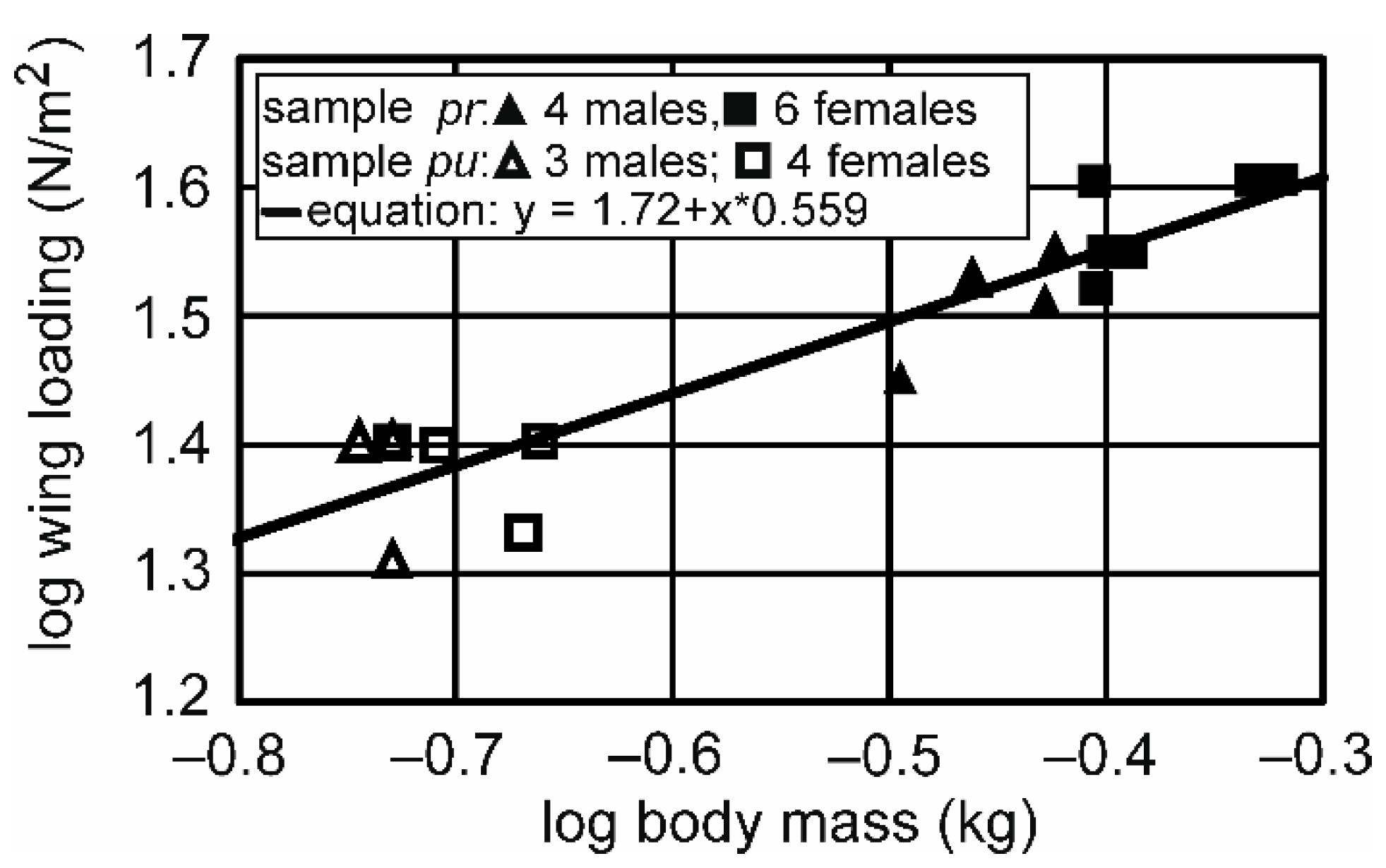
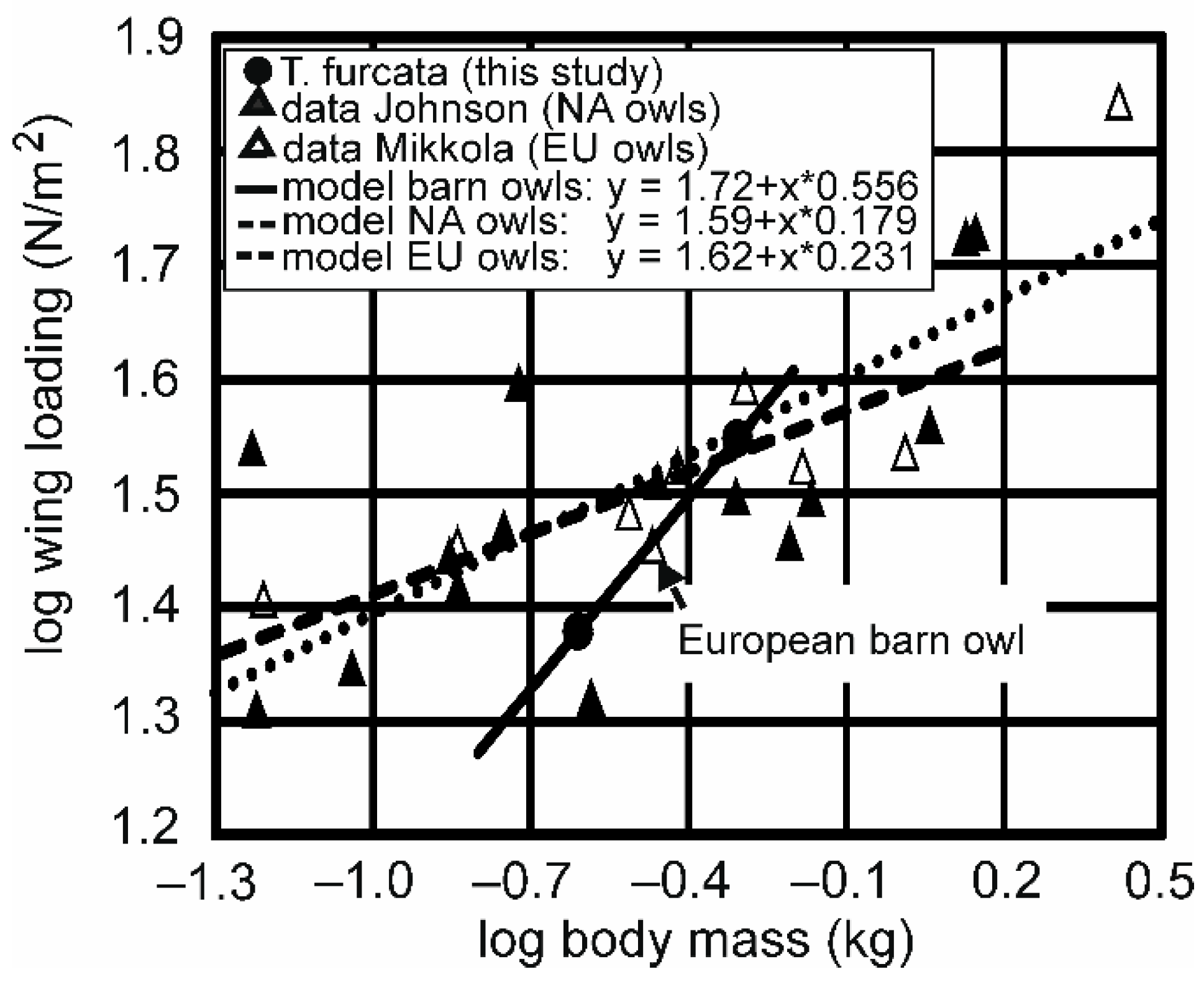
| Characteristic | Tyto furcata 1 pratincola (pr) | Tyto furcata 1 punctatissima (pu) | Factor pu/pr | Statistics 2 |
|---|---|---|---|---|
| Number of animals | 10 | 7 | ||
| Wing length (m) | 0.453 ± 0.01 | 0.367 ± 0.02 | 0.81 | U = 0; z = 3.3689; p = 0.0007456 |
| Wingspan (m) 3 | 1.01 ± 0.01 | 0.813 ± 0.05 | 0.81 | Follows wing length |
| Chord length (m) | 0.172 ± 0.01 | 0.154 ± 0.01 | 0.90 | U = 6.5; z = 2.7359; p = 0.006221 |
| Total wing area (m2) | 0.1378 ± 0.009 | 0.1015 ± 0.013 | 0.74 | U = 1; z = 3.2713; p = 0.001071 |
| Body mass (kg) | 0.498 ± 0.061 | 0.246 ± 0.019 | 0.49 | U = 0; z = 3.751; p = 0.000738 |
| Wing loading (N/m2) | 35.5 ± 3.9 | 24 ± 2.1 | 0.68 | U = 0; z = 3.731; p = 0.000743 |
| Chord/length c/l | 0.381 ± 0.01 | 0.417 ± 0.03 | 1.09 | U = 4; z = 2.9783; p = 0.002898 |
| Aspect ratio | 5.73 ± 0.1 | 5.35 ± 0.4 | 0.93 | U = 12; z = 2.1985; p = 0.0297 |
| Parameter | Slope 1 | Slope | CI 2 of Slope | CI of Slope | ||
|---|---|---|---|---|---|---|
| Predicted | Measured | 95% CI | 99% CI | |||
| lower | upper | lower | upper | |||
| Wing length | 0.333 | 0.290 | 0.222 | 0.358 | 0.196 | 0.384 |
| Chord length | 0.333 | 0.162 | 0.088 | 0.235 | 0.060 | 0.264 |
| Total area | 0.667 | 0.441 | 0.319 | 0.564 | 0.272 | 0.611 |
| Wing loading | 0.333 | 0.559 | 0.436 | 0.681 | 0.389 | 0.728 |
| Chord/length | 0 | −0.116 | −0.186 | −0.045 | −0.213 | −0.018 |
| Aspect ratio | 0 | 0.090 | 0.022 | 0.158 | −0.004 | 0.184 |
| Characteristic 1 | Male (m) | Female (f) | Factor m/f | Statistics |
|---|---|---|---|---|
| Number of animals | 4 | 6 | ||
| Wing length (m) | 0.451 ± 0.01 | 0.454 ± 0.02 | 0.99 | U = 9; z = 0.5108; p = 0.6095 |
| Wingspan (m) | 1.00 ± 0.02 | 1.01 ± 0.03 | 0.99 | follows wing length |
| Chord length (m) | 0.169 ± 0.01 | 0.174 ± 0.01 | 0.97 | U = 5; z = 1.39: p = 0.1645 |
| Total wing area (m2) Data in [12] 2 | 0.1348 ± 0.008 0.1576 | 0.1397 ± 0.010 0.1663 | 0.96 0.95 | U = 6; z = 1.1762; p = 0.2395 |
| Body mass (kg) Data in [12] 2 | 0.446 ± 0.034 0.474 | 0.533 ± 0.049 0.566 | 0.84 0.84 | U = 0; z = 2.5927; p = 0.00952 |
| Wing loading (N/m2) Data in [12] 2 | 32.5 ± 3.1 29.3 | 37.5 ± 3.2 33.4 | 0.87 0.88 | U = 5; z = 1.3943; p = 0.1632 |
| Chord/length | 0.376 ± 0.01 | 0.383 ± 0.01 | 0.98 | U = 6; z = 1.1762; p = 0.2395 |
| Aspect ratio | 5.80 ± 0.1 | 5.68 ± 0.1 | 1.02 | U = 5.5; z = 1.287; p = 0.1981 |
| Characteristic 1 | Male (m) | Female (f) | Factor m/f | Statistics |
|---|---|---|---|---|
| Number of animals | 3 | 4 | ||
| Wing length (m) | 0.364 ± 0.02 | 0.369 ± 0.03 | 0.99 | U = 5.5; z = 0; p = 1 |
| Wingspan (m) | 0.81 ± 0.05 | 0.82 ± 0.05 | 0.99 | follows wing length |
| Chord length (m) | 0.151 ± 0.01 | 0.157 ± 0.01 | 0.96 | U = 4; z = 0.4837; p = 0.6286 |
| Total wing area (m2) | 0.0973 ± 0.014 | 0.1046 ± 0.014 | 0.93 | U = 3; z = 0.8416; p = 0.4 |
| Body mass (kg) | 0.232 ± 0.005 | 0.257 ± 0.019 | 0.90 | U = 1; z = 1.651; p = 0.09873 |
| Wing loading (N/m2) | 23.7 ± 2.8 | 24.2 ± 1.9 | 0.98 | U = 5.5; z = 0; p = 1 |
| Chord/length c/l | 0.413 ± 0.02 | 0.419 ± 0.04 | 0.99 | U = 5; z = 0.18; p = 0.8571 |
| Aspect ratio | 5.39 ± 0.3 | 5.32 ± 0.4 | 1.01 | U = 5; z = 0.18; p = 0.8571 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, H.; Piedrahita, P.M. A Comparison of Aerodynamic Parameters in Two Subspecies of the American Barn Owl (Tyto furcata). Animals 2022, 12, 2532. https://doi.org/10.3390/ani12192532
Wagner H, Piedrahita PM. A Comparison of Aerodynamic Parameters in Two Subspecies of the American Barn Owl (Tyto furcata). Animals. 2022; 12(19):2532. https://doi.org/10.3390/ani12192532
Chicago/Turabian StyleWagner, Hermann, and Paolo Michael Piedrahita. 2022. "A Comparison of Aerodynamic Parameters in Two Subspecies of the American Barn Owl (Tyto furcata)" Animals 12, no. 19: 2532. https://doi.org/10.3390/ani12192532
APA StyleWagner, H., & Piedrahita, P. M. (2022). A Comparison of Aerodynamic Parameters in Two Subspecies of the American Barn Owl (Tyto furcata). Animals, 12(19), 2532. https://doi.org/10.3390/ani12192532







