1. Introduction
Traditional Chinese medicine (TCM) is used under the guidance of ancient Chinese medicinal philosophies [
1]. TCM has played a marked role in disease prevention and health improvement and has been investigated for thousands of years in China [
2,
3,
4]. With a desperate worldwide need to reduce antibiotic usage in human and veterinary medicine, research into the therapeutic effects of TCM has attracted much attention in recent years. TCM has been demonstrated to exert a therapeutic effect on various diseases, such as diabetes [
2], hypertension [
5], gastric cancer [
6], ulcerative colitis [
7], colorectal cancer [
8], etc. Knowledge about the underlying pharmacological mechanisms of TCM is still scarce.
It has been suggested that the therapeutic effect of TCM is closely related to the gut microbiota [
1,
9], which is the bridge between the body and the external environment, as there is a reciprocal link between the two. On the one hand, the improvement produced by the pharmacological activity of TCM depends on the gut microbiota [
6,
7]. The gut microbiota can promote the transformation and metabolism of TCM components by metabolizing TCM into specific molecules, such as alkaloids, flavonoids, and polysaccharides, which are easily absorbed in the intestine [
2,
6]. For example, paeoniflorin can be catalyzed into paeoniflorgenin and paeoniflorin by
Lactobacillus brevis and
Bacteroides fragilis, and puerarin is converted into daidzein by
Bifidobacterium and
E. faecalis, and aconitine can be decomposed into lipoaconitine by
Clostridium butyricum and
B. fragilis [
6]. On the other hand, when present in the digestive tract, TCM can promote the growth of probiotic bacteria and inhibit pathogens, as well as prevent bacterial transmission, thus regulating the microenvironment and maintaining the balance of the microflora [
6,
7]. For example, flavonoids, polysaccharides, and saponins in TCM serve as prebiotics that regenerates the gut microbiota.
Escherichia coli can be directly inhibited by cinnamon essential oil [
8]. The omics technologies, such as microbiomics and metabolomics, have been considered pivotal tools to help us understand the underlying mechanisms between TCM and gut microbiota.
Apparent nutrient digestibility indicates the digestibility of feed ingredients by animals and is also an important indicator used to evaluate the nutritional value of feed ingredients in diets. Previous studies had reported that dietary supplementation with TCM improved the growth performance of heat-stressed beef cattle by increasing the apparent digestibility of organic matter (OM), crude protein (CP), and acid detergent fiber (ADF) when TCM plus γ-aminobutyric acid (GABA) was used in the diet, and the apparent EE and neutral detergent fiber (NDF) digestibility also increased [
10,
11]. Furthermore, in lambs and hogs, dietary TCM increased the apparent digestibility of DM, OM, CP, and NDF [
12]. The research results cited above may suggest that TCM supplementation of the diet would be favorable for improving the apparent nutrient digestibility of feed. Similar studies on pig models have rarely been reported.
During weaning, piglets have to face a number of challenges that are critical and stressful [
13]. On the one hand, to accelerate the pace of banning the use of antibiotic growth promoters (AGPs) in China, the addition of TCM to the feed has been considered a substitute for antibiotics as a feed additive [
14]. On the other hand, physiological characteristics after weaning indicate that the weaned piglets may have unique intestinal microflora [
15]. Thus, at this particular stage, studies on the effect of dietary TCM on the gut microbiota and the apparent nutrient digestibility of weaned piglets have an important scientific value. Studies unraveling the relationship between the two related research strands have not been reported so far.
The present study was conducted in two stages. First, TCMs with potent antibacterial properties were selected for further application in the production of fermented feed. Second, the growth performance, apparent nutrient digestibility, and fecal microbiota of weaned piglets were investigated. This study focused on the correlation between gut microbial communities and apparent nutrient digestibility in piglets.
4. Discussion
With the implementation of the policy to remove AGPs in animal production in China, natural plants and their application as AGP substitutes have gained increasing interest in the research community because of their safety, efficiency, and availability [
38]. TCMs are considered better alternatives for improving animal health and resisting infectious diseases.
Fructus mume (“wumei” in Chinese) has long been used in China to treat chronic coughs, expectoration, ulceration, chronic diarrhea, and gastrointestinal diseases [
39,
40,
41,
42,
43,
44]. This medicinal effect is due to its antioxidant [
44], antibacterial [
45], and anti-inflammatory properties [
43,
44], and its protective ability against gastrointestinal diseases via the opsonization of intestinal commensal bacteria, as well as its ability to alleviate epithelial injury and inflammation [
39].
Scutellaria baicalensis Georgi (“huangqin” in Chinese) is also an old and well-known component of TCM and is widely used for the treatment of bronchitis, hepatitis, tumors, and inflammatory diseases [
46,
47,
48,
49,
50]. Numerous research studies have indicated that the therapeutic effects of
Scutellaria baicalensis Georgi are due to its various pharmacological activities, including its antiangiogenic, anti-inflammatory, antimicrobial, immunoenhancing, and antioxidative effects [
51,
52,
53,
54]. Very little was found in the literature on the effects of dietary TCM in weaned piglets. Our study systematically investigated the data and aimed to ascertain the effects of these two TCM feed additives on the growth performance, apparent nutrient digestibility, and fecal microbiota of weaned piglets.
Prior studies have reported that fermented feed significantly increases the body weight and ADG of piglets [
55], laying hen chicks [
56], and geese [
57]. Several reports have shown that TCM additives significantly improved the final BW, ADG, and FCR in lambs and hogs [
12] and promoted growth performance in heat-stressed beef cattle, which was associated with better physiological status [
11]. These findings are contrary to a previous study showing that dietary TCM led to greater feed intake but no significant differences in the final BW, ADG, or F:G ratio [
58]. There were few reports on the effects of fermentation with TCM mixtures on the growth performance of weaned piglets. In our study, dietary supplementation with
Fructus mume and
Scutellaria baicalensis Georgi, fermented or not fermented, led to no significant differences in ADFI during the experiment period. The findings of the current study do not support previous research where the authors suggested that the inclusion of supplemental TCMs may improve pigs’ appetite [
58]. The results of this study showed that the final BW and ADG increased, while the F:G ratio and diarrhea rate decreased in weaned piglets in the TCM and F-TCM groups, suggesting that
Fructus mume and
Scutellaria baicalensis Georgi supplementation in the diet improves growth performance, leading to greater weight gain and improved health [
10,
11,
12]. However, fermentation with
Fructus mume and
Scutellaria baicalensis Georgi in the diet had no significant effect on the growth performance of piglets.
One interesting finding in our study was observed in the TCM and F-TCM groups, in which ADG was increased while ADFI was not significantly changed; considerably more work will need to be carried out to determine apparent nutrient digestibility in piglets. Previous studies have explored whether dietary TCM increases the apparent digestibility of CP in finishing pigs [
59] and CP, Ca, P, and NDF in weaned piglets [
60]. Dietary TCM has also been suggested to improve the apparent digestibility of OM, CP, ADF, Ca, and P in beef cattle, even under heat stress [
10,
11]. However, the findings of the current study do not support the previous research; as shown in
Table 6, there was no significant difference in the apparent digestibility of CP or CF among the three groups. In addition, the partial substitution of fermented feed in the diet increased the apparent digestibility of CP and CF in growing-finishing pigs [
61]; CP and EE in Xuefeng black-boned chickens [
62]; and DM, CP, CF, NDF, and ADF in lactating dairy cows [
63]. Data on fermented TCM feed are lacking; in our study, feed fermented with TCM increased the apparent digestibility of DM and EE compared with the non-fermented group (TCM group), suggesting that fermented feed with
Fructus mume and
Scutellaria baicalensis Georgi mixture increased the digestibility of DM and EE in weaned piglets.
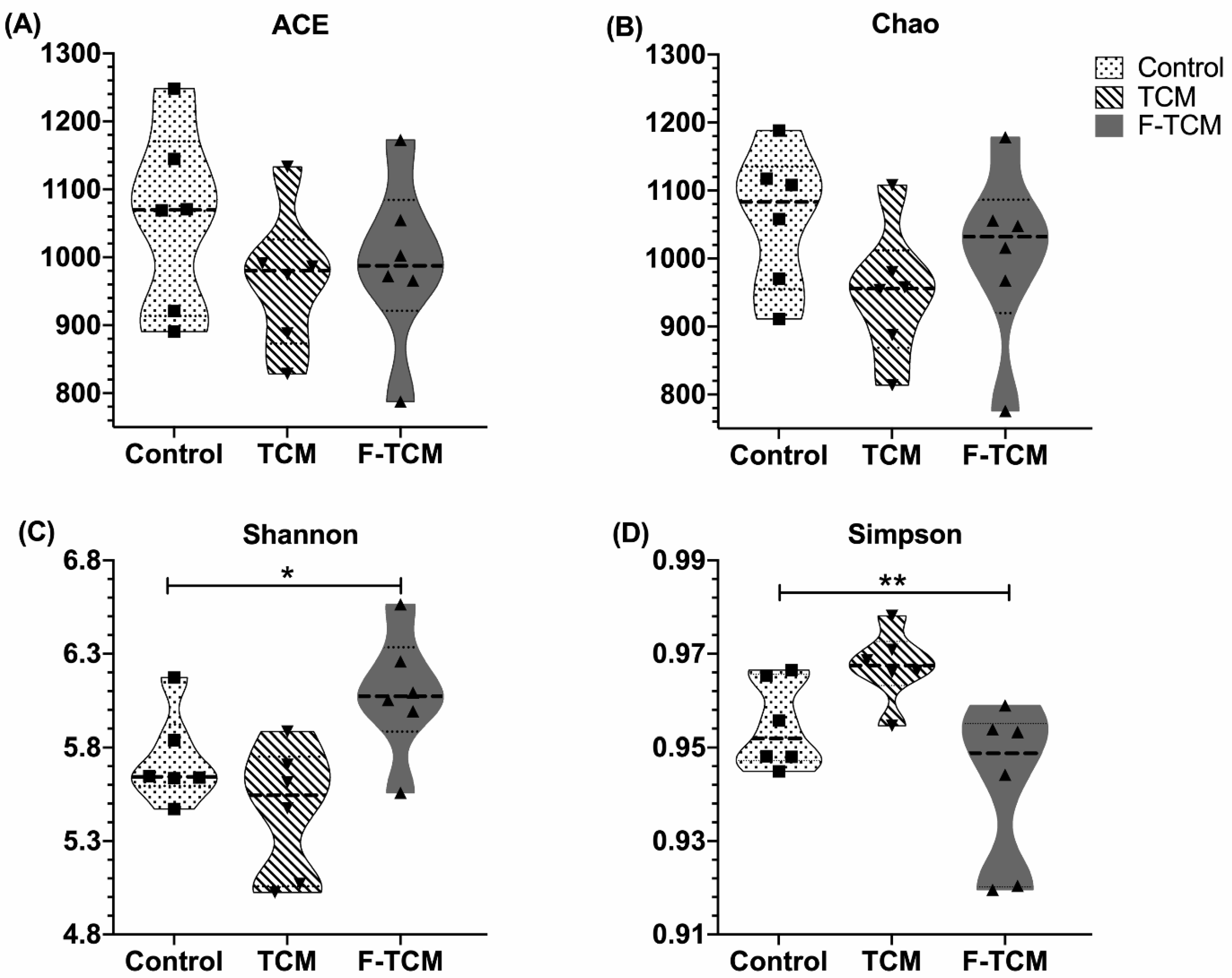
Recently, studies have found that the gut microbiota may explain the therapeutic effects of TCM [
6,
64]. An increasing number of studies have investigated the interactions between the gut microbiota and TCM, suggesting that the gut microbiota can directly affect the absorption, metabolism, and pharmacological activity of TCM [
2,
3,
65]. In our study, the composition of fecal microbiota in weaned piglets was analyzed. Dietary
Fructus mume and
Scutellaria baicalensis Georgi decreased the diversity but did not have a significant effect on the richness of fecal microbiota; this finding is inconsistent with that of Zou (2021), who reported that a Huangqin decoction (HQD) could increase the diversity of the intestinal microbiota of cholestatic mice [
66]. This inconsistency may be due to the different species. The current study also found that feed fermented with these two TCMs improved the diversity of fecal microbiota in piglets. Previous results suggested that eight Chinese herbs (Chinese name: “jian ji san”) fermented with
Zygosaccharomyces rouxii and their fermentation products increased the diversity of the foregut microbial community of broiler chickens [
67], which is in agreement with our results. According to these data, we can infer that dietary
Fructus mume and
Scutellaria baicalensis Georgi affected the fecal microbial composition by altering its diversity in weaned piglets.
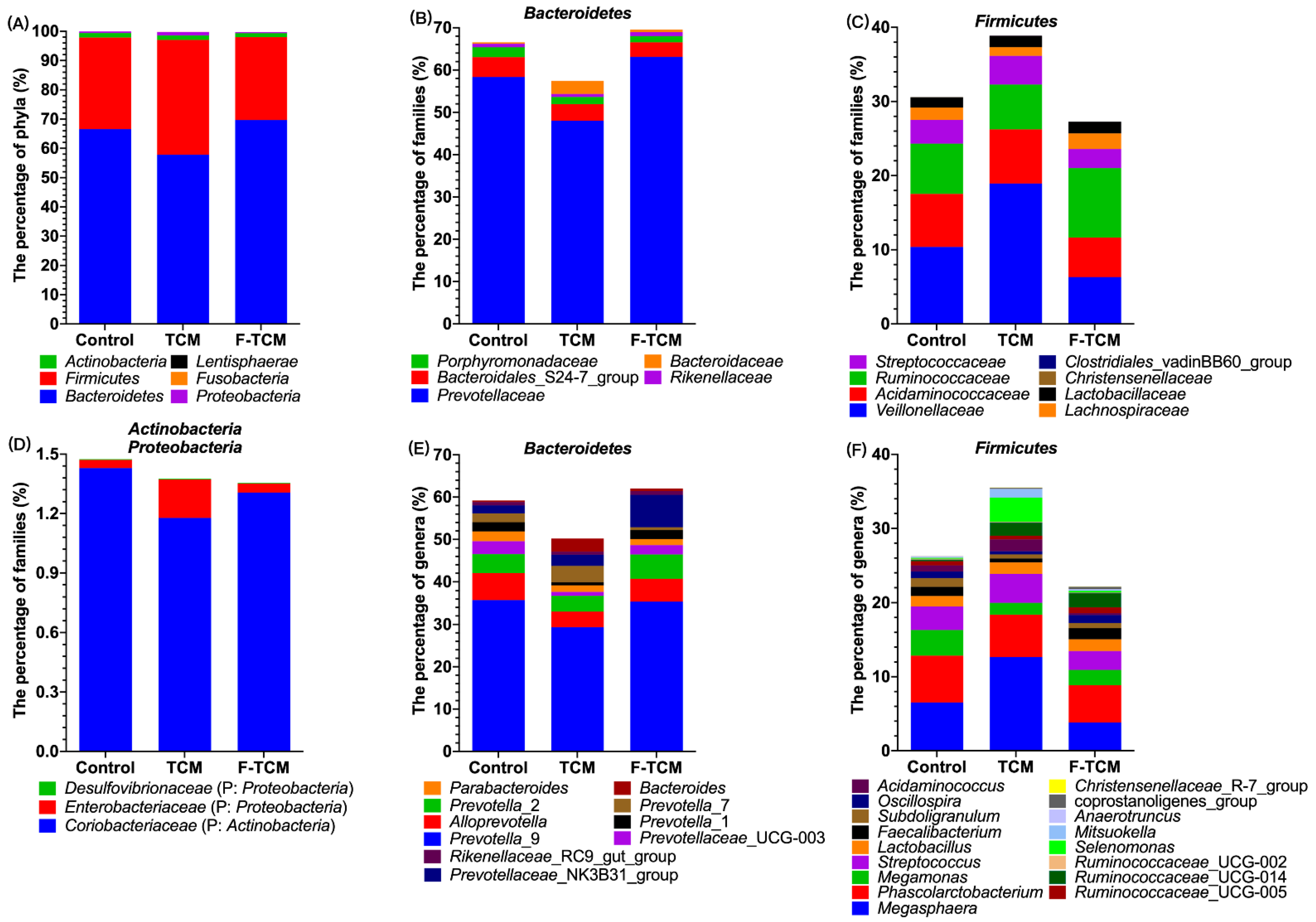
In our study,
Bacteroidetes and
Firmicutes were the predominant phyla in the fecal microbiota of piglets, similar to the findings of previous studies [
28,
68,
69]. As mentioned in the literature review, the gut microbial mediation of the potential therapeutic mechanism of TCMs can be attributed to the production of short-chain fatty acids (SCFAs) [
67,
70], mostly acetic, propionic, and butyric acids, which play an important role in maintaining the intestinal health of pigs [
70,
71]. In
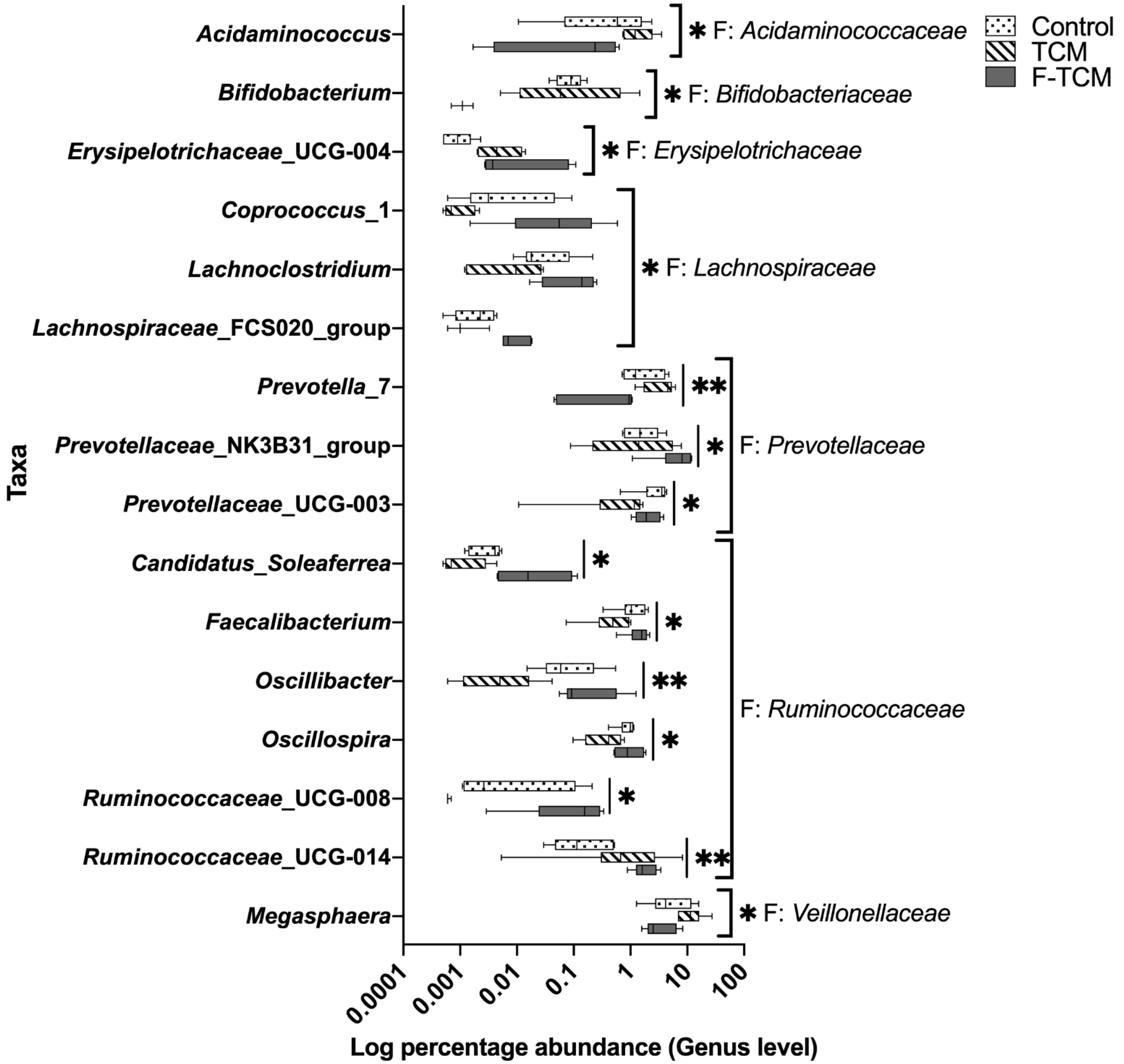
Figure 4, there is a clear trend of the increased relative abundance of
Acidaminococcus,
Prevotella, and
Megasphaera in the TCM group and a decreased abundance in the F-TCM group; all three genera are considered to be SCFA-producing bacteria [
70,
72], suggesting that the diet supplemented with
Fructus mume and
Scutellaria baicalensis Georgi increased the abundance of SCFA-producing bacteria in the hindgut, which may have promoted the intestinal health of the weaned piglets. However, the prefermentation of these two TCMs, dietary fibers, and other indigestible carbohydrates led to their degradation before being fed to the piglets [
70], explaining the decreasing tendency of apparent CF digestibility and the abundance of
Acidaminococcus,
Prevotella, and
Megasphaera in the F-TCM group. Another important finding in the current study was that the relative abundance of
Coprococcus,
Lachnospiraceae_FCS020 group, and
Oscillibacter were increased in the F-TCM group; these genera have been identified as the most active and healthy microbiome constituents in the intestinal environment in healthy adult humans and animals [
28,
73,
74,
75]. Thus, these findings suggest that fermentation with
Fructus mume and
Scutellaria baicalensis Georgi results in the improved healthy intestinal flora, which, in turn, could favor the intestinal health of weaned piglets.
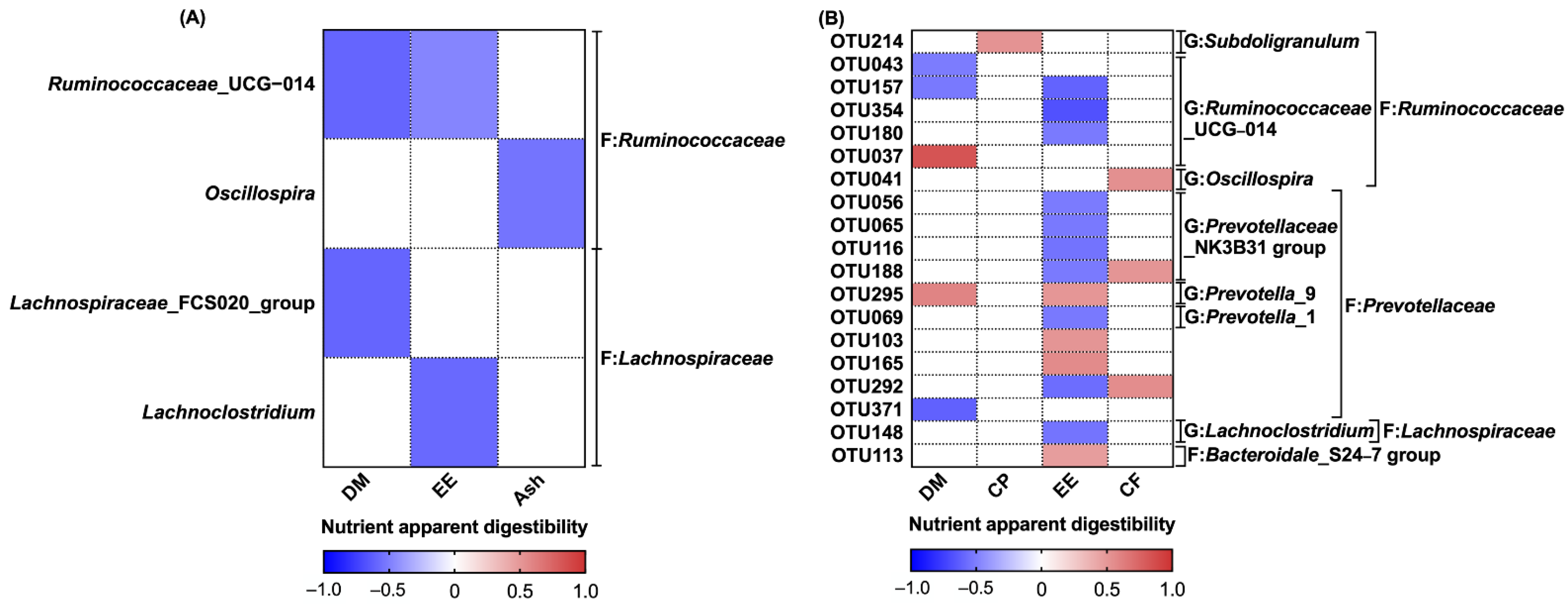
Another important objective of this study was to investigate the relationship between the gut microbial characteristics and the apparent nutrient digestibility in weaned piglets, so the correlation between the apparent nutrient digestibility and significant microbial genera and OTUs were further analyzed. Significant correlations between microbial diversity and apparent EE digestibility were observed, suggesting a potential link between changes in the intestinal flora and apparent EE digestibility. At the OTU level, the relative abundance of 10 OTUs that increased in the F-TCM group was positively correlated with apparent EE digestibility, while four OTUs for which the abundance was decreased in the F-TCM group were negatively correlated with apparent EE digestibility. Within these OTUs, most belong to the families
Ruminococcaceae and
Prevotellaceae, which are considered to be the core bacteria detected in 99% of fecal samples obtained from commercial swine worldwide [
76]. Prior studies have shown that the abundance of
Ruminococcaceae is negatively correlated with high-fat-diet (HFD)-induced obesity in a ripened pu-erh tea extract (PETe) intervention in mice [
77], while the relative abundance of
Prevotellaceae and
Prevotellaceae_NK3B31_group was observed to increase through supplementation with pea seed coats (PSCs) and stachyose in mice and rats fed an HFD [
78,
79]. According to these findings, we can infer that the digestion and absorption of nutritional lipids in the diet were closely related to the changes in the
Ruminococcaceae and
Prevotellaceae families. Our correlation results are consistent with previous studies focusing on the gut microbiota and the apparent nutrient digestibility of grower pigs and sows [
15,
80] and support the possible relationship between the gut microbiota and the regulation of dietary nutrient utilization in piglets.