Antibodies against Two Testudinid Herpesviruses in Pet Tortoises in Europe
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marschang, R.E.; Origgi, F.C.; Stenglein, M.D.; Hyndman, T.H.; Wellehan, J.F.X.; Jacobson, E.R. Viruses and viral diseases of reptiles. In Infectious Diseases and Pathology of Reptiles, Color Atlas and Text, 2nd ed.; Jacobson, E.R., Garner, M.M., Eds.; CRC Press: Boca Raton, LA, USA, 2021; Volume 1, pp. 575–703. [Google Scholar]
- Une, Y.; Uemura, K.; Nakano, Y.; Kamiie, J.; Ishibashi, T.; Nomura, Y. Herpesvirus infection in tortoises (Malacochersus tornieri and Testudo horsfieldii). Vet. Pathol. 1999, 36, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, E.; Obiegala, A.; Marschang, R.E. Detection of Mycoplasma spp., herpesviruses, topiviruses, and ferlaviruses in samples from chelonians in Europe. J. Vet. Diagn. Investig. 2017, 29, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Leineweber, C.; Müller, E.; Marschang, R.E. Herpesviruses in captive chelonians in Europe between 2016 and 2020. Front. Vet. Sci. 2021, 8, 733299. [Google Scholar] [CrossRef] [PubMed]
- Schüler, L.; Picquet, P.; Leineweber, C.; Dietz, J.; Müller, E.; Marschang, R.E. A testudinid herpesvirus 1 (TeHV1)-associated disease outbreak in a group of Horsfield’s tortoises (Testudo horsfieldii). Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2021, 49, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Pessier, A.P.; Wellehan, J.F.X.; Brown, R.; Jacobson, E.R. Identification of a novel herpesvirus from a California desert tortoise (Gopherus agassizii). J. Vet. Microbiol. 2005, 111, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bicknese, E.J.; Childress, A.L.; Wellehan, J.F., Jr. A novel herpesvirus of the proposed genus Chelonivirus from an asymptomatic bowsprit tortoise (Chersina angulata). J. Zoo Wildl. Med. 2010, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, E.; Mittenzwei, F.; Marschang, R.E. Detection of testudinid herpesvirus type 4 in a leopard tortoise (Stigmochelys pardalis). Tierärztl. Prax. Kleintiere 2016, 44, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Gatherer, D.; ICTV Herpesvirales Study Group. 18 New Species in the Family Herpesvirdae. 2018. Available online: https://talk.ictvonline.org//taxonomy/p/taxonomyhistory?taxnode_id=201856398 (accessed on 12 September 2019).
- Marschang, R.E.; Gravendyck, M.; Kaleta, E.F. Herpesviruses in tortoises: Investigations into virus isolation and the treatment of viral stomatitis in Testudo hermanni and T. graeca. J. Vet. Med. B 1997, 44, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Schneider, R.M. Antibodies against viruses in wild-caught spur-thighed tortoises (Testudo graeca) in Turkey. Vet. Rec. 2007, 161, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Milde, K.; Bellavista, M. Virus isolation and vaccination of Mediterranean tortoises against a chelonid herpesvirus in a chronically infected population in Italy. Dtsch. Tierarztl. Wochenschr. 2001, 108, 376–379. [Google Scholar] [PubMed]
- Origgi, F.C.; Klein, P.A.; Mathes, K.; Blahak, S.; Marschang, R.E.; Tucker, S.J.; Jacobson, E.R. An enzyme linked immunosorbent assay (ELISA) for detecting herpesvirus exposure in Mediterranean tortoises. J. Clin. Microbiol. 2001, 39, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Frost, J.W.; Gravendyck, M.; Kaleta, E.F. Comparison of 16 chelonid herpesviruses by virus neutralization tests and restriction endonuclease digestion of viral DNA. J. Vet. Med. B 2001, 48, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.R.; Berry, K.H.; Wellehan, J.F.X.; Origgi, F.; Childress, A.L.; Braun, J.; Schrenzel, M.; Yee, J.; Rideout, B. Serologic and molecular evidence for Testudinid herpesvirus 2 infection in wild Agassiz’s desert tortoises, Gopherus agassizii. J. Wildl. Dis. 2012, 48, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Gandar, F.; Wilkie, G.S.; Gatherer, D.; Kerr, K.; Marlier, D.; Diez, M.; Marschang, R.E.; Mast, J.; Dewals, B.G.; Davison, A.J.; et al. The genome of a tortoise herpesvirus (Testudinid herpesvirus 3) has a novel structure and contains a large region that is not required for replication in vitro or virulence in vivo. J. Virol. 2015, 89, 11438–11456. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Marenzoni, M.L.; Santoni, L.; Felici, A.; Maresca, C.; Stefanetti, V.; Sforna, M.; Franciosini, M.P.; Proietti, P.C.; Origgi, F.C. Clinical, virological and epidemiological characterization of an outbreak of Testudinid herpesvirus 3 in a chelonian captive breeding facility: Lessons learned and first evidence of TeHV3 vertical transmission. PLoS ONE 2018, 13, e0197169. [Google Scholar]
- Martel, A.; Blahak, S.; Vissenaekens, H.; Pasmans, F. Reintroduction of clinically healthy tortoises: The herpesvirus Torjan horse. J. Wildl. Dis. 2009, 45, 218–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stöhr, A.C.; Marschang, R.E. Detection of a tortoise herpesvirus type 1 in a Hermann’s tortoise (Testudo hermanni boettgeri) in Germany. J. Herpetol. Med. Surg. 2010, 20, 61–63. [Google Scholar] [CrossRef]
- Marschang, R.E. Viruses infecting reptiles. Viruses 2011, 3, 2087–2126. [Google Scholar] [CrossRef] [PubMed]
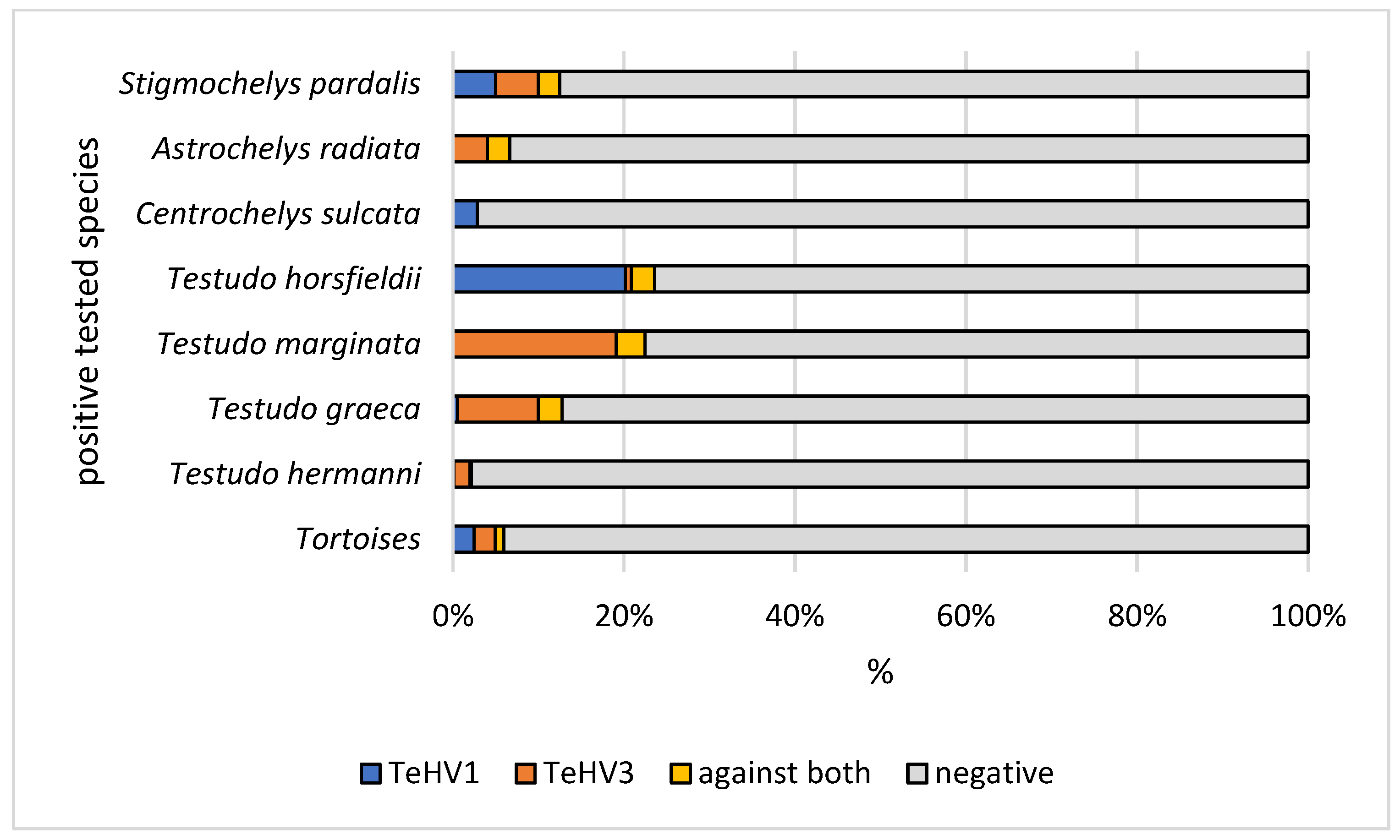
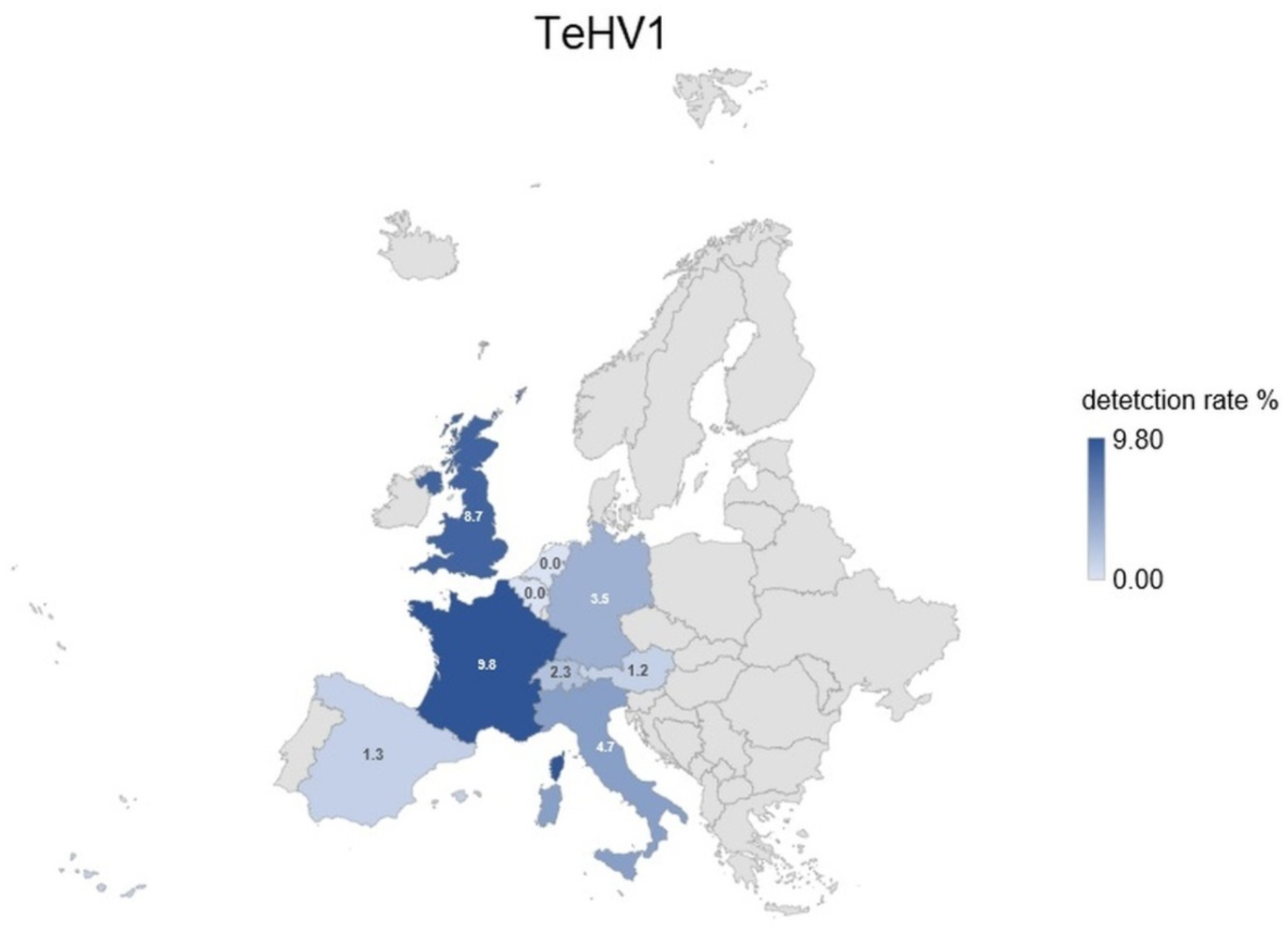
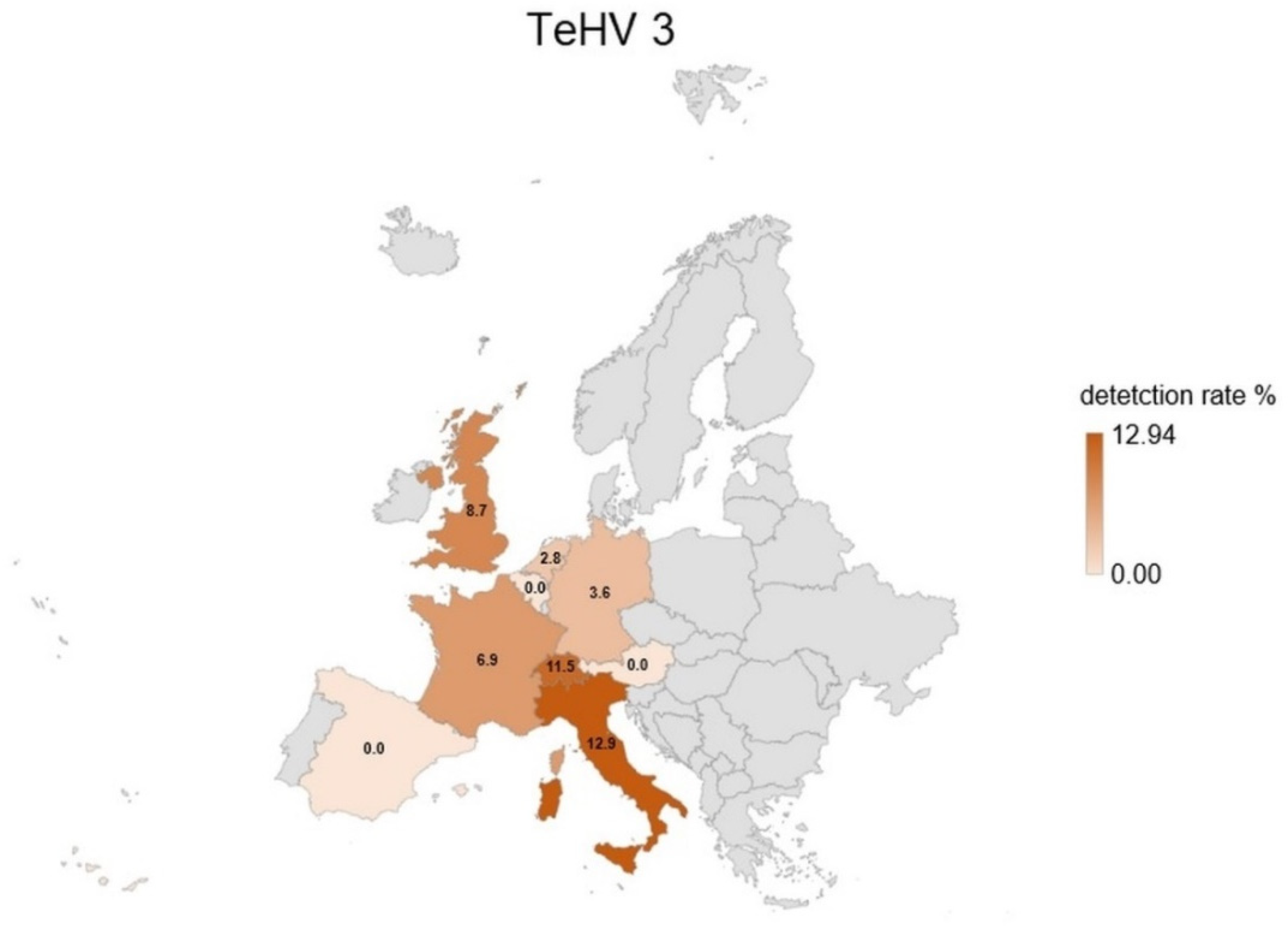
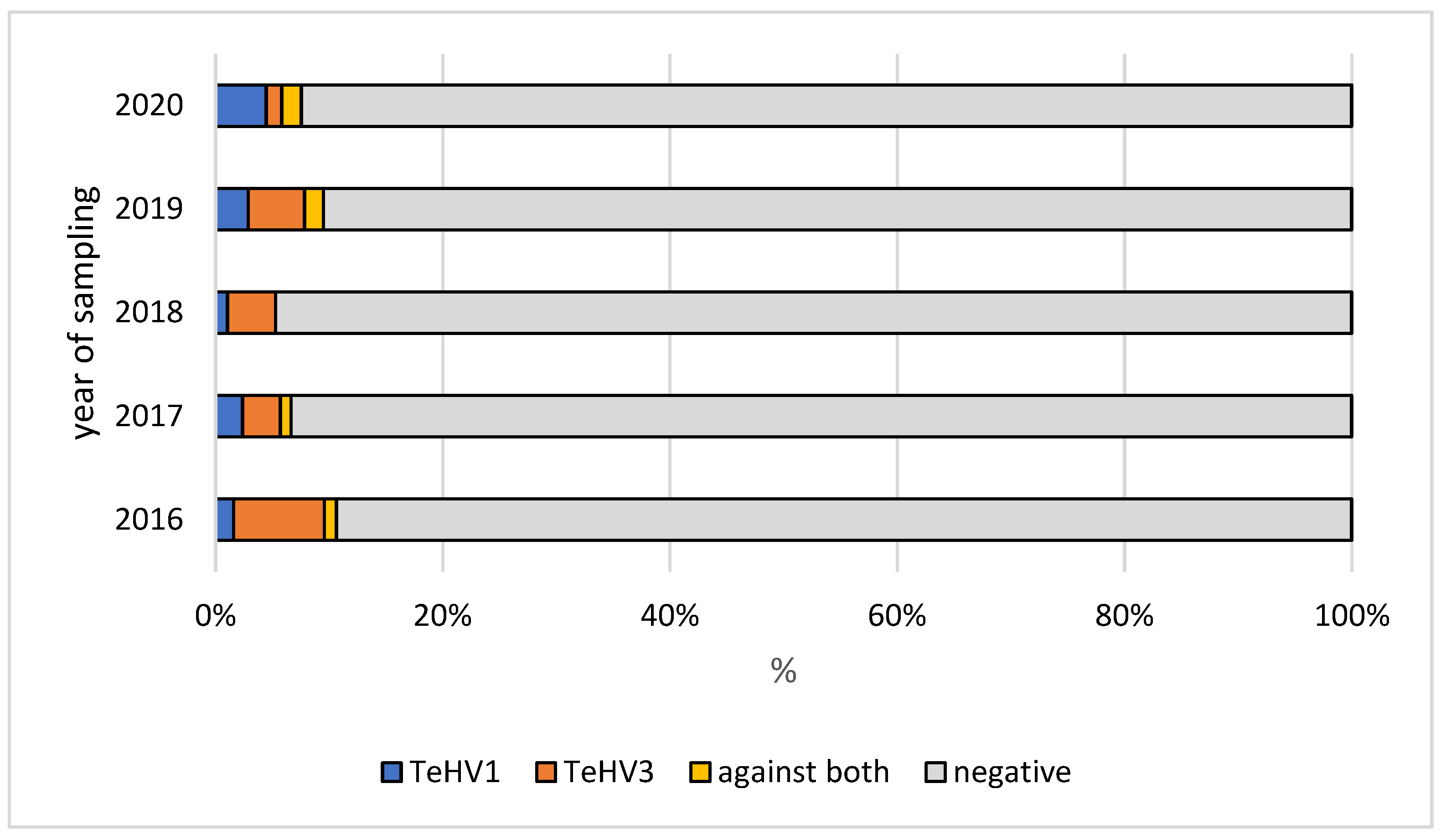

| Species | Total Tested | TeHV1 Antibodies | TeHV3 Antibodies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | |||
| Tortoise, species unknown | 285 | n | 275 | 3 | 3 | 4 | 275 | 1 | 5 | 4 |
| % | 96.50 | 1.05 | 1.05 | 1.40 | 96.50 | 0.35 | 1.75 | 1.40 | ||
| CI | 93.66–98.08 | 0.36–3.04 | 0.36–3.04 | 0.55–3.55 | 93.66–98.08 | 0.06–1.96 | 0.75–4.03 | 0.55–3.55 | ||
| Testudo hermanni | 797 | n | 795 | 2 | 0 | 0 | 781 | 10 | 2 | 4 |
| % | 99.75 | 0.25 | 0 | 0 | 98.00 | 1.25 | 0.25 | 0.50 | ||
| CI | 99.09–99.93 | 0.07–0.91 | 0–0.48 | 0–0.48 | 96.76–98.76 | 0.68–2.29 | 0.07–0.91 | 0.19–1.28 | ||
| Testudo graeca | 180 | n | 174 | 3 | 1 | 2 | 158 | 9 | 3 | 10 |
| % | 96.66 | 1.67 | 0.56 | 1.11 | 87.77 | 5.00 | 1.67 | 5.56 | ||
| CI | 92.92–98.47 | 0.57–4.79 | 0.10–3.09 | 0.30–3.96 | 82.19–91.79 | 2.65–9.23 | 0.57–4.79 | 3.05–9.93 | ||
| Testudo marginata | 89 | n | 86 | 3 | 0 | 0 | 69 | 11 | 1 | 8 |
| % | 96.63 | 3.37 | 0 | 0 | 77.53 | 12.36 | 1.12 | 8.99 | ||
| CI | 90.55–98.85 | 1.15–9.45 | 0–4.14 | 0–4.14 | 67.82–84.96 | 7.04–20.79 | 0.02–6.09 | 4.63–16.75 | ||
| Testudo horsfieldii | 144 | n | 111 | 11 | 6 | 16 | 139 | 3 | 1 | 1 |
| % | 77.08 | 7.64 | 4.17 | 11.11 | 96.54 | 2.08 | 0.69 | 0.69 | ||
| CI | 69.56–8.19 | 4.32–13.16 | 1.93–8.80 | 6.96–17.29 | 92.13–98.51 | 0.71–5.94 | 0.12–3.82 | 0.12–3.82 | ||
| Testudo kleinmanni | 5 | n | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 56.55–100 | 0–43.45 | 0–43.45 | 0–43.45 | 56.55–100 | 0–43.45 | 0–43.45 | 0–43.45 | ||
| Centrochelys sulcata | 35 | n | 34 | 1 | 0 | 0 | 35 | 0 | 0 | 0 |
| % | 97.14 | 2.86 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 85.46–99.49 | 0.51–14.54 | 0–9.89 | 0–9.89 | 90.11–100 | 0–9.89 | 0–9.89 | 0–9.89 | ||
| Astrochelys radiata | 75 | n | 73 | 1 | 0 | 1 | 70 | 3 | 1 | 1 |
| % | 97.34 | 1.33 | 0 | 1.33 | 93.34 | 4.00 | 1.33 | 1.33 | ||
| CI | 90.78–99.26 | 0.23–7.17 | 0–4.87 | 0.23–7.17 | 85.32–97.12 | 1.37–11.11 | 0.23–7.17 | 0.23–7.17 | ||
| Stigmochelys pardalis | 40 | n | 37 | 0 | 1 | 2 | 37 | 0 | 1 | 2 |
| % | 92.50 | 0 | 2.50 | 5.00 | 92.50 | 0 | 2.50 | 5.00 | ||
| CI | 80.14–97.42 | 0–8.76 | 0.44–12.88 | 1.38–16.50 | 80.14–97.42 | 0–8.76 | 0.44–12.88 | 1.38–16.50 | ||
| Aldabrachelys gigantea | 12 | n | 12 | 0 | 0 | 0 | 12 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 75.75–100 | 0–24.25 | 0–24.25 | 0–24.25 | 75.75–100 | 0–24.25 | 0–24.25 | 0–24.25 | ||
| Chelonoidis nigra | 2 | n | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | ||
| Chelonoidis carbonarius | 14 | n | 14 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 78.47–100 | 0–21.53 | 0–21.53 | 0–21.53 | 78.47–100 | 0–21.53 | 0–21.53 | 0–21.53 | ||
| Chelonoidis denticulatus | 3 | n | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 43.85–100 | 0–56.15 | 0–56.15 | 0–56.15 | 43.85–100 | 0–56.15 | 0–56.15 | 0–56.15 | ||
| Geochelone elegans | 9 | n | 9 | 0 | 0 | 0 | 9 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 70.09–100 | 0–29.91 | 0–29.91 | 0–29.91 | 70.09–100 | 0–29.91 | 0–29.91 | 0–29.91 | ||
| Geochelone platynota | 7 | n | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 64.57–100 | 0–35.43 | 0–35.43 | 0–35.43 | 64.57–100 | 0–35.43 | 0–35.43 | 0–35.43 | ||
| Astrochelys yniphora | 5 | n | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 56.55–100 | 0–43.45 | 0–43.45 | 0–43.45 | 56.55–100 | 0–43.45 | 0–43.45 | 0–43.45 | ||
| Kinixys sp. | 3 | n | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 43.85–100 | 0–56.15 | 0–56.15 | 0–56.15 | 43.85–100 | 0–56.15 | 0–56.15 | 0–56.15 | ||
| Indotestudo elongata | 4 | n | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 51.01–100 | 0–48.99 | 0–48.99 | 0–48.99 | 51.01–100 | 0–48.99 | 0–48.99 | 0–48.99 | ||
| Gopherus berlandieri | 1 | n | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | ||
| Malacochersus tornieri | 6 | n | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 60.97–100 | 0–39.03 | 0–39.03 | 0–39.03 | 60.97–100 | 0–39.03 | 0–39.03 | 0–39.03 | ||
| Homopus sp. | 1 | n | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | ||
| Manouria sp. | 1 | n | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | 20.65–100 | 0–79.35 | 0–79.35 | 0–79.35 | ||
| Pyxis sp. | 6 | n | 6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 60.97–100 | 0–39.03 | 0–39.03 | 0–39.03 | 60.97–100 | 0–39.03 | 0–39.03 | 0–39.03 | ||
| Chersina angulata | 2 | n | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | ||
| Psammobates sp. | 2 | n | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| % | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | ||
| CI | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | 34.24–100 | 0–65.76 | 0–65.76 | 0–65.76 | ||
| Total | 1728 | n | 1668 | 24 | 11 | 25 | 1647 | 37 | 14 | 30 |
| % | 96.53 | 1.39 | 0.64 | 1.45 | 95.31 | 2.14 | 0.81 | 1.74 | ||
| CI | 95.56–97.29 | 0.94–2.06 | 0.36–1.14 | 0.98–2.13 | 94.21–96.21 | 1.56–2.94 | 0.48–1.36 | 1.22–2.47 | ||
| Year | Total Tested | TeHV1 Antibodies | TeHV3 Antibodies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | |||
| 2016 | 305 | n | 299 | 2 | 1 | 3 | 285 | 5 | 5 | 10 |
| % | 98.03 | 0.66 | 0.33 | 0.98 | 93.44 | 1.64 | 1.64 | 3.28 | ||
| CI | 95.77–99.09 | 0.18–2.37 | 0.06–1.84 | 0.33–2.85 | 90.09–95.71 | 0.70–3.78 | 0.70–3.78 | 1.79–5.93 | ||
| 2017 | 303 | n | 292 | 4 | 3 | 4 | 290 | 6 | 2 | 5 |
| % | 96.37 | 1.32 | 0.99 | 1.32 | 95.71 | 1.98 | 0.66 | 1.65 | ||
| CI | 93.62–97.96 | 0.51–3.34 | 0.34–2.87 | 0.51–3.34 | 92.80–97.48 | 0.91–4.25 | 0.18–2.70 | 0.71–3.80 | ||
| 2018 | 381 | n | 377 | 1 | 1 | 2 | 369 | 6 | 0 | 6 |
| % | 98.96 | 0.26 | 0.26 | 0.52 | 96.86 | 1.57 | 0.0 | 1.57 | ||
| CI | 97.33–99.59 | 0.05–1.47 | 0.05–1.47 | 0.14–1.89 | 94.58–98.19 | 0.72–3.39 | 0–1.00 | 0.72–3.39 | ||
| 2019 | 350 | n | 331 | 9 | 4 | 6 | 326 | 13 | 5 | 6 |
| % | 94.58 | 2.57 | 1.14 | 1.71 | 93.15 | 3.71 | 1.43 | 1.71 | ||
| CI | 91.68–96.50 | 1.36–4.81 | 0.44–2.90 | 0.79–3.68 | 90.00–95.35 | 2.18–6.24 | 0.61–3.30 | 0.79–3.68 | ||
| 2020 | 389 | n | 369 | 8 | 2 | 10 | 377 | 7 | 2 | 3 |
| % | 94.86 | 2.06 | 0.51 | 2.57 | 96.92 | 1.80 | 0.51 | 0.77 | ||
| CI | 92.19–96.65 | 1.05–4.01 | 0.14–1.85 | 1.40–4.67 | 94.69–98.23 | 0.87–3.67 | 0.14–1.85 | 0.26–2.24 | ||
| Total | 1728 | 1668 | 24 | 11 | 25 | 1647 | 37 | 14 | 30 | |
| Season | Total Tested | TeHV1 Antibodies | TeHV3 Antibodies | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | Titer < 2 | Titer 2 to 4 | Titer 8 | Titer ≥ 16 | |||
| Spring | 529 | n | 515 | 5 | 5 | 4 | 505 | 9 | 6 | 9 |
| % | 97.34 | 0.95 | 0.95 | 0.76 | 95.47 | 1.70 | 1.13 | 1.70 | ||
| CI | 95.60–98.41 | 0.41–2.20 | 0.41–2.20 | 0.30–1.93 | 93.33–96.93 | 0.90–3.20 | 0.52–2.45 | 0.90–3.20 | ||
| Summer | 680 | n | 642 | 17 | 6 | 15 | 642 | 17 | 5 | 16 |
| % | 94.41 | 2.50 | 0.88 | 2.21 | 94.41 | 2.50 | 0.74 | 2.35 | ||
| CI | 92.42–95.90 | 1.57–3.97 | 0.40–1.91 | 1.34–3.61 | 92.42–95. 90 | 1.57–3.97 | 0.32–1.72 | 1.45–3.78 | ||
| Fall | 402 | n | 397 | 2 | 0 | 3 | 384 | 11 | 3 | 4 |
| % | 98.75 | 0.50 | 0 | 0.75 | 95.51 | 2.74 | 0.75 | 1.00 | ||
| CI | 97.13–99.47 | 0.14–1.80 | 0–0.95 | 0.26–2.18 | 93.03–97.15 | 1.54–4.84 | 0.26–2.18 | 0.39–2.54 | ||
| Winter | 117 | n | 114 | 0 | 0 | 3 | 116 | 0 | 0 | 1 |
| % | 97.44 | 0 | 0 | 2.56 | 99.15 | 0 | 0 | 0.85 | ||
| CI | 92.74–99.13 | 0–3.18 | 0–3.18 | 0.87–7.26 | 95.32–99.85 | 0–3.18 | 0–3.18 | 0.15–4.68 | ||
| Total | 1728 | 1668 | 24 | 11 | 25 | 1647 | 37 | 14 | 30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leineweber, C.; Marschang, R.E. Antibodies against Two Testudinid Herpesviruses in Pet Tortoises in Europe. Animals 2022, 12, 2298. https://doi.org/10.3390/ani12172298
Leineweber C, Marschang RE. Antibodies against Two Testudinid Herpesviruses in Pet Tortoises in Europe. Animals. 2022; 12(17):2298. https://doi.org/10.3390/ani12172298
Chicago/Turabian StyleLeineweber, Christoph, and Rachel E. Marschang. 2022. "Antibodies against Two Testudinid Herpesviruses in Pet Tortoises in Europe" Animals 12, no. 17: 2298. https://doi.org/10.3390/ani12172298
APA StyleLeineweber, C., & Marschang, R. E. (2022). Antibodies against Two Testudinid Herpesviruses in Pet Tortoises in Europe. Animals, 12(17), 2298. https://doi.org/10.3390/ani12172298





