Molecular Characterization, Expression Profiles of SMAD4, SMAD5 and SMAD7 Genes and Lack of Association with Litter Size in Tibetan Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. cDNA Cloning and Sequence Analysis
2.3. Gene Expression Analysis
2.4. SNP Identification and Genotyping
2.5. Statistical Analysis
3. Results
3.1. Expression Profiles of Genes in Sheep
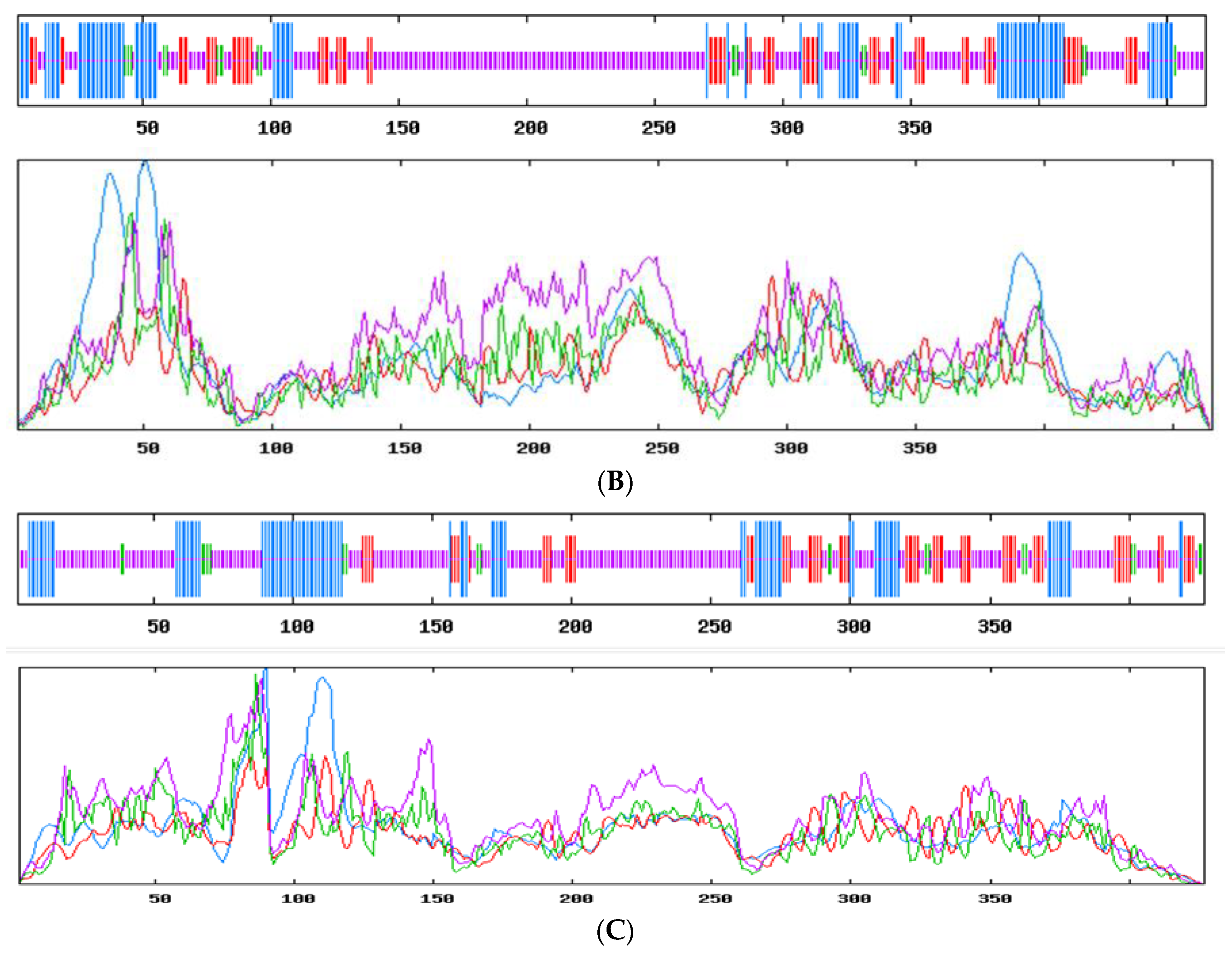
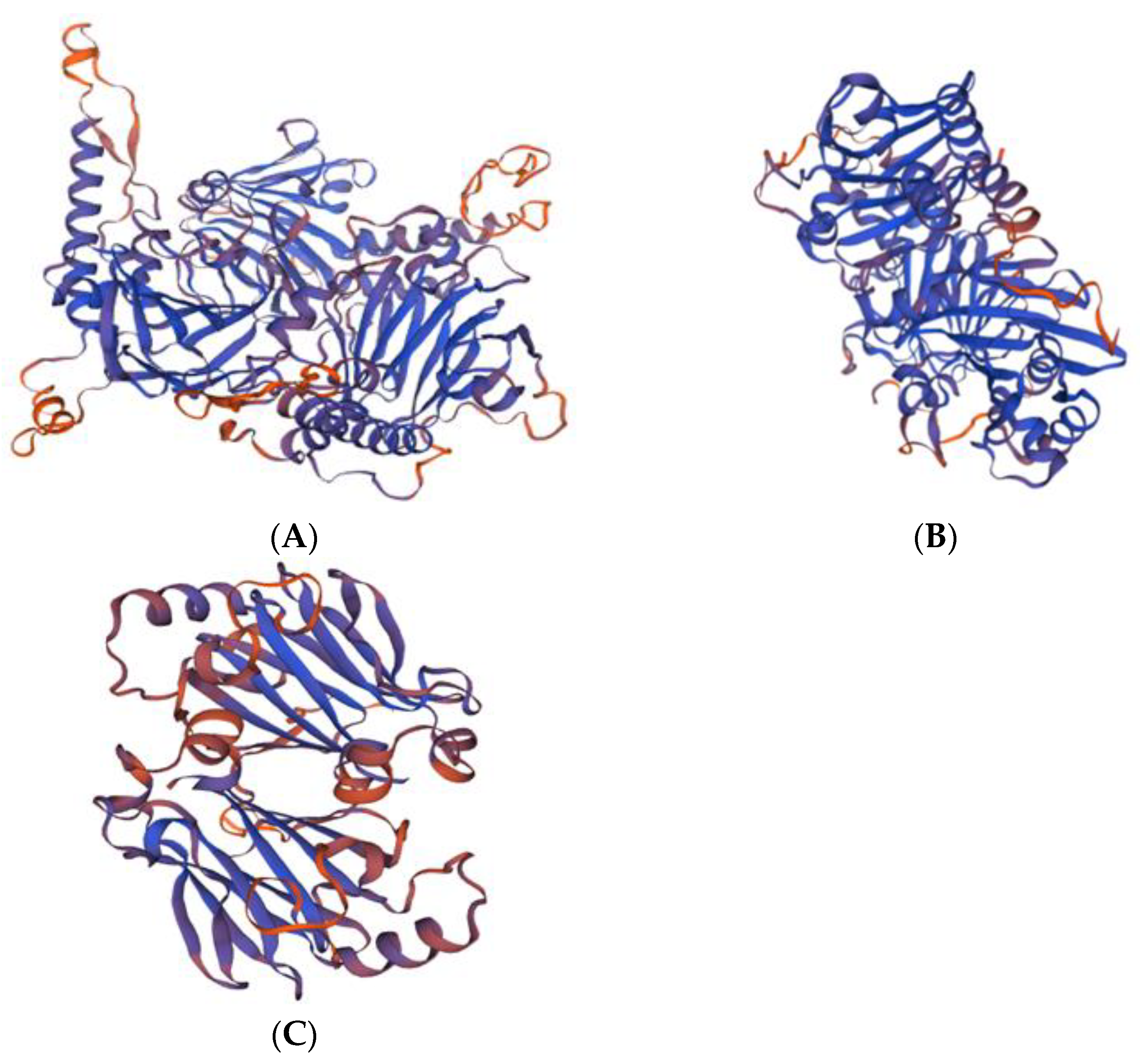
3.2. cDNA Cloning and Bioinformatic Analysis of Genes
3.3. Polymorphism of Genes in Sheep
3.4. Association between the Snp Loci of Genes and Litter Size of Tibetan Sheep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.; Yuan, C.; Li, J.; Guo, T.; Yue, Y.; Niu, C.; Liu, J.; Yang, B. Comprehensive analysis of long non-coding RNA and mRNA transcriptomes related to hypoxia adaptation in Tibetan sheep. Front. Vet. Sci. 2020, 8, 801278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; He, Z.; Xi, Q.; Sun, H.; Luo, Y.; Wang, J.; Li, S. Variations in HIF-1a contributed to high altitude Hypoxia adaptation via affected oxygen metabolism in Tibetan sheep. Animals 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Ma, P.; Wei, Y.; Li, R.; Chen, Z.; Li, Y. Molecular detection of anaplasma spp.; babesia spp. and theileria spp. in yaks (Bos grunniens) and Tibetan sheep (Ovis aries) on the Qinghai-Tibetan Plateau, China. Parasites Vectors 2021, 14, 613. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, X.; Sha, Y.; Shi, H.; Wei, H.; Luo, Y.; Pu, X. Rumen fermentation-microbiota-host gene expression interactions to reveal the adaptability of Tibetan sheep in different periods. Animals 2021, 11, 3529. [Google Scholar] [CrossRef]
- Frick, C.L.; Yarka, C.; Nunns, H.; Goentoro, L. Sensing relative signal in the Tgf-β/Smad pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E2975–E2982. [Google Scholar] [CrossRef]
- Kaivo-Oja, N.; Jeffery, L.A.; Ritvos, O.; Mottershead, D.G. Smad signalling in the ovary. Reprod. Biol. Endocrinol. 2006, 4, 21. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Shi, L.L. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef]
- Gaarenstroom, T.; Hill, C.S. TGF-β signaling to chromatin: How Smads regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 2014, 32, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Brorson, M.; Hougaard, D.; Nielsen, J.; Tornehave, D.; Larsson, L.-I. Expression of SMAD signal transduction molecules in the pancreas. Histochem. Cell Biol. 2001, 116, 263–267. [Google Scholar] [CrossRef]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Su, R.; Lv, X.Y.; Gao, W.; Chen, L.; Bao, J.J.; Sun, W. Analysis of the spatiotemporal expression of major genes in the TGF-β/Smad signaling pathway and correlation analysis using Hu sheep muscle tissue. Genet. Mol. Res. GMR 2016, 15, 15028133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, X.H.; Wu, R.Y.; Derynck, R. Receptor-associated mad homologues synergize as effectors of the TGF-β response. Nature 1996, 383, 168–172. [Google Scholar] [CrossRef]
- Massagué, J.; Wotton, D. Transcriptional control by the TGF/Smad signalling system. EMBO J. 2000, 19, 4360–4369. [Google Scholar] [CrossRef]
- Rodriguez, A.; Tripurani, S.K.; Burton, J.C.; Clementi, C.; Larina, I.; Pangas, S.A. Faculty Opinions recommendation of SMAD signaling is required for structural integrity of the female reproductive tract and uterine function during early pregnancy in mice. Biol. Reprod. 2016, 95, 44. [Google Scholar] [CrossRef]
- Sharum, I. Regulation of TGF/Smad Signalling during Early Follicle Development in the Mouse Ovary. Ph.D. Thesis, The University of Sheffield, Sheffield, UK, 2016. [Google Scholar]
- Chu, M.X.; Liu, Z.H.; Jiao, C.L.; He, Y.Q.; Fang, L.; Ye, S.C.; Wang, J.Y. Mutations in BMPR-IB and BMP-15 genes are associated with litter size in Small Tailed Han sheep (Ovis aries). J. Anim. Sci. 2007, 85, 598–603. [Google Scholar] [CrossRef]
- Bodin, L.; Di Pasquale, E.; Fabre, S.; Bontoux, M.; Monget, P.; Persani, L.; Mulsant, P. A Novel Mutation in the Bone Morphogenetic Protein 15 Gene Causing Defective Protein Secretion Is Associated with Both Increased Ovulation Rate and Sterility in Lacaune Sheep. Endocrinology 2007, 148, 393–400. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, Y.F. Study on the Relationship between the Expression of Genes Related to Smad Signal Transduction Pathway and High Fertility of Hu Sheep. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2010. [Google Scholar]
- Li, Z.F.; Sun, W.; Chu, M.X. Expression analysis of TGF-β pathway-related genes in sheep gonad axis tissues. J. Northeast. Agric. Univ. 2021, 52, 43–49. [Google Scholar]
- Wang, Y.H.; Xu, Y.F.; Niu, J.Q. Tissue expression of Smad4 gene mRNA in yak. Chin. J. Anim. Sci. Vet. Med. 2017, 48, 1882–1891. [Google Scholar]
- Ning, Y. Regulation of SMAD5 Gene on Proliferation and Differentiation of Qinchuan Cattle Myoblasts and Correlation Analysis with Beef Quality Traits. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]
- Chen, Q.R.; Li, J.; Wang, Y.J. Cloning, sequence analysis and tissue expression mapping of porcine Smad7 and Smad9 genes. J. Sichuan Univ. Nat. Sci. Ed. 2015, 52, 157–162. [Google Scholar]
- Tanekhy, M.; Kono, T.; Sakai, M. Cloning, characterization, and expression analysis of Toll-like receptor-7 cDNA from common carp, Cyprinus carpio L. Comp. Biochem. Physiol. Part D Genom. Proteom. 2010, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.C.; Johnson, L.C.; Herman, M.A. Ecological genomics: Understanding gene and genome function in the natural environment. Heredity 2007, 100, 178–183. [Google Scholar] [CrossRef]
- Diederichs, S.; Bartsch, L.; Berkmann, J.C.; Fröse, K.; Heitmann, J.; Hoppe, C.; Iggena, D.; Jazmati, D.; Karschnia, P.; Linsenmeier, M.; et al. The dark matter of the cancer genome: Aberrations in regulatory elements, untranslated regions, splice sites, non-coding RNA and synonymous mutations. EMBO Mol. Med. 2016, 8, 442–457. [Google Scholar] [CrossRef]
- Sharma, Y.; Miladi, M.; Dukare, S.; Boulay, K.; Caudron-Herger, M.; Groß, M.; Backofen, R.; Diederichs, S. A pan-cancer analysis of synonymous mutations. Nat. Commun. 2019, 10, 2569. [Google Scholar] [CrossRef]
- Lin, C.; Li, F.; Zhang, X.; Zhang, D.; Li, X.; Zhang, Y.; Wang, W. Expression and polymorphisms of CD8B gene and its associations with body weight and size traits in sheep. Anim. Biotechnol. 2021, 1–9, Online ahead of print. [Google Scholar] [CrossRef]
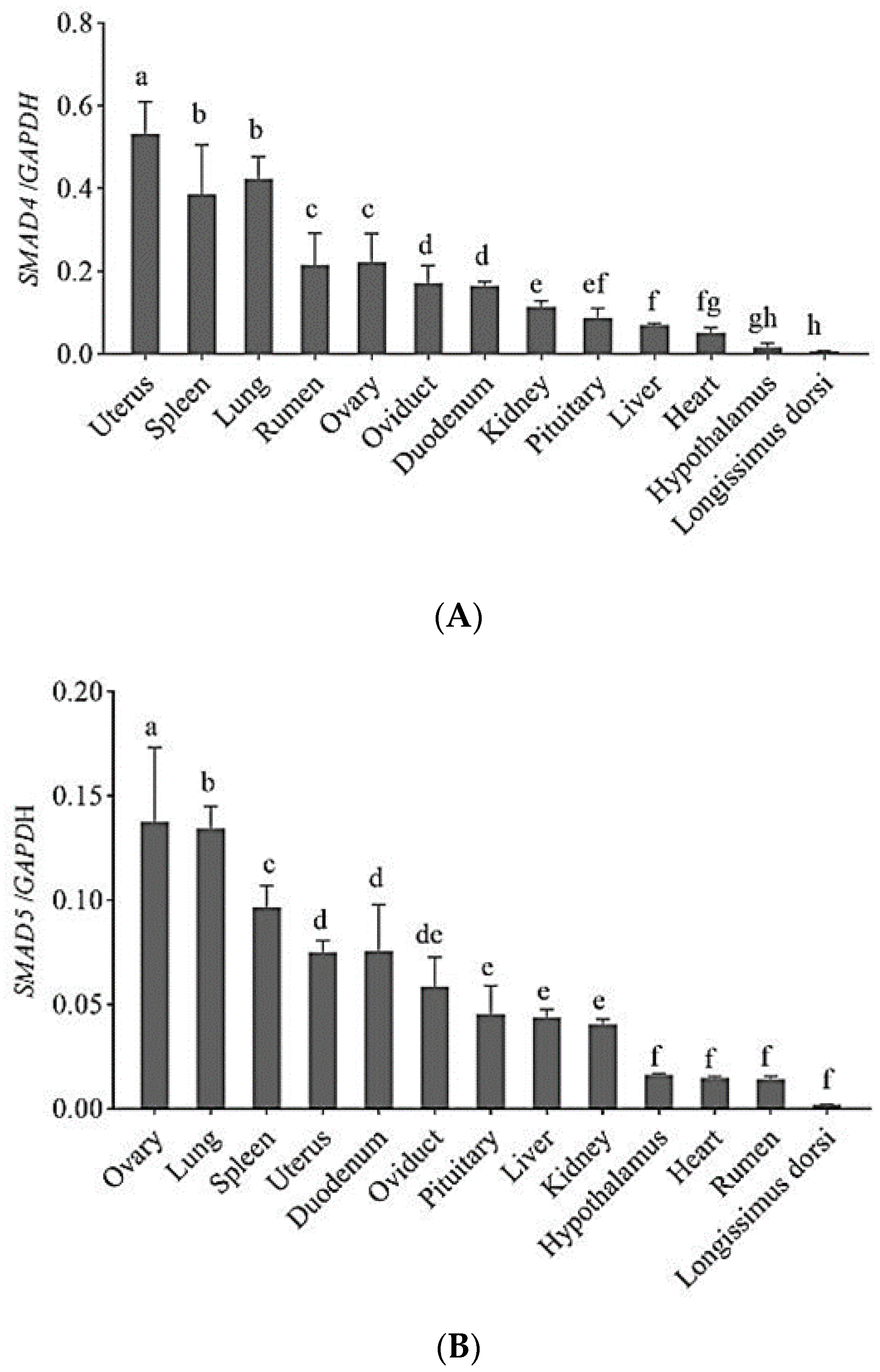

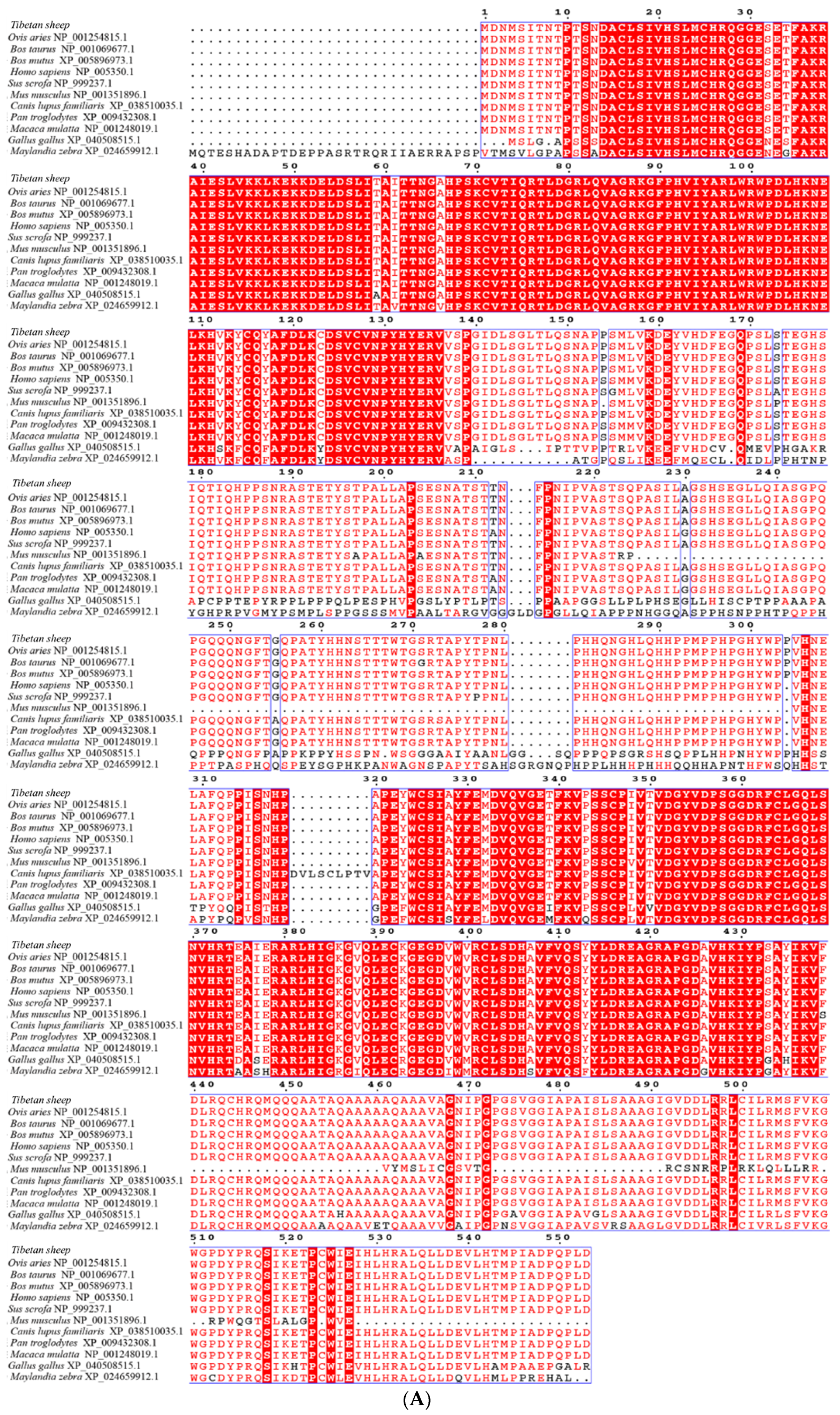

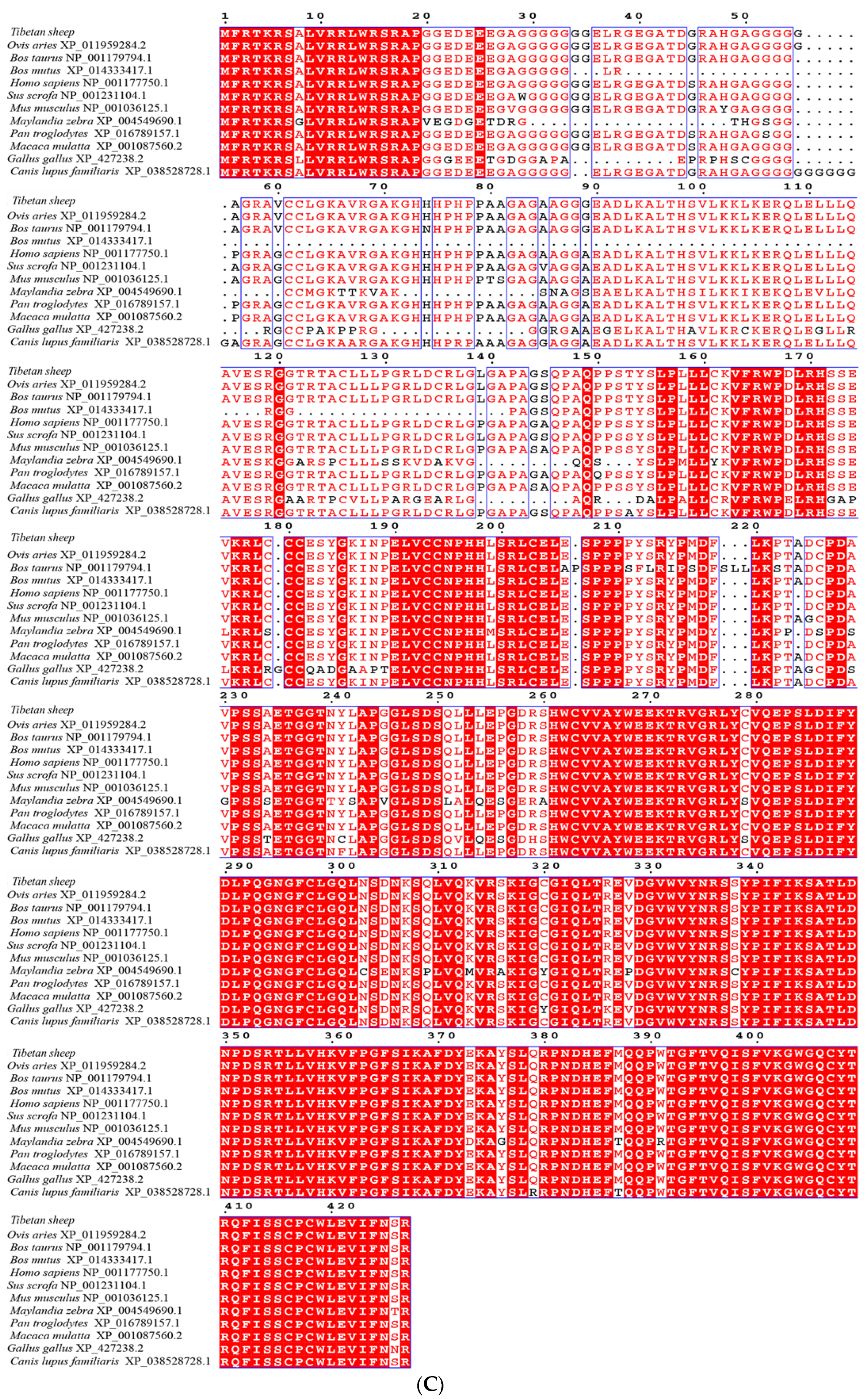


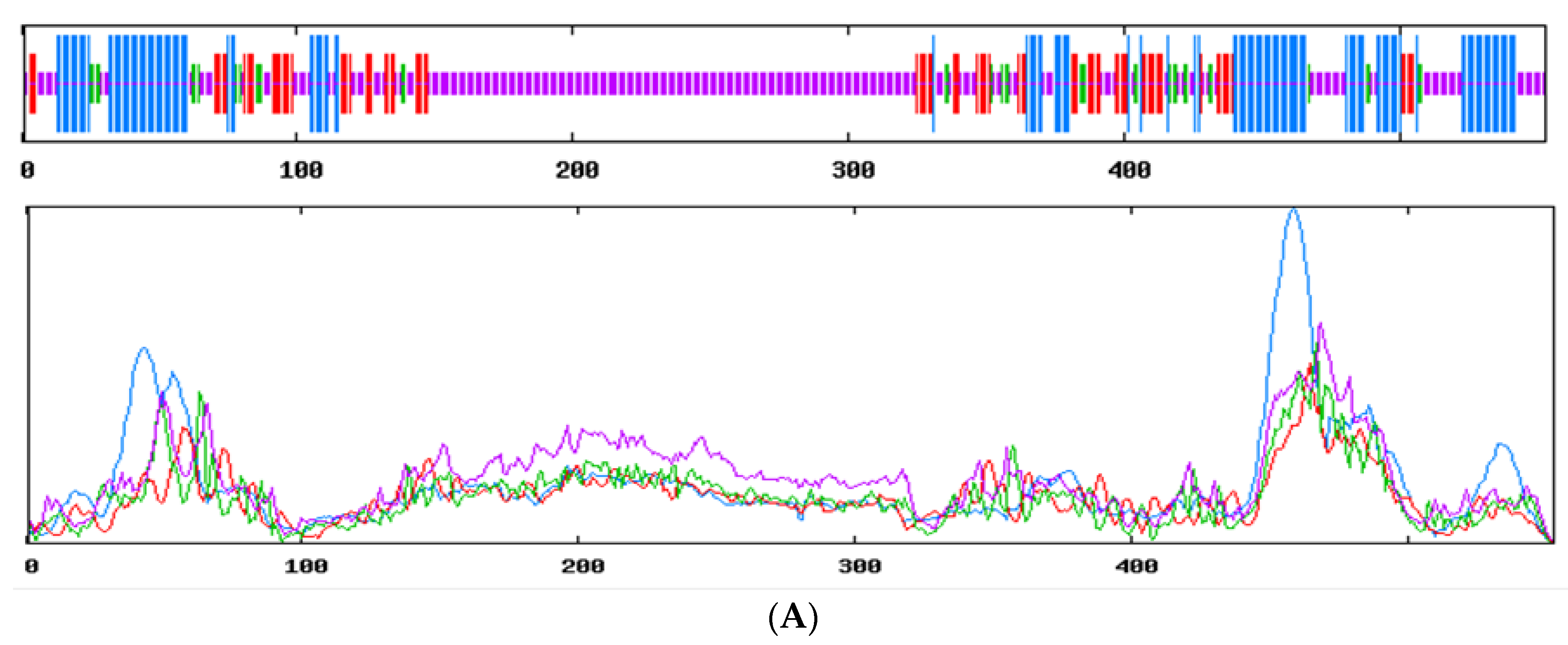
| Gene | Primer Name | Primer Sequence (5′~3′) | Product Length/bp | Tem/°C |
|---|---|---|---|---|
| SMAD4 | SMAD4-CDS1-F SMAD4-CDS1-R | CGAATACACCAACAAGTAATGATGC AACACCTTCGGTGTCCTTACAA | 648 | 61 |
| SMAD4 | SMAD4-CDS2-F SMAD4-CDS2-R | ATCCAGCATCCACCAAGTAATCG AGTGGAAATGTAAGGTTGACGTGTG | 641 | 64 |
| SMAD4 | SMAD4-CDS3-F SMAD4-CDS3-R | CTGGAGGAGATCGCTTTTGCTTG TTCCGACACCCAGCCGTTATC | 582 | 65 |
| SMAD5 | SMAD5-CDS-S SMAD5-CDS-A | CCCTTCCTTTTAAATTGCGAC AATACTTACTCTGCACCGTTC | 1667 | 55 |
| SMAD7 | SMAD7-CDS-S SMAD7-CDS-A | CCCGACTTCTTCATGGTGT TGTTCTGCCAACCATACCAC | 1408 | 61 |
| SMAD4 | SMAD4-expression-S SMAD4-expression-A | ACGGAAGGCTTCAGGTGGCTG AGGCCACCTCCAGAGACGGG | 71 | 64 |
| SMAD5 | SMAD5-expression-S SMAD5-expression-A | TCCCAGCCCATGGATACAAGCA GGGCTCTTCATAGGCGACAGGC | 93 | 63 |
| SMAD7 | SMAD7-expression-S SMAD7-expression-A | ATGCTGTGCCTTCCTCCGCT CCACGCACCAGTGTGACCGA | 111 | 64 |
| GAPDH | GAPDH-expression-S GAPDH-expression-A | GCGAGATCCTGCCAACATCAAGT CCCTTCAGGTGAGCCCCAGC | 105 | 64 |
| Gene | Locus | SNP Type | Genotype | Genotype Frequency(no.) | Allele | Allele Frequency | Exon |
|---|---|---|---|---|---|---|---|
| SMAD5 | g.51537A>G | Synonymous type | AA | 0.59(256) | A | 0.76 | 3 |
| AG | 0.35(150) | G | 0.24 | ||||
| GG | 0.06(27) | ||||||
| SMAD7 | g.319C>T | Synonymous type | CC | 0.94(409) | C | 0.97 | 3 |
| CT | 0.06(24) | T | 0.03 |
| Gene | Locus | Homozygosity (Ho) | Heterozygosity (He) | Effective Allele Numbers (Ne) | Polymorphic Information Content (PIC) | χ2 Test |
|---|---|---|---|---|---|---|
| SMAD5 | g.51537A>G | 0.64 | 0.36 | 1.56 | 0.30 | 0.43 |
| SMAD7 | g.319C>T | 0.95 | 0.05 | 1.06 | 0.05 | 1.00 |
| Gene | Locus | Genotype | No. of Individuals | Litter Size |
|---|---|---|---|---|
| SMAD5 | g.51537A>G | AA | 256 | 1.07 ± 0.26 |
| AG | 150 | 1.07 ± 0.27 | ||
| GG | 27 | 1.00 ± 0.00 | ||
| SMAD7 | g.319C>T | CC | 409 | 1.07 ± 0.25 |
| CT | 24 | 1.08 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Li, M.; He, N.; Wen, X.; Zhang, J. Molecular Characterization, Expression Profiles of SMAD4, SMAD5 and SMAD7 Genes and Lack of Association with Litter Size in Tibetan Sheep. Animals 2022, 12, 2232. https://doi.org/10.3390/ani12172232
Sun R, Li M, He N, Wen X, Zhang J. Molecular Characterization, Expression Profiles of SMAD4, SMAD5 and SMAD7 Genes and Lack of Association with Litter Size in Tibetan Sheep. Animals. 2022; 12(17):2232. https://doi.org/10.3390/ani12172232
Chicago/Turabian StyleSun, Ruizhe, Mingming Li, Na He, Xiaocheng Wen, and Junxia Zhang. 2022. "Molecular Characterization, Expression Profiles of SMAD4, SMAD5 and SMAD7 Genes and Lack of Association with Litter Size in Tibetan Sheep" Animals 12, no. 17: 2232. https://doi.org/10.3390/ani12172232
APA StyleSun, R., Li, M., He, N., Wen, X., & Zhang, J. (2022). Molecular Characterization, Expression Profiles of SMAD4, SMAD5 and SMAD7 Genes and Lack of Association with Litter Size in Tibetan Sheep. Animals, 12(17), 2232. https://doi.org/10.3390/ani12172232




