Rearing Environment during the Endogenous Feeding Stage of Acipenser baerii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Larval Parameters and Sampling
2.3. Lipid Content and Fatty Acid Composition
2.4. Muscle Histometry
2.5. Muscle Immunohistochemistry
2.6. RNA Extraction and cDNA Synthesis
2.7. Gene Identification and Primer Design
2.8. Gene Expression Profiles
2.9. Statistical Analysis
3. Results
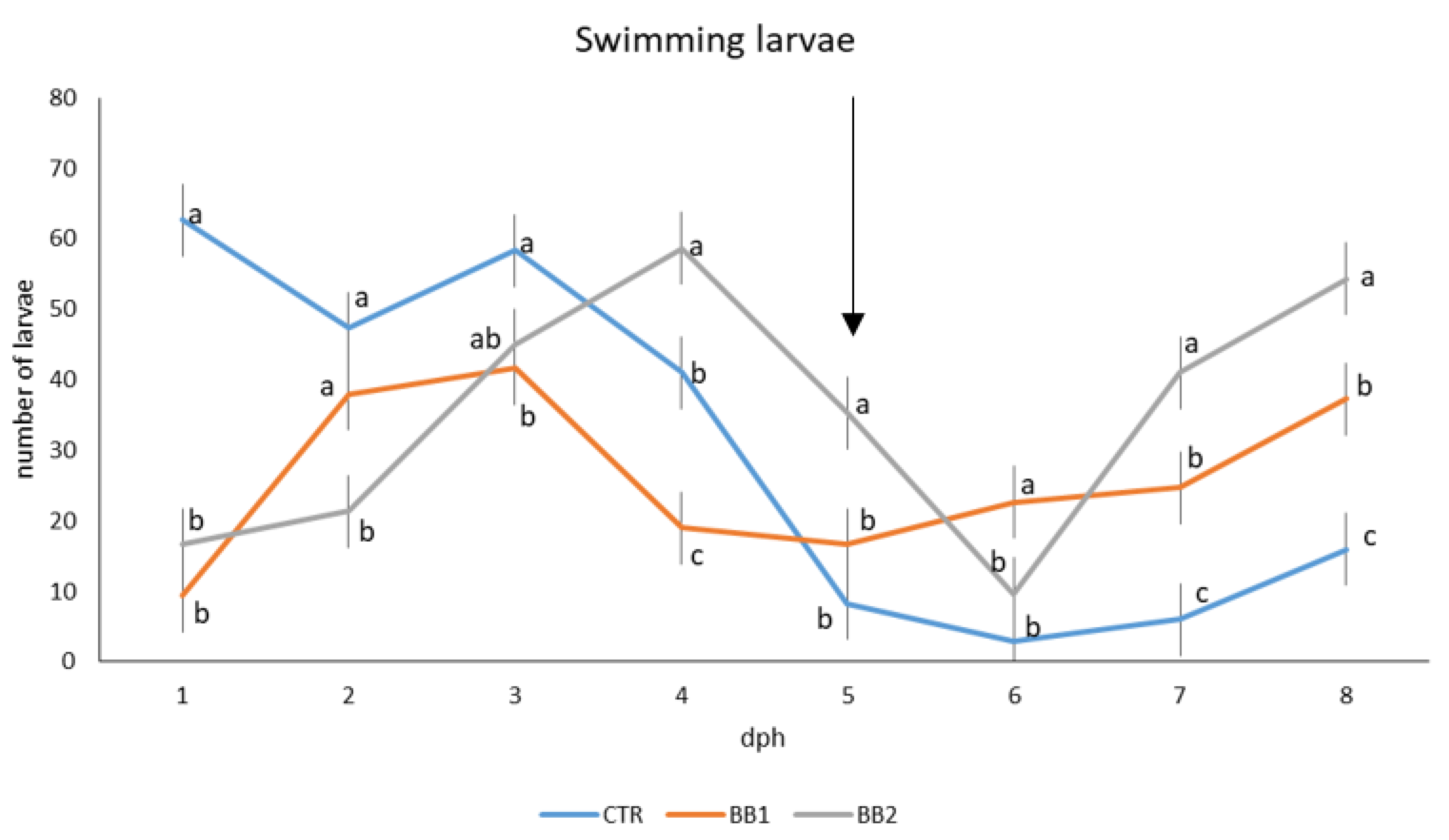
3.1. Development, Behaviour, Survival and Growth
3.2. Lipid Content and Fatty Acid Composition
3.3. Histological and Histometrical Analyses
3.4. Immunohistochemistry and Cell Counts
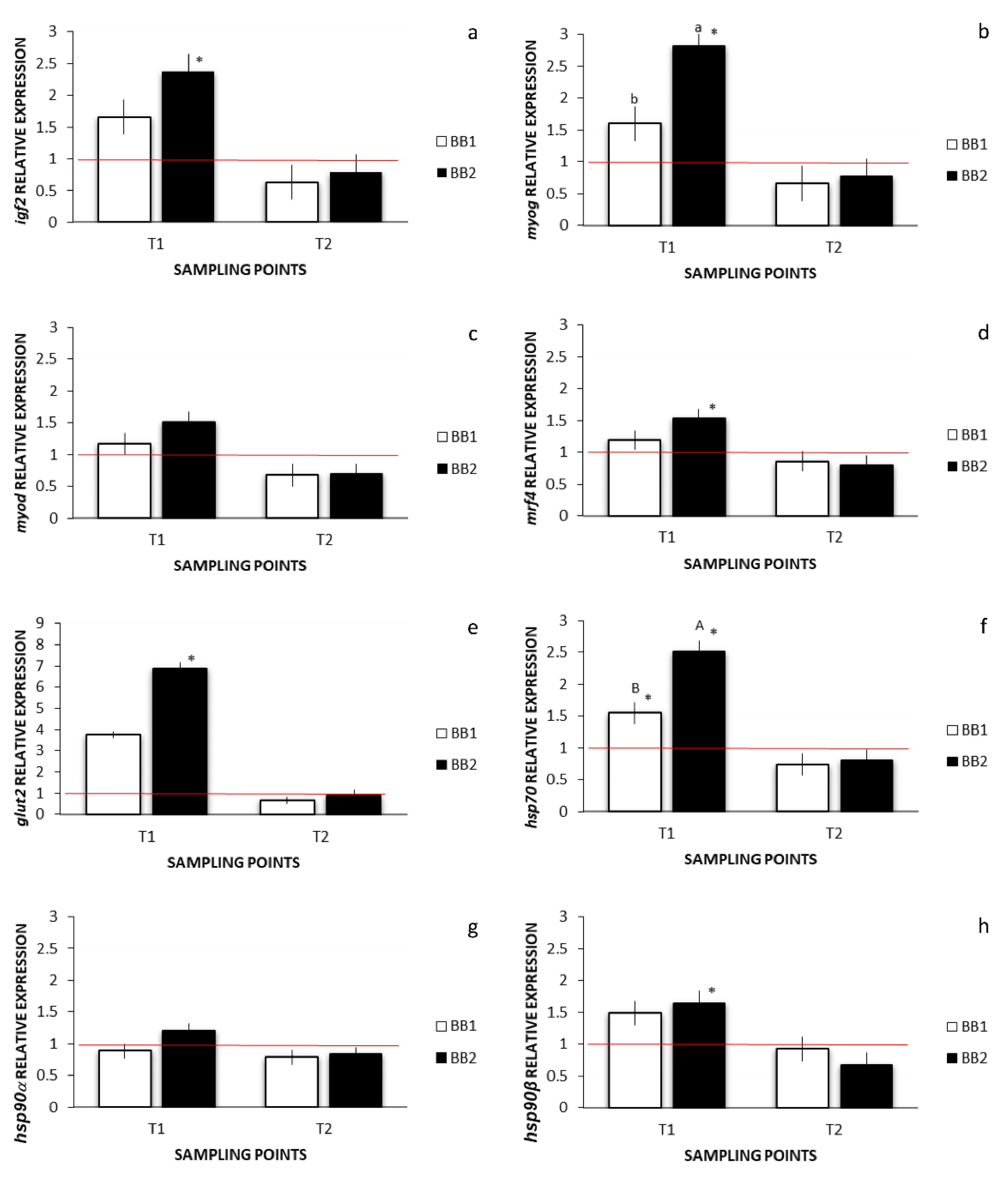
3.5. Environmental Stress, Muscle Development, and Growth-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronzi, P.; Rosenthal, H. Present and Future Sturgeon and Caviar Production and Marketing: A Global Market Overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon Meat and Caviar Production: Global Update 2017. J. Appl. Ichthyol. 2019, 35, 257–266. [Google Scholar] [CrossRef]
- Ruban, G.I. The Siberian Sturgeon Acipenser Baerii Brandt: Species Structure and Ecology; Books on Demand: Norderstedt, Germany, 2005; ISBN 978-3-8334-4038-0. [Google Scholar]
- Ruban, G.I. Species Structure, Contemporary Distribution and Status of the Siberian Sturgeon, Acipenser baerii. In Sturgeon Biodiversity and Conservation; Birstein, V.J., Waldman, J.R., Bemis, W.E., Eds.; Developments in Environmental Biology of Fishes; Springer: Dordrecht, The Netherlands, 1997; pp. 221–230. ISBN 978-0-306-46854-4. [Google Scholar]
- Gisbert, E.; Ruban, G.I. Ontogenetic Behavior of Siberian Sturgeon, Acipenser baerii: A Synthesis between Laboratory Tests and Field Data. Environ. Biol. Fishes 2003, 67, 311–319. [Google Scholar] [CrossRef]
- Devitsina, G.V.; Kazhlayev, A.A. Development of Chemosensory Organs in Siberian Sturgeon, Acipenser baeri, and Stellate Sturgeon, Acipenser Stellatus. J. Appl. Ichthyol. 1993, 33, 9–19. [Google Scholar]
- Gisbert, E. Early Development and Allometric Growth Patterns in Siberian Sturgeon and Their Ecological Significance. J. Fish Biol. 1999, 54, 852–862. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. Environmental Enrichment for Fish in Captive Environments: Effects of Physical Structures and Substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental Enrichment in Fish Aquaculture: A Review of Fundamental and Practical Aspects. Rev. Aquac. 2021, 14, 704–728. [Google Scholar] [CrossRef]
- McAdam, S.O. Effects of Substrate Condition on Habitat Use and Survival by White Sturgeon (Acipenser transmontanus) Larvae and Potential Implications for Recruitment. Can. J. Fish. Aquat. Sci. 2011, 68, 812–822. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological Changes in Fish from Stress in Aquaculture with Emphasis on the Response and Effects of Corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Peterson, R.H.; Martin-Robichaud, D.J. Yolk Utilization by Atlantic Salmon (Salmo salar L.) Alevins in Response to Temperature and Substrate. Aquac. Eng. 1995, 14, 85–99. [Google Scholar] [CrossRef]
- Gessner, J.; Kamerichs, C.M.; Kloas, W.; Wuertz, S. Behavioural and Physiological Responses in Early Life Phases of Atlantic Sturgeon (Acipenser oxyrinchus Mitchill 1815) towards Different Substrates. J. Appl. Ichthyol. 2009, 25, 83–90. [Google Scholar] [CrossRef]
- Baker, D.W.; McAdam, D.S.O.; Boucher, M.; Huynh, K.T.; Brauner, C.J. Swimming Performance and Larval Quality Are Altered by Rearing Substrate at Early Life Phases in White Sturgeon, Acipenser transmontanus (Richardson, 1836). J. Appl. Ichthyol. 2014, 30, 1461–1472. [Google Scholar] [CrossRef]
- Bates, L.C.; Boucher, M.A.; Shrimpton, J.M. Effect of Temperature and Substrate on Whole Body Cortisol and Size of Larval White Sturgeon (Acipenser transmontanus Richardson, 1836). J. Appl. Ichthyol. 2014, 30, 1259–1263. [Google Scholar] [CrossRef]
- Zubair, S.N.; Peake, S.J.; Hare, J.F.; Anderson, W.G. The Effect of Temperature and Substrate on the Development of the Cortisol Stress Response in the Lake Sturgeon, Acipenser fulvescens, Rafinesque (1817). Environ. Biol. Fishes 2012, 93, 577–587. [Google Scholar] [CrossRef]
- Moriyama, S.; Ayson, F.G.; Kawauchi, H. Growth Regulation by Insulin-like Growth Factor-I in Fish. Biosci. Biotechnol. Biochem. 2000, 64, 1553–1562. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Valdés, J.A.; Molina, A.; Björnsson, B.T. Regulation of Skeletal Muscle Growth in Fish by the Growth Hormone—Insulin-like Growth Factor System. Gen. Comp. Endocrinol. 2013, 192, 136–148. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Moutou, K.A.; Conceição, L.E.C.; Engrola, S.; Fernandes, J.M.O.; Johnston, I.A. What Determines Growth Potential and Juvenile Quality of Farmed Fish Species? Rev. Aquac. 2013, 5, S168–S193. [Google Scholar] [CrossRef]
- Eissa, N.; Wang, H.-P. Transcriptional Stress Responses to Environmental and Husbandry Stressors in Aquaculture Species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Bischoff, A.A.; Kubitz, M.; Wranik, C.M.; Pfefferkorn, H.; Augustin, C.B.; Hagen, W.; Palm, H.W. Fatty Acid Utilization of Pikeperch (Sander lucioperca (Linnaeus, 1758)) Larvae under Starvation Conditions during Early Development. Bull. Fish Biol. 2017, 17, 59–73. [Google Scholar]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Aidos, L.; Valente, L.M.P.; Sousa, V.; Lanfranchi, M.; Domeneghini, C.; Giancamillo, A.D. Effects of Different Rearing Temperatures on Muscle Development and Stress Response in the Early Larval Stages of Acipenser baerii. Eur. J. Histochem. 2017, 61, 4. [Google Scholar] [CrossRef] [PubMed]
- Aidos, L.; Cafiso, A.; Bertotto, D.; Bazzocchi, C.; Radaelli, G.; Giancamillo, A.D. How Different Rearing Temperatures Affect Growth and Stress Status of Siberian Sturgeon Acipenser baerii Larvae. J. Fish Biol. 2020, 96, 913–924. [Google Scholar] [CrossRef]
- Aidos, L.; Vasconi, M.; Abbate, F.; Valente, L.M.P.; Lanfranchi, M.; Di Giancamillo, A. Effects of Stocking Density on Reared Siberian Sturgeon (Acipenser baerii) Larval Growth, Muscle Development and Fatty Acids Composition in a Recirculating Aquaculture System. Aquac. Res. 2019, 50, 588–598. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Fisheries and Aquaculture Department—Species Fact Sheets. Available online: www.fao.org (accessed on 5 December 2021).
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis, 3rd ed.; Woodhead Publishing Limited: Bridgwater, UK, 2003. [Google Scholar]
- Vasconi, M.; Aidos, L.; Di Giancamillo, A.; Bellagamba, F.; Domeneghini, C.; Moretti, V.M. Effect of Temperature on Fatty Acid Composition and Development of Unfed Siberian Sturgeon (A. baerii) Larvae. J. Appl. Ichthyol. 2019, 35, 296–302. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Rossi, R.; Vitari, F.; Pastorelli, G.; Corino, C.; Domeneghini, C. Dietary Conjugated Linoleic Acids Decrease Leptin in Porcine Adipose Tissue. J. Nutr. 2009, 139, 1867–1872. [Google Scholar] [CrossRef]
- Aidos, L.; Cafiso, A.; Serra, V.; Vasconi, M.; Bertotto, D.; Bazzocchi, C.; Radaelli, G.; Di Giancamillo, A. How Different Stocking Densities Affect Growth and Stress Status of Acipenser baerii Early Stage Larvae. Animals 2020, 10, 1289. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gisbert, E.; Williot, P.; Castelló-Orvay, F. Behavioural Modifications in the Early Life Stages of Siberian Sturgeon (Acipenser baerii, Brandt). J. Appl. Ichthyol. 1999, 15, 237–242. [Google Scholar] [CrossRef]
- Gebauer, T.; Gebauer, R.; Císař, P.; Tran, H.Q.; Tomášek, O.; Podhorec, P.; Prokešová, M.; Rebl, A.; Stejskal, V. The Effect of Different Feeding Applications on the Swimming Behaviour of Siberian Sturgeon: A Method for Improving Restocking Programmes. Biology 2021, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Rescan, P.Y. Regulation and Functions of Myogenic Regulatory Factors in Lower Vertebrates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 1–12. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-Like Growth Factor Signaling in Fish. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 243, pp. 215–285. ISBN 978-0-12-364647-7. [Google Scholar]
- Patruno, M.; Sivieri, S.; Poltronieri, C.; Sacchetto, R.; Maccatrozzo, L.; Martinello, T.; Funkenstein, B.; Radaelli, G. Real-Time Polymerase Chain Reaction, in Situ Hybridization and Immunohistochemical Localization of Insulin-like Growth Factor-I and Myostatin during Development of Dicentrarchus labrax (Pisces: Osteichthyes). Cell Tissue Res. 2008, 331, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.A.; McAdam, S.O.; Shrimpton, J.M. The Effect of Temperature and Substrate on the Growth, Development and Survival of Larval White Sturgeon. Aquaculture 2014, 430, 139–148. [Google Scholar] [CrossRef]
- Boucher, M.A.; Baker, D.W.; Brauner, C.J.; Shrimpton, J.M. The Effect of Substrate Rearing on Growth, Aerobic Scope and Physiology of Larval White Sturgeon Acipenser transmontanus. J. Fish Biol. 2018, 92, 1731–1746. [Google Scholar] [CrossRef]
- Luo, L.; Ai, L.; Li, T.; Xue, M.; Wang, J.; Li, W.; Wu, X.; Liang, X. The Impact of Dietary DHA/EPA Ratio on Spawning Performance, Egg and Offspring Quality in Siberian Sturgeon (Acipenser baeri). Aquaculture 2015, 437, 140–145. [Google Scholar] [CrossRef]
- Bell, J.G.; Ashton, I.; Secombes, C.J.; Weitzel, B.R.; Dick, J.R.; Sargent, J.R. Dietary Lipid Affects Phospholipid Fatty Acid Compositions, Eicosanoid Production and Immune Function in Atlantic Salmon (Salmo salar). Prostaglandins Leukot Essent Fat. Acids 1996, 54, 173–182. [Google Scholar] [CrossRef]
- Lee, S.-M. Review of the Lipid and Essential Fatty Acid Requirements of Rockfish (Sebastes schlegeli). Aquac. Res. 2001, 32, 8–17. [Google Scholar] [CrossRef]
- Yanes-Roca, C.; Rhody, N.; Nystrom, M.; Main, K.L. Effects of Fatty Acid Composition and Spawning Season Patterns on Egg Quality and Larval Survival in Common Snook (Centropomus undecimalis). Aquaculture 2009, 287, 335–340. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Masuda, R.; Uematsu, K.; Shimizu, K.; Arimoto, M.; Takeuchi, T. The Effect of Dietary Docosahexaenoic Acid on Schooling Behaviour and Brain Development in Larval Yellowtail. J. Fish Biol. 2001, 58, 1691–1703. [Google Scholar] [CrossRef]
- Navarro, J.C.; Sargent, J.R. Behavioural Differences in Starving Herring Clupea harengus L. Larvae Correlate with Body Levels of Essential Fatty Acids. J. Fish Biol. 1992, 41, 509–513. [Google Scholar] [CrossRef]
- Hauville, M.R.; Main, K.L.; Migaud, H.; Gordon Bell, J. Fatty Acid Utilization during the Early Larval Stages of Florida Pompano (Trachinotus carolinus) and Common Snook (Centropomus undecimalis). Aquac. Res. 2016, 47, 1443–1458. [Google Scholar] [CrossRef]
- Rowlerson, A.; Veggetti, A. Cellular Mechanisms of Post-Embryonic Muscle Growth in Aquaculture Species. Muscle Dev. Growth 2001, 18, 103–140. [Google Scholar]
- Alami-Durante, H. Growth of Organs and Tissues in Carp (Cyprinus carpio L.) Larvae. Growth Dev. Aging 1990, 54, 109–116. [Google Scholar] [PubMed]
- Galloway, T.F.; Kjorsvik, E.; Kryvi, H. Muscle Growth in Yolk-Sac Larvae of the Atlantic Halibut as Influenced by Temperature in the Egg and Yolk-Sac Stage. J. Fish Biol. 1999, 55, 26–43. [Google Scholar] [CrossRef]
- Nathanailides, C.; Stickland, N.C.; Lopez-Albors, O. Influence of Prehatch Temperature on the Development of Muscle Cellularity in Posthatch Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1995, 52, 675–680. [Google Scholar] [CrossRef]
- Steinbacher, P.; Haslett, J.R.; Sänger, A.M.; Stoiber, W. Evolution of Myogenesis in Fish: A Sturgeon View of the Mechanisms of Muscle Development. Anat. Embryol. 2006, 211, 311–322. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Guo, H.; Zhang, X. Effect of Environmental Enrichment on the Stress Response of Juvenile Black Rockfish Sebastes schlegelii. Aquaculture 2021, 533, 736088. [Google Scholar] [CrossRef]
- Murtaza, M.u.H.; Zuberi, A.; Ahmad, M.; Amir, I.; Kamran, M.; Ahmad, M. Influence of Early Rearing Environment on Water-Borne Cortisol and Expression of Stress-Related Genes in Grass Carp (Ctenopharyngodon idella). Mol. Biol. Rep. 2020, 47, 5051–5060. [Google Scholar] [CrossRef]
- Maradonna, F.; Bavestrello, G.; Cardinali, M.; Olivotto, I.; Cerrano, C.; Giovine, M.; Carnevali, O. Role of Substrate on Larval Development of the Freshwater Teleost Pelvicachromis pulcher. Mol. Reprod. Dev. 2003, 66, 256–263. [Google Scholar] [CrossRef]
- Johnston, I.A. Environment and Plasticity of Myogenesis in Teleost Fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| T0 | T1 | T2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTR | BB1 | BB2 | Sign | T1 Mean | CTR | BB1 | BB2 | Sign | T2 Mean | Sign T | ||
| Total lipid | 3.36 ± 0.05 A | 3.01 ± 0.16 | 3.05 ± 0.29 | 2.81 ± 0.27 | ns | 2.96 ± 0.14A | 2.29 ± 0.52 | 2.23 ± 0.33 | 2.36 ± 0.22 | ns | 2.29 ± 0.08 B | <0.001 |
| Fatty acid profile (g 100 g−1 of total fatty acids) | ||||||||||||
| 14:0 | 0.84 ± 0.06 A | 0.86 ± 0.04 | 0.80 ± 0.01 | 0.81 ± 0.01 | ns | 0.83 ± 0.02 A | 0.69 ± 0.00 b | 0.73 ± 0.12 a | 0.74 ± 0.01 a | <0.001 | 0.72 ± 0.01 B | <0.001 |
| 16:0 | 19.45 ± 0.07 C | 19.96 ± 0.12 | 19.84 ± 0.07 | 19.94 ± 0.07 | ns | 19.91 ± 0.05 B | 20.29 ± 0.06 | 20.54 ± 0.05 | 20.49 ± 0.17 | ns | 20.44 ± 0.06 A | <0.001 |
| 18:0 | 4.00 ± 0.02 C | 4.71 ± 0.08 | 4.70 ± 0.06 | 4.79 ± 0.08/ | ns | 4.73 ± 0.17B | 5.65 ± 0.07 a,b | 5.80 ± 0.06 a | 5.50 ± 0.06 b | <0.05 | 5.65 ± 0.19 A | |
| SFA | 24.29 ± 0.11 C | 25.53 ± 0.24 | 25.34 ± 0.11 | 25.54 ± 0.14 | ns | 25.47 ± 0.10 B | 26.63 ± 0.11 | 27.07 ± 0.09 | 26.73 ± 0.20 | ns | 26.81 ± 0.09 A | <0.001 |
| 16:1 n-7 | 3.00 ± 0.02 A | 2.85 ± 0.01 a,b | 2.81 ± 0.02 b | 2.87 ± 0.01 a | <0.05 | 2.84 ± 0.01 B | 2.61 ± 0.02 a,b | 2.57 ± 0.02b | 2.66 ± 0.02 a | <0.01 | 2.61 ± 0.01 C | <0.001 |
| 18:1 n-9 | 34.63 ± 0.10 A | 34.01 ± 0.12 | 33.95 ± 0.10 | 33.97 ± 0.08 | ns | 33.97 ± 0.06 B | 32.89 ± 0.10 | 32.57 ± 0.10 | 32.94 ± 0.13 | ns | 32.80 ± 0.07 C | <0.001 |
| 18:1 n-7 | 2.65 ± 0.01 B | 2.66 ± 0.01 b | 2.71 ± 0.01 a | 2.67 ± 0.00 b | <0.001 | 2.68 ± 0.01 | 2.76 ± 0.01 | 2.78 ± 0.01 | 2.79 ± 0.01 | ns | 2.78 ± 0.01 A | <0.001 |
| 20:1 n-9 | 1.11 ± 0.05 A | 1.08 ± 0.01 | 1.06 ± 0.01 | 1.06 ± 0.01 | ns | 1.07 ± 0.01 B | 1.01 ± 0.01 | 0.97 ± 0.02 | 1.01 ± 0.01 | ns | 1.00 ± 0.01 C | |
| MUFA | 41.39 ± 0.10 A | 40.59 ± 0.14 | 40.52 ± 0.13 | 40.57 ± 0.09 | ns | 40.56 ± 0.07 B | 39.27 ± 0.11 a,b | 38.89 ± 0.11 b | 39.40 ± 0.15 a | <0.05 | 39.19 ± 0.09 C | |
| 18:2 n-6 | 12.01 ± 0.05 A | 11.39 ± 0.05 a | 11.26 ± 0.02 b | 11.41 ± 0.03 a | <0.05 | 11.35 ± 0.02 B | 10.77 ± 0.05 a,b | 10.64 ± 0.06 b | 10.86 ± 0.02 a | <0.01 | 10.76 ± 0.03 C | <0.001 |
| 18:3 n-6 | 2.13 ± 0.01 A | 2.06 ± 0.01 | 1.99 ± 0.03 | 2.05 ± 0.01 | <0.05 | 2.03 ± 0.01 B | 1.89 ± 0.01 a,b | 1.86 ± 0.01 b | 1.91 ± 0.02 a | <0.05 | 1.89 ± 0.01 C | <0.001 |
| 18:3 n-3 | 1.75 ± 0.08 A | 1.62 ± 0.02 a,b | 1.59 ± 0.01 b | 1.63 ± 0.01 a | <0.05 | 1.61 ± 0.01 B | 1.46 ± 0.01 | 1.43 ± 0.01 | 1.48 ± 0.01 | ns | 1.45 ± 0.01 C | <0.001 |
| 20:2 n-6 | 0.54 ± 0.01 B | 0.54 ± 0.00 b | 0.56 ± 0.00 a | 0.54 ± 0.00 a,b | <0.05 | 0.50 ± 0.00 B | 0.56 ± 0.01 b | 0.58 ± 0.00 a | 0.56 ± 0.00 b | <0.01 | 0.57 ± 0.00 A | <0.001 |
| 20:3 n-6 | 0.64 ± 0.01 A,B | 0.60 ± 0.01 a,b | 0.61 ± 0.00 a | 0.59 ± 0.00 b | <0.05 | 0.60 ± 0.00 B | 0.62 ± 0.01 | 0.63 ± 0.01 | 0.63 ± 0.01 | ns | 0.63 ± 0.01 A | <0.001 |
| 20:4 n-6 | 3.20 ± 0.02 C | 3.31 ± 0.03 a,b | 3.39 ± 0.02 a | 3.29 ± 0.01 b | <0.05 | 3.33 ± 0.02 B | 3.61 ± 0.02 | 3.72 ± 0.04 | 3.63 ± 0.03 | ns | 3.66 ± 0.02 A | <0.001 |
| 20:5 n-3 | 2.20 ± 0.01 A | 2.15 ± 0.01 | 2.17 ± 0.01 | 2.15 ± 0.01 | ns | 2.15 ± 0.01 B | 2.17 ± 0.01 | 2.20 ± 0.01 | 2.18 ± 0.02 | ns | 2.19 ± 0.01 A | <0.01 |
| 22:5 n-3 | 0.74 ± 0.01 | 0.72 ± 0.00 | 0.74 ± 0.01 | 0.73 ± 0.01 | ns | 0.73 ± 0.00 | 0.70 ± 0.00 | 0.72 ± 0.01 | 0.73 ± 0.01 | ns | 0.72 ± 0.01 | ns |
| 22:6 n-3 | 11.12 ± 0.05 C | 11.50 ± 0.13 b | 11.84 ± 0.07 a | 11.49 ± 0.05 b | <0.05 | 11.31 ± 0.06 B | 12.30 ± 0.09 | 12.46 ± 0.11 | 12.26 ± 0.16 | ns | 12.34 ± 0.07 A | <0.001 |
| PUFA | 34.33 ± 0.07 A | 33.88 ± 0.19 | 34.13 ± 0.09 | 33.89 ± 0.07 | ns | 33.97 ± 0.07 B | 34.10 ± 0.09 | 34.23 ± 0.15 | 34.25 ± 0.21 | ns | 34.19 ± 0.09 A,B | <0.05 |
| DHA/EPA | 5.06 ± 0.01 C | 5.35 ± 0.03 b | 5.46 ± 0.01 a | 5.35 ± 0.03 b | <0.05 | 5.39 ± 0.02 B | 5.66 ± 0.02 | 5.66 ± 0.03 | 5.61 ± 0.03 | ns | 5.64 ± 0.02 A | <0.001 |
| ARA/EPA | 1.44 ± 0.00 C | 1.54 ± 0.01 a,b | 1.56 ± 0.01 a | 1.53 ± 0.01 b | <0.05 | 1.54 ± 0.00 B | 1.66 ± 0.00 | 1.69 ± 0.01 | 1.66 ± 0.0 | ns | 1.67 ± 0.00 A | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aidos, L.; Cafiso, A.; Lopez, A.; Vasconi, M.; Valente, L.M.P.; Bazzocchi, C.; Di Giancamillo, A. Rearing Environment during the Endogenous Feeding Stage of Acipenser baerii. Animals 2022, 12, 2205. https://doi.org/10.3390/ani12172205
Aidos L, Cafiso A, Lopez A, Vasconi M, Valente LMP, Bazzocchi C, Di Giancamillo A. Rearing Environment during the Endogenous Feeding Stage of Acipenser baerii. Animals. 2022; 12(17):2205. https://doi.org/10.3390/ani12172205
Chicago/Turabian StyleAidos, Lucia, Alessandra Cafiso, Annalaura Lopez, Mauro Vasconi, Luisa M. P. Valente, Chiara Bazzocchi, and Alessia Di Giancamillo. 2022. "Rearing Environment during the Endogenous Feeding Stage of Acipenser baerii" Animals 12, no. 17: 2205. https://doi.org/10.3390/ani12172205
APA StyleAidos, L., Cafiso, A., Lopez, A., Vasconi, M., Valente, L. M. P., Bazzocchi, C., & Di Giancamillo, A. (2022). Rearing Environment during the Endogenous Feeding Stage of Acipenser baerii. Animals, 12(17), 2205. https://doi.org/10.3390/ani12172205






