Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Growth Performance and Organ Indices
2.3. Analyses of Serum Biochemical Indicators
2.4. Microbial Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of HS and MCE on Growth Performance
3.2. Effects of HS and MCE on Organ Indices
3.3. Effects of HS and MCE on Serum Biochemical Indicators
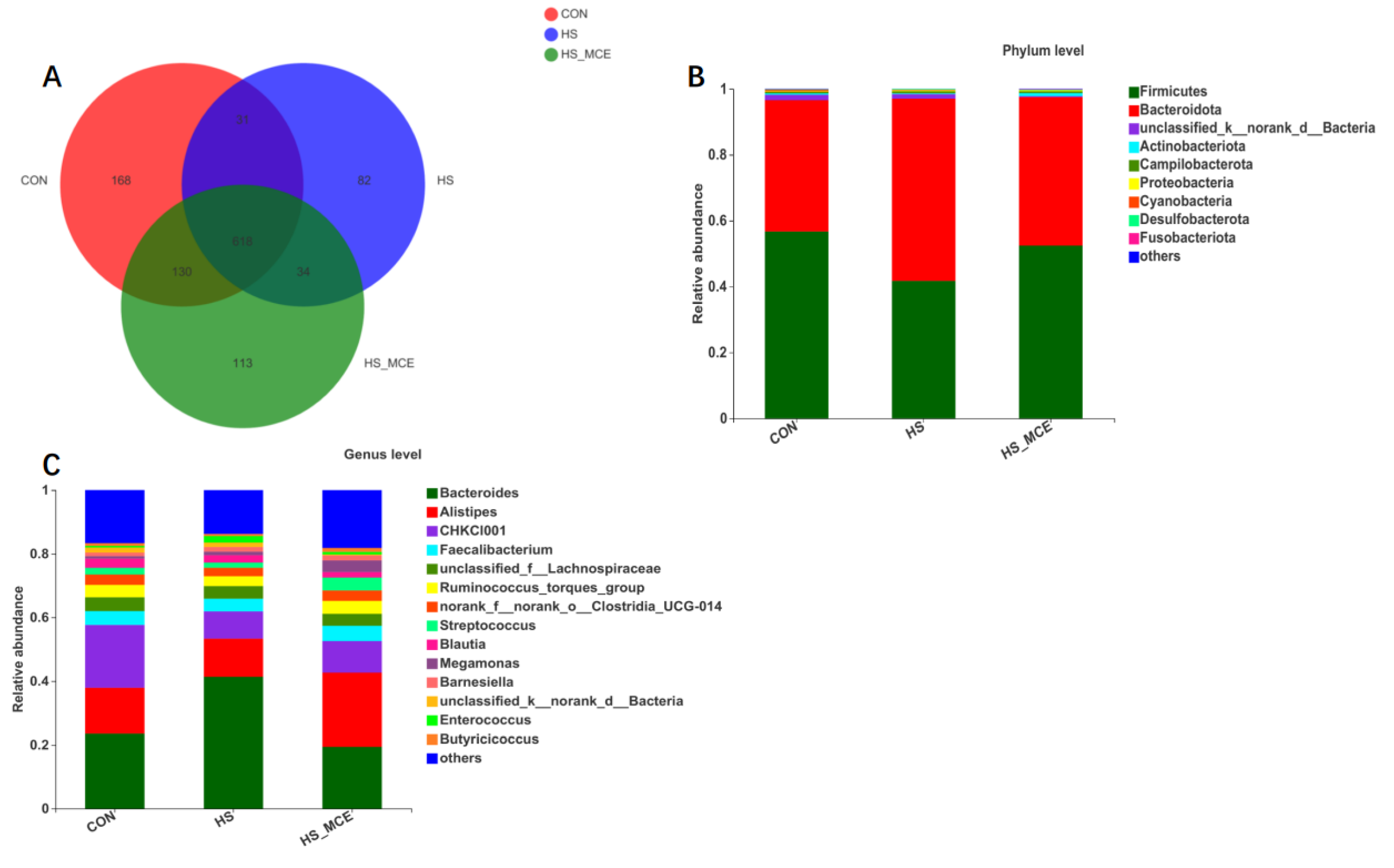
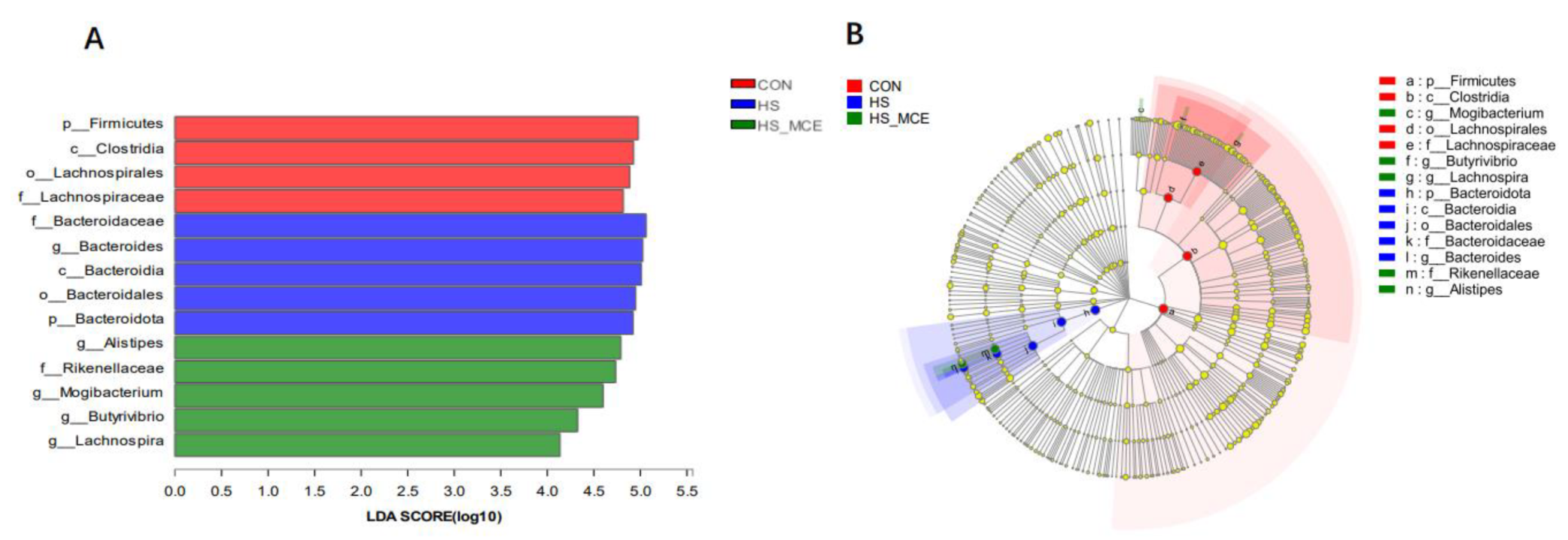
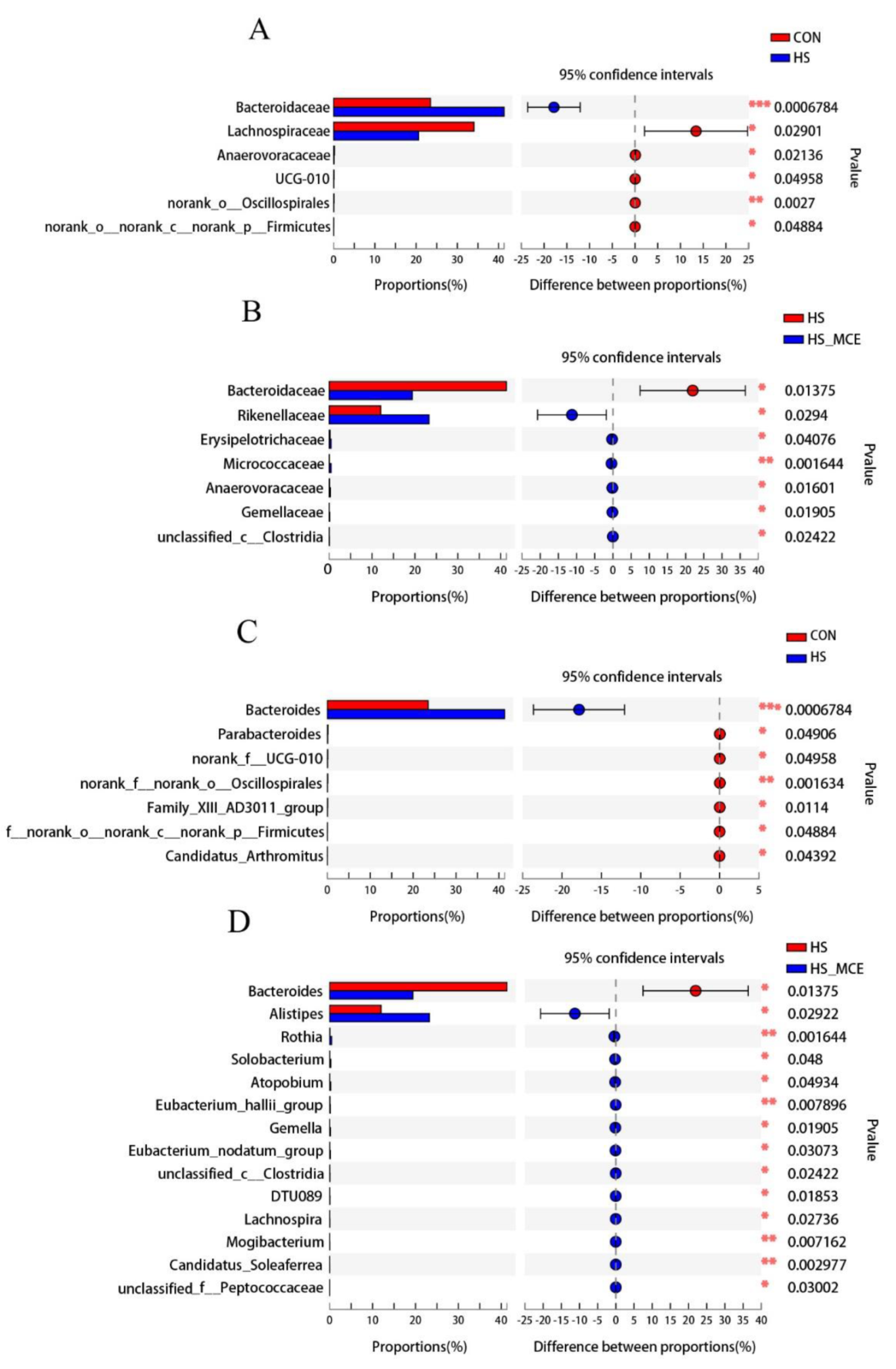
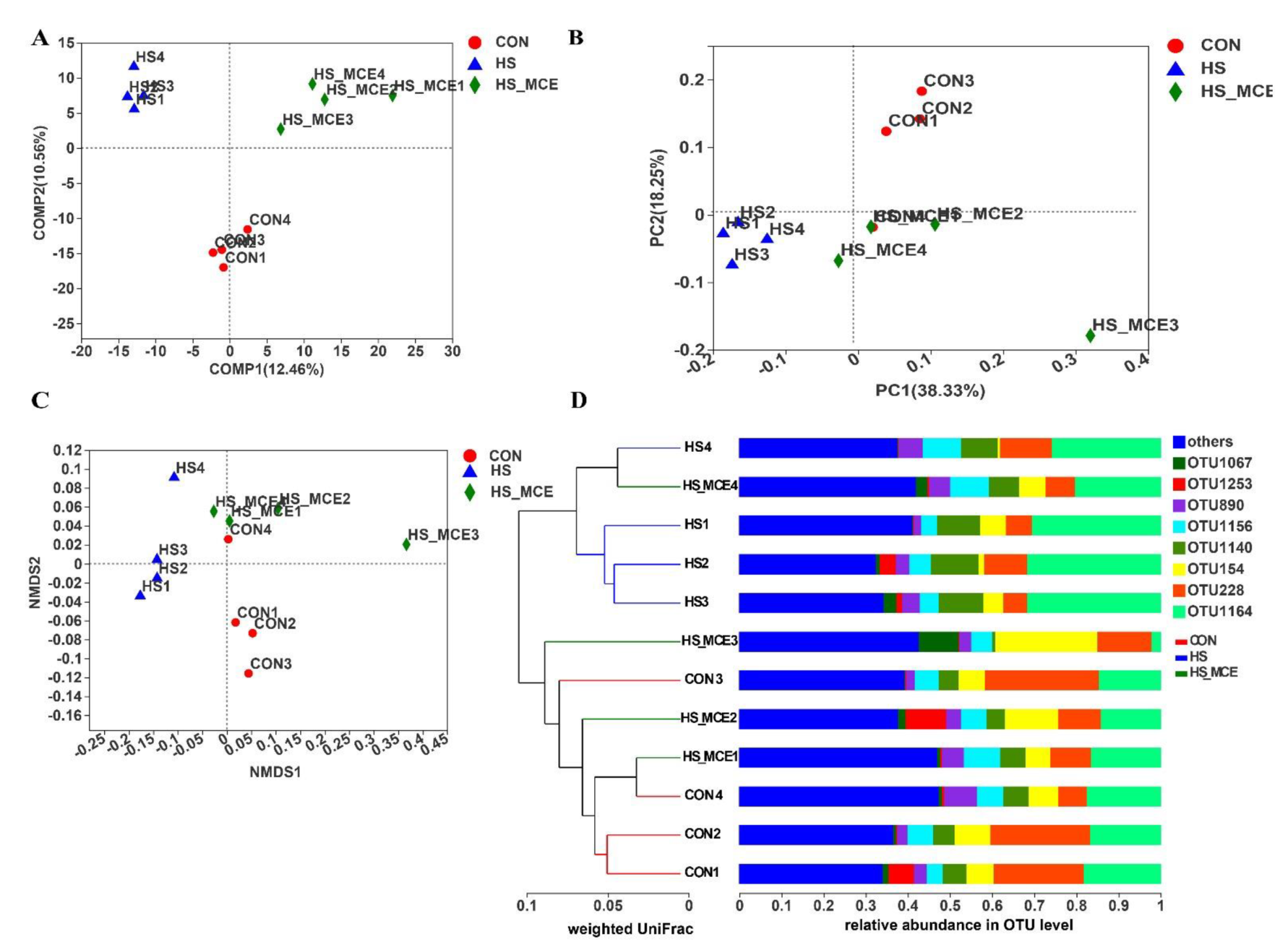
3.4. Effects of HS and MCE on Intestinal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Xue, X.; Liu, Q.; Zhang, S.; Peng, M.; Zhou, J.; Chen, L.; Fang, F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019, 85, 102420. [Google Scholar] [CrossRef]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Mohammed, A.; Jacobs, J.; Murugesan, G.; Cheng, H. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018, 97, 1101–1108. [Google Scholar] [CrossRef]
- Patra, A.K.; Kar, I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Technol. 2021, 63, 211–247. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, K.; Zheng, X.; Ahmad, H.; Zhang, L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, M.; Huang, X.; Zheng, X.; Song, Z.; Zhang, L.; Wang, T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2018, 98, 187–193. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, H.; Ma, T.; Yan, Z.; Zhang, Y.; Geng, Y.; Zhu, Y.; Shi, Y. Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 2020, 91, 102619. [Google Scholar] [CrossRef]
- Bercik, P.; Collins, S.M.; Verdu, E.F. Microbes and the gut-brain axis. Neurogastroenterol. Motil. 2012, 24, 405–413. [Google Scholar] [CrossRef]
- Cao, C.; Chowdhury, V.S.; Cline, M.A.; Gilbert, E.R. The Microbiota-Gut-Brain Axis During Heat Stress in Chickens: A Review. Front. Physiol. 2021, 12, 752265. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Sun, C.; Balasubramanian, B.; Zhao, Z.; An, L. Effects of Dietary Betaine on Growth Performance, Digestive Function, Carcass Traits, and Meat Quality in Indigenous Yellow-Feathered Broilers under Long-Term Heat Stress. Animals 2019, 9, 506. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, Y.; Sun, C.; Balasubramanian, B.; Zhao, Z.; An, L. Effects of Dietary Supplementation of Algae-Derived Polysaccharides on Morphology, Tight Junctions, Antioxidant Capacity and Immune Response of Duodenum in Broilers under Heat Stress. Animals 2021, 11, 2279. [Google Scholar] [CrossRef]
- Liu, W.-C.; Huang, M.-Y.; Balasubramanian, B.; Jha, R. Heat Stress Affects Jejunal Immunity of Yellow-Feathered Broilers and Is Potentially Mediated by the Microbiome. Front. Physiol. 2022, 13, 913696. [Google Scholar] [CrossRef]
- Dong, Z.; Tang, S.-S.; Ma, X.-L.; Li, C.-H.; Tang, Z.-S.; Yang, Z.-H.; Zeng, J.-G. Preclinical Safety Evaluation of Macleaya Cordata Extract: A Re-Assessment of General Toxicity and Genotoxicity Properties in Rodents. Front. Pharmacol. 2022, 13, 3165. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, M.; Zhong, X.; Ou, X.; Yun, X.; Wang, M.; Ren, S.; Qing, Z.; Zeng, J. Identification of the Impurities in Bopu Powder(®) and Sangrovit(®) by LC-MS Combined with a Screening Method. Molecules 2021, 26, 3851. [Google Scholar] [CrossRef]
- Kumar, G.S.; Hazra, S. Sanguinarine, a promising anticancer therapeutic: Photochemical and nucleic acid binding properties. RSC Adv. 2014, 4, 56518–56531. [Google Scholar] [CrossRef]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antimicrobial activity of combinations of sanguinarine and EDTA with vancomycin against multidrug resistant bacteria. Drug Metab. Lett. 2014, 8, 119–128. [Google Scholar] [CrossRef]
- Xue, G.D.; Wu, S.B.; Choct, M.; Pastor, A.; Steiner, T.; Swick, R.A. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017, 96, 3581–3585. [Google Scholar] [CrossRef]
- Wang, P.-Q.; Yin, Z.-H.; Kang, W.-Y. Advance in studies on pharmacological activities of chelerythrine. Zhongguo Zhong Yao Za Zhi 2013, 38, 2745–2749. [Google Scholar]
- Li, W.; Li, H.; Yao, H.; Mu, Q.; Zhao, G.; Li, Y.; Hu, H.; Niu, X. Pharmacokinetic and anti-inflammatory effects of sanguinarine solid lipid nanoparticles. Inflammation 2014, 37, 632–638. [Google Scholar] [CrossRef]
- Hu, N.-X.; Chen, M.; Liu, Y.-S.; Shi, Q.; Yang, B.; Zhang, H.-C.; Cheng, P.; Tang, Q.; Liu, Z.-Y.; Zeng, J.-G. Pharmacokinetics of sanguinarine, chelerythrine, and their metabolites in broiler chickens following oral and intravenous administration. J. Vet. Pharmacol. Ther. 2019, 42, 197–206. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhong, L.; Chen, T.; Shi, Y.; Hu, Y.; Zeng, J.-G.; Liu, H.-Y.; Xu, S.-D. Dietary sanguinarine supplementation on the growth performance, immunity and intestinal health of grass carp (Ctenopharyngodon idellus) fed cottonseed and rapeseed meal diets. Aquaculture 2020, 528, 735521. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Chu, Q.; Xu, F.; Liang, T.; Zhou, B. Macleaya cordata extracts suppressed the increase of a part of antibiotic resistance genes in fecal microorganism of weaned pigs. Can. J. Anim. Sci. 2018, 98, 884–887. [Google Scholar] [CrossRef]
- Michels, A.; Neumann, M.; Leão, G.F.M.; Reck, A.M.; Bertagnon, H.G.; Lopes, L.S.; De Souza, A.M.; Dos Santos, L.C.; Júnior, E.S.S. Isoquinoline alkaloids supplementation on performance and carcass traits of feedlot bulls. Asian-Australas J. Anim. Sci. 2018, 31, 1474–1480. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, X.-L.; Ou, S.-Q.; Hou, D.-X.; He, J.-H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS ONE 2020, 15, e0234920. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yin, Y.; Yang, M.; Chen, J.; Fu, C.; Huang, K. Effects of Combined Supplementation of Macleaya cordata Extract and Benzoic Acid on the Growth Performance, Immune Responses, Antioxidant Capacity, Intestinal Morphology, and Microbial Composition in Weaned Piglets. Front. Vet. Sci. 2021, 8, 708597. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, B.; Zhao, Y.; Yao, K.; Fu, C. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1666–1674. [Google Scholar] [CrossRef]
- He, S.; Li, S.; Arowolo, M.A.; Yu, Q.; Chen, F.; Hu, R.; He, J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019, 90, 401–411. [Google Scholar] [CrossRef]
- Khadem, A.; Soler, L.; Everaert, N.; Niewold, T.A. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br. J. Nutr. 2014, 112, 1110–1118. [Google Scholar] [CrossRef]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef]
- Moustafa, E.; Alsanie, W.; Gaber, A.; Kamel, N.; Alaqil, A.; Abbas, A. Blue-Green Algae (Spirulina platensis) Alleviates the Negative Impact of Heat Stress on Broiler Production Performance and Redox Status. Animals 2021, 11, 1243. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Mack, L.A.; Felver-Gant, J.N.; Dennis, R.L.; Cheng, H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013, 92, 285–294. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lei, J.; Liu, L.; Qu, X.; Li, P.; Liu, X.; Guo, Y.; Gao, Q.; Lan, F.; Xiao, B.; et al. Effects of Macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poult. Sci. 2021, 100, 101031. [Google Scholar] [CrossRef]
- Bussabong, P.; Rairat, T.; Chuchird, N.; Keetanon, A.; Phansawat, P.; Cherdkeattipol, K.; Pichitkul, P.; Kraitavin, W. Effects of isoquinoline alkaloids from Macleaya cordata on growth performance, survival, immune response, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0251343. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Y.; Cheng, Y.; Yan, Q.; Zhou, C.; He, Z.; Zeng, J.; He, J.; Tan, Z. Supplementation of Lactobacillus plantarum or Macleaya cordata Extract Alleviates Oxidative Damage Induced by Weaning in the Lower Gut of Young Goats. Animals 2020, 10, 548. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Cui, Y.; Sun, R.-Y.; Liang, W.-W.; Wang, L.-J.; Wang, W.-Y.; Lv, Q.; Hu, J. Effects of Taurine on Bowel Inflammatory Factor of Small Intestinal Mucosa Impaired by Heat Stress in Broilers. Adv. Exp. Med. Biol. 2019, 1155, 1049–1056. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, L.; Chen, Y.-Z.; Guo, Y.-X.; Sun, Y.; Guo, Q.; Duan, G.-X.; Li, C.; Tang, Z.-B.; Zhang, Z.-X.; et al. Preventive effect of sanguinarine on intestinal injury in mice exposed to whole abdominal irradiation. Biomed Pharm. 2022, 146, 112496. [Google Scholar] [CrossRef]
- Niu, X.-F.; Zhou, P.; Li, W.-F.; Xu, H.-B. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 2011, 82, 620–625. [Google Scholar] [CrossRef]
- Bitterman, S.; Ben Shahar, Y.; Pollak, Y.; Bitterman, N.; Halabi, S.; Coran, A.G.; Bitterman, A.; Sukhotnik, I. Effect of Chelerythrine on Intestinal Cell Turnover following Intestinal Ischemia-Reperfusion Injury in a Rat Model. Eur. J. Pediatr. Surg. 2017, 27, 36–43. [Google Scholar] [CrossRef]
- Gogoi, S.; Kolluri, G.; Tyagi, J.S.; Marappan, G.; Manickam, K.; Narayan, R. Impact of heat stress on broilers with varying body weights: Elucidating their interactive role through physiological signatures. J. Therm. Biol. 2021, 97, 102840. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Ding, S.; Yin, Y.; Duraipandiyan, V.; Al-Dhabi, N.A.; Liu, G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci. China Life Sci. 2019, 62, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, Y.; Wen, C.; Zhou, Y. Protective effects of dietary mannan oligosaccharide on heat stress-induced hepatic damage in broilers. Environ. Sci. Pollut. Res. Int. 2020, 27, 29000–29008. [Google Scholar] [CrossRef]
- Bavarsadi, M.; Mahdavi, A.H.; Mahyari, S.A.; Jahanian, E. Effects of different levels of sanguinarine on antioxidant indices, immunological responses, ileal microbial counts and jejunal morphology of laying hens fed diets with different levels of crude protein. J. Anim. Physiol. Anim. Nutr. 2017, 101, 936–948. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Liu, Y.; Hu, Z.-F.; Zhang, Y.; Ni, J.; Ma, Z.-G.; Liao, H.-H.; Wu, Q.-Q.; Tang, Q.-Z. Sanguinarine Attenuates Lipopolysaccharide-induced Inflammation and Apoptosis by Inhibiting the TLR4/NF-κB Pathway in H9c2 Cardiomyocytes. Curr. Med. Sci. 2018, 38, 204–211. [Google Scholar] [CrossRef]
- Elazab, S.; Elshater, N.; Kishaway, A.; Ei-Emam, H. Cinnamon Extract and Probiotic Supplementation Alleviate Copper-Induced Nephrotoxicity via Modulating Oxidative Stress, Inflammation, and Apoptosis in Broiler Chickens. Animals 2021, 11, 1609. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, G.; Wang, G.; Liu, Y.; Zhang, L.; Wang, W.; Chen, N. Association of serum uric acid levels with the incident of kidney disease and rapid eGFR decline in Chinese individuals with eGFR > 60 mL/min/1.73 m(2) and negative proteinuria. Clin. Exp. Nephrol. 2019, 23, 871–879. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, S.; Yin, B.; Xu, J.; Di, L.; Zhang, J.; Bao, E. Heat stress-induced renal damage in poultry and the protective effects of HSP60 and HSP47. Cell Stress Chaperones 2018, 23, 1033–1040. [Google Scholar] [CrossRef]
- Lamp, O.; Derno, M.; Otten, W.; Mielenz, M.; Nürnberg, G.; Kuhla, B. Metabolic Heat Stress Adaption in Transition Cows: Differences in Macronutrient Oxidation between Late-Gestating and Early-Lactating German Holstein Dairy Cows. PLoS ONE 2015, 10, e0125264. [Google Scholar] [CrossRef]
- Liu, G.; Aguilar, Y.M.; Zhang, L.; Ren, W.; Chen, S.; Guan, G.; Xiong, X.; Liao, P.; Li, T.; Huang, R.; et al. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs. J. Anim. Sci. 2016, 94 (Suppl. 3), 75–78. [Google Scholar] [CrossRef][Green Version]
- Dršata, J.; Ulrichová, J.; Walterová, D. Sanguinarine and Chelerythrine as Inhibitors of Aromatic Amino Acid Decarboxylase. J. Enzym. Inhib. 1996, 10, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mellor, S. Natural appetisers from plants. Feed. Mix. 2001, 9, 29–31. [Google Scholar]
- Vieira, S.L.; Oyarzabal, O.; Freitas, D.M.; Berres, J.; Peña, J.E.M.; Torres, C.A.; Coneglian, J.L.B. Performance of Broilers Fed Diets Supplemented with Sanguinarine-Like Alkaloids and Organic Acids1. J. Appl. Poult. Res. 2008, 17, 128–133. [Google Scholar] [CrossRef]
- Herbert, J.-M.; Savi, P.; Laplace, M.-C.; Dumas, A.; Dol, F. Chelerythrine, a selective protein kinase C inhibitor, counteracts pyrogen-induced expression of tissue factor without effect on thrombomodulin down-regulation in endothelial cells. Thromb. Res. 1993, 71, 487–493. [Google Scholar] [CrossRef]
- Nowak, G.; Takacsova-Bakajsova, D.; Megyesi, J. Deletion of protein kinase C-ε attenuates mitochondrial dysfunction and ameliorates ischemic renal injury. Am. J. Physiol. Renal. Physiol. 2017, 312, F109–F120. [Google Scholar] [CrossRef]
- Ozer, E.K.; Goktas, M.T.; Kilinc, I.; Bariskaner, H.; Ugurluoglu, C.; Iskit, A.B. Celecoxib administration reduced mortality, mesenteric hypoperfusion, aortic dysfunction and multiple organ injury in septic rats. Biomed Pharm. 2017, 86, 583–589. [Google Scholar] [CrossRef]
- Hosseini-Vashan, S.J.; Golian, A.; Yaghobfar, A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016, 60, 1183–1192. [Google Scholar] [CrossRef]
- Wan, X.; Jiang, L.; Zhong, H.; Lu, Y.; Zhang, L.; Wang, T. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim. Sci. J. 2017, 88, 1239–1246. [Google Scholar] [CrossRef]
- Ke, W.; Lin, X.; Yu, Z.; Sun, Q.; Zhang, Q. Molluscicidal activity and physiological toxicity of Macleaya cordata alkaloids components on snail Oncomelania hupensis. Pestic. Biochem. Physiol. 2017, 143, 111–115. [Google Scholar] [CrossRef]
- Liu, X.-W.; Tang, C.-L.; Zheng, H.; Wu, J.-X.; Wu, F.; Mo, Y.-Y.; Liu, X.; Zhu, H.-J.; Yin, C.-L.; Cheng, B.; et al. Investigation of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced liver fibrosis in rats by (1)H-NMR-based metabonomics and network pharmacology approaches. J. Pharm. Biomed. Anal. 2018, 159, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Huang, Y.-J.; Zhang, Z.-Y.; Liu, Y.-S.; Liu, Z.-Y. Metabolism and Tissue Distribution of Chelerythrine and Effects of Macleaya Cordata Extracts on Liver NAD(P)H Quinone Oxidoreductase. Front. Vet. Sci. 2021, 8, 659771. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, L.; Wang, Y.; Liu, L.; Zhong, M.; He, X.; Liu, Y. Experimental study on antagonizing liver fibrosis of Macleaya cordata extract. Chin. J. Exp. Tradit. Med. Formulae 2012, 1, 135–140. [Google Scholar]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Ryu, S.-T.; Park, B.-S.; Bang, H.-T.; Kang, H.-K.; Hwangbo, J. Effects of anti-heat diet and inverse lighting on growth performance, immune organ, microorganism and short chain fatty acids of broiler chickens under heat stress. J. Environ. Biol. 2016, 37, 185–192. [Google Scholar] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.; Pragna, P.; Lees, A.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef]
- Xie, J.; Tang, L.; Lu, L.; Zhang, L.; Lin, X.; Liu, H.-C.; Odle, J.; Luo, X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015, 94, 1635–1644. [Google Scholar] [CrossRef]
- Dvořák, Z.; Vrzal, R.; Maurel, P.; Ulrichová, J. Differential effects of selected natural compounds with anti-inflammatory activity on the glucocorticoid receptor and NF-kappaB in HeLa cells. Chem. Biol. Interact. 2006, 159, 117–128. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Gong, L.; Xiao, G.; Zheng, L.; Yan, X.; Qi, Q.; Zhu, C.; Feng, X.; Huang, W.; Zhang, H. Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers. Animals 2021, 11, 3425. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Zhang, M.; Li, X.; Ma, D.; Chang, S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018, 97, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Wu, N.; Zhu, G.; Yang, C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019, 103, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, H.; Song, Y.; Sun, C.; Zhang, Z.; Chen, S. Community composition of cecal microbiota in commercial yellow broilers with high and low feed efficiencies. Poult. Sci. 2021, 100, 100996. [Google Scholar] [CrossRef]
- Torok, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacAlpine, R.; Percy, N.J.; Ophel-Keller, K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Hart, M.L.; Ericsson, A.C.; Franklin, C.L. Differing Complex Microbiota Alter Disease Severity of the IL-10(-/-) Mouse Model of Inflammatory Bowel Disease. Front. Microbiol. 2017, 8, 792. [Google Scholar] [CrossRef]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C.; et al. Evaluation of the Epithelial Barrier Function and Ileal Microbiome in an Established Necrotic Enteritis Challenge Model in Broiler Chickens. Front. Vet. Sci. 2018, 5, 199. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.L.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Microb. Ecol. Divers. 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Van Immerseel, F. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003, 85, 237–248. [Google Scholar] [CrossRef]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Hu, Z.P.; Lu, C.H.; Yang, M.X.; Zhang, L.L.; Wang, T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015, 93, 1656–1665. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Le Sciellour, M.; Zemb, O.; Hochu, I.; Riquet, J.; Gilbert, H.; Giorgi, M.; Billon, Y.; Gourdine, J.-L.; Renaudeau, D. Effect of chronic and acute heat challenges on fecal microbiota composition, production, and thermoregulation traits in growing pigs1,2. J. Anim. Sci. 2019, 97, 3845–3858. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Shabbir, M.Z.; Ijaz, A.; Rehman, H. Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian. Pathol. 2015, 44, 67–74. [Google Scholar] [CrossRef]
- Xing, S.; Wang, X.; Diao, H.; Zhang, M.; Zhou, Y.; Feng, J. Changes in the cecal microbiota of laying hens during heat stress is mainly associated with reduced feed intake. Poult. Sci. 2019, 98, 5257–5264. [Google Scholar] [CrossRef]
- Goel, A.; Kim, B.-J.; Ncho, C.-M.; Jeong, C.-M.; Gupta, V.; Jung, J.-Y.; Ha, S.-Y.; Lee, D.-H.; Yang, J.-K.; Choi, Y.-H. Dietary Supplementation of Shredded, Steam-Exploded Pine Particles Decreases Pathogenic Microbes in the Cecum of Acute Heat-Stressed Broilers. Animals 2021, 11, 2252. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, P.; Wang, Q.; Ling, H.; Jiang, S.; Zhang, G. Effects of veterinary boluohui powder on the growth of commonly used probiotics. Feed. Ind. 2018, 39, 40–44. [Google Scholar] [CrossRef]

| Item | Amount |
|---|---|
| Ingredient (%) | |
| Corn | 63.26 |
| Soybean meal | 28.00 |
| Corn gluten meal | 2.00 |
| Soybean oil | 2.50 |
| Limestone | 1.41 |
| Dicalcium phosphate | 1.33 |
| DL-Met | 0.15 |
| L-Lys-HCl | 0.18 |
| Wheat middling | 0.17 |
| Vitamin-mineral premix 1 | 1.00 |
| Calculated nutrient composition 2 | |
| ME (MJ/kg) | 12.54 |
| CP (%) | 18.63 |
| Lys (%) | 1.00 |
| Met (%) | 0.46 |
| Ca (%) | 0.88 |
| Available p (%) | 0.40 |
| Item | Treatment | ||
|---|---|---|---|
| CON | HS | HS-MCE | |
| Initial BW, g | 365.00 ± 0.29 | 365.06 ± 0.32 | 365.33 ± 0.40 |
| Final BW, g | 838.88 ± 6.29 a | 745.88 ± 11.81 c | 769.38 ± 2.47 b |
| ADFI, g/d | 64.21 ± 1.22 a | 51.61 ± 0.99 c | 54.86 ± 0.59 b |
| ADG, g/d | 33.85 ± 0.43 a | 27.20 ± 0.84 c | 28.85 ± 0.17 b |
| FCR, g/g | 1.90 ± 0.02 | 1.91 ± 0.05 | 1.90 ± 0.02 |
| Item (% BW) | Treatment | ||
|---|---|---|---|
| CON | HS | HS-MCE | |
| Bursa index | 0.24 ± 0.03 | 0.18 ± 0.02 | 0.19 ± 0.02 |
| Spleen index | 0.19 ± 0.007 a | 0.14 ± 0.004 c | 0.16 ± 0.007 b |
| Liver index | 2.51 ± 0.06 a | 2.18 ± 0.10 b | 2.44 ± 0.11 a |
| Item | Treatment | ||
|---|---|---|---|
| CON | HS | HS-MCE | |
| Uric acid (mmol/L) | 184.50 ± 11.20 | 207.67 ± 16.59 | 208.33 ± 20.07 |
| Creatinine (mmol/L) | 3.14 ± 0.54 ab | 4.49 ± 0.38 a | 2.27 ± 0.61 b |
| AST(U/L) | 252.01 ± 10.31 b | 283.89 ± 6.43 a | 255.00 ± 9.85 b |
| ALT(U/L) | 7.40 ± 0.90 b | 11.55 ± 1.14 a | 8.05 ± 0.54 b |
| ALP(U/L) | 1657.56 ± 64.57 a | 1307.57 ± 144.14 b | 1880.54 ± 113.55 a |
| LDH(U/L) | 1191.33 ± 100.99 b | 1729.17 ± 108.84 a | 1297.33 ± 88.89 b |
| TP (g/L) | 35.89 ± 1.41 | 33.17 ± 1.30 | 36.71 ± 1.60 |
| TC (mmol/L) | 3.04 ± 0.26 b | 3.60 ± 0.15 a | 3.20 ± 0.09 ab |
| LDL-C (mmol/L) | 0.98 ± 0.07 b | 1.16 ± 0.04 a | 1.02 ± 0.06 ab |
| HDL-C (mmol/L) | 2.27 ± 0.12 | 1.98 ± 0.16 | 2.22 ± 0.05 |
| GLU, mol/L | 9.28 ± 0.24 a | 8.09 ± 0.37 b | 9.24 ± 0.45 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, J.; Huang, X.; Liu, Y.; Zeng, J. Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress. Animals 2022, 12, 2197. https://doi.org/10.3390/ani12172197
Wang M, Zhang J, Huang X, Liu Y, Zeng J. Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress. Animals. 2022; 12(17):2197. https://doi.org/10.3390/ani12172197
Chicago/Turabian StyleWang, Mingcan, Junkai Zhang, Xiuqiong Huang, Yisong Liu, and Jianguo Zeng. 2022. "Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress" Animals 12, no. 17: 2197. https://doi.org/10.3390/ani12172197
APA StyleWang, M., Zhang, J., Huang, X., Liu, Y., & Zeng, J. (2022). Effects of Dietary Macleaya cordata Extract on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers Subjected to Chronic Heat Stress. Animals, 12(17), 2197. https://doi.org/10.3390/ani12172197







