Blood Transfusion in Equids—A Practical Approach and Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Disorders Requiring Transfusion
2.1. Whole Blood Loss
2.1.1. Traumatic Blood Loss
2.1.2. Surgical Blood Loss
2.1.3. Hemostatic Disorders
2.2. Anemia
Anemia of Chronic Disease
2.3. Other Disorders Requiring Transfusion
3. Indications for Transfusion
4. Practicalrfttttities of Transfusion
4.1. Donor Selection
4.2. Blood Donor Disease Screening
4.3. Biological Products and Hepacivirues
4.4. Cross Matching and Blood Typing
4.4.1. Blood Typing
4.4.2. Cross Matching
4.5. Other Tests
4.6. Blood Volume Calculations
4.7. Collection and Transfusion Practicalities
Washed Red Cells
5. Contraindications and Complications of Transfusion
5.1. Transfusion Reactions
5.2. Incompatible Blood
5.3. Long Term Complications
6. Neonatal Alloimmune Disorders
7. Donkey Factor
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farley, A.; Hendry, C.; McLafferty, E. Blood components. Nurs. Stand. 2012, 27, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Robie, S.M.; Janson, C.H.; Smith, S.C.; O’Connor, J.T. Equine serum lipids: Lipid composition and electrophoretic mobility of equine serum lipoprotein fractions. Am. J. Vet. Res. 1975, 36, 1715–1717. [Google Scholar]
- Ramsey, G. Hemostatic Efficacy of Pathogen-Inactivated Blood Components. Semin. Thromb. Hemost. 2015, 42, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Cap, A.P.; Beckett, A.; Benov, A.; Borgman, M.; Chen, J.; Corley, J.B.; Doughty, H.; Fisher, A.; Glassberg, E.; Gonzales, R.; et al. Whole Blood Transfusion. Mil. Med. 2018, 183 (Suppl. S2), 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, C.L.; Magdesian, K.G. Review of Packed Cell Volume and Total Protein for Use in Equine Practice. AAEP Proc. 2011, 4, 321. [Google Scholar]
- Wickler, S.J.; Anderson, T.P. Hematological changes and athletic performance in horses in response to high altitude (3800 m). Am. J. Physiol. Integr. Comp. Physiol. 2000, 279, R1176–R1181. Available online: https://www.physiology.org/doi/10.1152/ajpregu.2000.279.4.R1176 (accessed on 12 January 2022). [CrossRef]
- de Solis, C.N.; Foreman, J.; Byron, C.; Carpenter, R. Ultrasonographic measurement of spleen volume in horses. Comp. Exerc. Physiol. 2012, 8, 19–25. Available online: https://www.wageningenacademic.com/doi/10.3920/CEP11017 (accessed on 12 January 2022). [CrossRef]
- Mudge, M.C. Acute Hemorrhage and Blood Transfusions in Horses. Vet. Clin. N. Am. Equine Pract. 2022, 30, 427–436. Available online: https://www.sciencedirect.com/science/article/pii/S0749073914000285 (accessed on 1 June 2022). [CrossRef]
- Sharma, S.K.; Sharma, P.; Tyler, L.N. Transfusion of blood and blood products: Indications and complications. Am. Fam. Physician 2011, 83, 719–724. [Google Scholar]
- Hooper, N.; Armstrong, T.J. Hemorrhagic Shock. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470382/ (accessed on 2 May 2022).
- Dobesova, O.; Schwarz, B.; Velde, K.; Jahn, P.; Zert, Z.; Bezdekova, B. Guttural pouch mycosis in horses: A retrospective study of 28 cases. Vet. Rec. 2012, 171, 561. Available online: https://veterinaryrecord.bmj.com/content/171/22/561 (accessed on 14 May 2019). [CrossRef]
- Perkins, G.; Ainsworth, D.M.; Yeager, A. Hemothorax in 2 horses. J. Vet. Intern. Med. 1999, 13, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Dechant, J.E.; Nieto, J.E.; Le Jeune, S.S. Hemoperitoneum in horses: 67 cases (1989–2004). J. Am. Vet. Med. Assoc. 2006, 229, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.E.; Payne, M.; Thompson, J.A.; Slovis, N.M.; Bain, F.T. Periparturient hemorrhage in mares: 73 cases (1998–2005). J. Am. Vet. Med. Assoc. 2008, 232, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, M.A.; Nambo, Y.; Miyamoto, M.; Miura, H.; Kikuchi, M.; Ohnami, Y. Postpartum Massive Hematoma within the Broad Ligament of the Uterus in a Broodmare Possibly Caused by Rupture of the Uterine Artery. J. Equine Sci. 2009, 20, 41–46. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4013962/ (accessed on 28 June 2022). [CrossRef]
- Ueno, T.; Nambo, Y.; Tajima, Y.; Umemura, T. Pathology of lethal peripartum broad ligament haematoma in 31 Thoroughbred mares. Equine Vet. J. 2010, 42, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Laing, J. Hutchins Progressive ethmoidal haematoma in horses. Aust. Vet. J. 1992, 69, 57–58. [Google Scholar] [CrossRef]
- Matsuda, Y.; Nakanishi, Y.; Mizuno, Y. Occlusion of the Internal Carotid Artery by Means of Microcoils for Preventing Epistaxis Caused by Guttural Pouch Mycosis in Horses. J. Vet. Med. Sci. 1999, 61, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Lording, P.M. Erythrocytes. Vet. Clin. N. Am. Equine Pract. 2008, 24, 225–237. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Ahern, B.; Parente, E.J. Surgical Complications of the Equine Upper Respiratory Tract. Vet. Clin. N. Am. Equine Pract. 2008, 24, 465–484. [Google Scholar] [CrossRef]
- Mudge, M.C. Hemostasis, Surgical Bleeding, and Transfusion. In Equine Surgery, 5th ed.; Saunders Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–53. [Google Scholar]
- Corona, D.; Brunisholz, H.; Junge, H.; Bettschart-Wolfensberger, R. Management of acute bleeding in a shetland pony during surgery for foreign body removal. Vet. Rec. Case Rep. 2019, 7, e000737. [Google Scholar] [CrossRef]
- Leise, B.S. Complications of Surgery of the Equine Foot. Complicat. Equine Surg. 2021, 47, 667–682. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119190332.ch47 (accessed on 24 January 2022).
- Pezzanite, L.; Dvm, J.T.E. Complications Following Surgery of the Equine Nasal Passages and Paranasal Sinuses. Complicat. Equine Surg. 2021, 33, 413–426. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119190332.ch33 (accessed on 24 January 2022).
- Pezzanite, L.M.; Hackett, E.S.; McCready, E.; Easley, J.T. Outcomes following single, caudally based bilateral versus unilateral frontonasal sinusotomy for treatment of equine paranasal sinus disease. Vet. Med. Sci. 2021, 7, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Mudge, M.C. Complications of Blood Transfusion. In Complications in Equine Surgery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 64–69. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119190332.ch8 (accessed on 13 January 2022).
- Verwilghen, D. Surgical Techniques. In Equine Surgery, 5th ed.; Saunders Elsevier: Amsterdam, The Netherlands, 2018; pp. 198–213. [Google Scholar]
- Dahlgren, A.R.; Tablin, F.; Finno, C.J. Genetics of equine bleeding disorders. Equine Vet. J. 2020, 53, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Epstein, K.L. Coagulopathies in Horses. Vet. Clin. Equine Pract. 2014, 30, 437–452. Available online: https://www.vetequine.theclinics.com/article/S0749-0739(14)00026-1/abstract (accessed on 15 January 2019). [CrossRef]
- Pusterla, N.; Watson, J.L.; Affolter, V.K.; Magdesian, K.G.; Wilson, W.D.; Carlson, G.P. Purpura haemorrhagica in 53 horses. Vet. Rec. 2003, 153, 118–121. [Google Scholar] [CrossRef]
- Barcellini, W.; Fattizzo, B. Clinical Applications of Hemolytic Markers in the Differential Diagnosis and Management of Hemolytic Anemia. Dis. Mark. 2015, 2015, 635670. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4706896/ (accessed on 1 June 2022). [CrossRef] [Green Version]
- Ramaiah, S.K.; Harvey, J.W.; Giguère, S.; Franklin, R.P.; Crawford, P.C. Intravascular Hemolysis Associated with Liver Disease in a Horse with Marked Neutrophil Hypersegmentation. J. Vet. Intern. Med. 2003, 17, 360–363. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1939-1676.2003.tb02463.x (accessed on 1 June 2022). [CrossRef]
- Mair, T.S.; Taylor, F.G.; Hillyer, M.H. Autoimmune haemolytic anaemia in eight horses. Vet. Rec. 1990, 126, 51–53. [Google Scholar]
- Underwood, C.; Southwood, L.L. Haemolytic anaemia as a complication following colic surgery in a 10-year-old Arabian stallion. Equine Vet. Educ. 2008, 20, 422–426. Available online: http://doi.wiley.com/10.2746/095777308X321134 (accessed on 1 June 2022). [CrossRef]
- Dickinson, C.E.; Traub-Dargatz, J.L.; Dargatz, A.D.; Bennett, D.G.; Knight, A.P. Rattlesnake venom poisoning in horses: 32 cases (1973–1993). J. Am. Vet. Med. Assoc. 1996, 208, 1866–1871. [Google Scholar] [PubMed]
- Fielding, C.L.; Pusterla, N.; Magdesian, K.G.; Higgins, J.C.; Meier, C.A. Rattlesnake envenomation in horses: 58 cases (1992–2009). J. Am. Vet. Med. Assoc. 2011, 238, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.J.; Mollat, W.H.; Bryant, J.E. Overview of Clinical Pathology and the Horse. Vet. Clin. N. Am. Equine Pract. 2015, 31, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Hardang, I.M.; Lilleholt, K.; Hagve, T.A. Anemia of chronic disease. Tidsskr. Nor. Laegeforen. 2017, 137, 1011–1023. [Google Scholar]
- Guan, F.; Uboh, C.E.; Soma, L.R.; Maylin, G.; Jiang, Z.; Chen, J. Confirmatory Analysis of Continuous Erythropoietin Receptor Activator and Erythropoietin Analogues in Equine Plasma by LC−MS for Doping Control. Anal. Chem. 2010, 82, 9074–9081. [Google Scholar] [CrossRef] [PubMed]
- Piercy, R.J.; Swardson, C.J.; Hinchcliff, K. Erythroid hypoplasia and anemia following administration of recombinant human erythropoietin to two horses. J. Am. Vet. Med. Assoc. 1998, 212, 244–247. [Google Scholar]
- Timms, M.; Steel, R. Defining the specificity of recombinant human erythropoietin confirmation in equine samples by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2021, 14, 676–689. [Google Scholar] [CrossRef]
- Tennent-Brown, B. Plasma therapy in foals and adult horses. Compendium 2011, 33, E1–E4. [Google Scholar]
- Slovis, N.M.; Murray, G. How to Approach Whole Blood Transfusions in Horses. AAEP Proc. 2001, 47, 266–269. [Google Scholar]
- McLellan, S.A.; Walsh, T.S. Oxygen delivery and haemoglobin. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 123–126. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1743181617306066 (accessed on 15 May 2022). [CrossRef]
- Durham, A.E. Blood and plasma transfusion in the horse. Equine Vet. Educ. 1996, 8, 8–12. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.2042-3292.1996.tb01643.x (accessed on 13 January 2022). [CrossRef]
- Arai, S.; Stotts, N.; Puntillo, K. Thirst in Critically Ill Patients: From Physiology to Sensation. Am. J. Crit. Care 2013, 22, 328–335. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3718499/ (accessed on 10 May 2022). [CrossRef] [PubMed] [Green Version]
- Gerhardt, R.T.; Tactical Combat Casualty Care Rsch Group. Abstract 170: Correlation of Thirst and Vasopressin to Central Hypovolemia in a Lower-Body Negative Pressure Model of Hemorrhagic Shock. Circulation 2012, 126 (Suppl. S21), A170. Available online: https://www.ahajournals.org/doi/10.1161/circ.126.suppl_21.A170 (accessed on 10 May 2022).
- Gonzales, G.L. How to Establish an Equine Blood Donor Protocol. In Proceedings of the Annual Convention of the American Association of Equine Practicioners (AAEP), San Diego, CA, USA, 6–10 December 2001; p. 4. [Google Scholar]
- Radcliffe, R.M.; Bookbinder, L.C.; Liu, S.Y.; Tomlinson, J.E.; Cook, V.L.; Hurcombe, S.D.A.; Divers, T.J. Collection and administration of blood products in horses: Transfusion indications, materials, methods, complications, donor selection, and blood testing. J. Vet. Emerg. Crit. Care 2022, 32, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Divers, T.J.; Tennant, B.C.; Kumar, A.; McDonough, S.; Cullen, J.; Bhuva, N.; Jain, K.; Chauhan, L.S.; Scheel, T.; Lipkin, W.I.; et al. New Parvovirus Associated with Serum Hepatitis in Horses after Inoculation of Common Biological Product. Emerg. Infect. Dis. 2018, 24, 303–310. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Tennant, B.C.; Struzyna, A.; Mrad, D.; Browne, N.; Whelchel, D.; Johnson, P.J.; Jamieson, C.; Löhr, C.V.; Bildfell, R.; et al. Viral testing of 10 cases of Theiler’s disease and 37 in-contact horses in the absence of equine biologic product administration: A prospective study (2014–2018). J. Vet. Intern. Med. 2018, 33, 258–265. [Google Scholar] [CrossRef]
- Becht, J.L.; Semrad, S.D. Hematology, Blood Typing, and Immunology of the Neonatal Foal. Vet. Clin. N. Am. Equine Pract. 1985, 1, 91–116. [Google Scholar] [CrossRef]
- Bowling, A. The use and efficacy of horse blood typing tests. J. Equine Vet. Sci. 1985, 5, 195–199. Available online: https://www.sciencedirect.com/science/article/pii/S0737080685800964 (accessed on 12 January 2022). [CrossRef]
- Proverbio, D.; Perego, R.; Baggiani, L.; Ferrucci, F.; Zucca, E.; Nobile, F.; Spada, E. Prevalence of Ca Blood Type and Alloantibodies in a Population of Horses from Italy. Animals 2020, 10, 1179. [Google Scholar] [CrossRef]
- Tomlinson, J.; Taberner, E.; Boston, R.; Owens, S.; Nolen-Walston, R. Survival Time of Cross-Match Incompatible Red Blood Cells in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.S.; Bortsie-Aryee, A.D.; Perkins, G.A.; Mann, S.; Tomlinson, J.E.; Wood, E.M.; Mix, S.E.; Stokol, T. Agreement of stall-side and laboratory major crossmatch tests with the reference standard method in horses. J. Vet. Intern. Med. 2020, 34, 941–948. [Google Scholar] [CrossRef]
- Young, N.A.F. Direct compatibility testing. J. Clin. Pathol. 1958, 11, 311–315. Available online: http://jcp.bmj.com/cgi/doi/10.1136/jcp.11.4.311 (accessed on 12 January 2022). [CrossRef] [PubMed] [Green Version]
- Wardrop, K.J. The Coombs’ test in veterinary medicine: Past, present, future. Vet. Clin. Pathol. 2005, 34, 325–334. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1939-165X.2005.tb00057.x (accessed on 13 May 2022). [CrossRef]
- Kähn, W.; Vaala, W.; Palmer, J. Neonatal isoerythrolysis in newborn foals. Tierarztliche Prax. 1991, 19, 521–529. [Google Scholar]
- Short, J.L.; Diehl, S.; Seshadri, R.; Serrano, S. Accuracy of formulas used to predict post-transfusion packed cell volume rise in anemic dogs. J. Vet. Emerg. Crit. Care 2012, 22, 428–434. [Google Scholar] [CrossRef]
- Niinistö, K.; Raekallio, M.; Sankari, S. Storage of equine red blood cells as a concentrate. Vet. J. 2008, 176, 227–231. [Google Scholar] [CrossRef]
- Schmidt, A.; Refaai, M.; Kirkley, S.; Blumberg, N. Proven and potential clinical benefits of washing red blood cells before transfusion: Current perspectives. Int. J. Clin. Transfus. Med. 2016, 4, 79–88. Available online: https://www.dovepress.com/proven-and-potential-clinical-benefits-of-washing-red-blood-cells-befo-peer-reviewed-article-IJCTM (accessed on 13 May 2022). [CrossRef] [Green Version]
- Polkes, A.; Giguère, S.; Lester, G.; Bain, F. Factors Associated with Outcome in Foals with Neonatal Isoerythrolysis (72 Cases, 1988–2003). J. Vet. Intern. Med. 2008, 22, 1216–1222. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1939-1676.2008.0171.x (accessed on 13 January 2022). [CrossRef]
- Suddock, J.T.; Crookston, K.P. Transfusion Reactions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482202/ (accessed on 13 January 2022).
- Swiderski, C.E. Hypersensitivity Disorders in Horses. Vet. Clin. N. Am. Equine Pract. 2000, 16, 131–151. Available online: https://www.sciencedirect.com/science/article/pii/S0749073917301232 (accessed on 12 May 2022). [CrossRef]
- Radcliffe, R.M. Anaphylaxis. In Equine Clinical Immunology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 31–38. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119086512.ch04 (accessed on 13 January 2022).
- Orsini, J.A.; Divers, T.J. (Eds.) Emergency Drug Charts in Equine Emergencies, 4th ed.; Saunders: St. Louis, MO, USA, 2014; p. IBC6. Available online: https://www.sciencedirect.com/science/article/pii/B9781455708925000672 (accessed on 8 March 2022).
- Toy, P.; Lowell, C. TRALI—Definition, mechanisms, incidence and clinical relevance. Best Pract. Res. Clin. Anaesthesiol. 2007, 21, 183–193. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2767181/ (accessed on 13 January 2022). [CrossRef] [PubMed] [Green Version]
- Cho, M.S.; Modi, P.; Sharma, S. Transfusion-related Acute Lung Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507846/ (accessed on 13 January 2022).
- King, K.E.; Shirey, R.S.; Thoman, S.K.; Bensen-Kennedy, D.; Tanz, W.S.; Ness, P.M. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion 2004, 44, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radtke, A.V.; Goodale, M.B.; Fortier, L.A. Platelet and Leukocyte Concentration in Equine Autologous Conditioned Plasma Are Inversely Distributed by Layer and Are Not Affected by Centrifugation Rate. Front. Vet. Sci. 2020, 7, 173. [Google Scholar] [CrossRef]
- Rasel, M.; Mahboobi, S.K. Transfusion Iron Overload. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK562146/ (accessed on 13 January 2022).
- McKinnon, A.O.; Squires, E.L.; Vaala, W.E.; Varner, D.D. Equine Reproduction; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 7740. [Google Scholar]
- Becht, J.L.; Page, E.H.; Morter, R.L.; Boon, G.D.; Thacker, H.L. Evaluation of a series of testing procedures to predict neonatal isoerythrolysis in the foal. Cornell Vet. 1983, 73, 390–402. [Google Scholar]
- Felippe, J.B. Equine Neonatal Isoerythrolysis. In Interpretation of Equine Laboratory Diagnostics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 251–255. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118922798.ch42 (accessed on 13 May 2022).
- McClure, J.J.; Koch, C.; Traub-Dargatz, J. Characterization of a red blood cell antigen in donkeys and mules associated with neonatal isoerythrolysis. Anim. Genet. 1994, 25, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Choi, S.K.; Cho, Y.J.; Cho, G.J. Neonatal isoerythrolysis in Thoroughbred foals. Korean J. Veter Res. 2011, 51, 55–58. Available online: http://www.kjvr.org/journal/view.php?doi=10.14405/kjvr.2011.51.1.055 (accessed on 13 January 2022). [CrossRef]
- Blackmer, J.M. Strategies for prevention of neonatal isoerythrolysis in horses and mules. Equine Vet. Educ. 2010, 15, 6–10. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.2042-3292.2003.tb01806.x (accessed on 13 January 2022). [CrossRef]
- Perkins, G.; Miller, W.; Divers, T.; Clark, C.; Belgrave, R.; Sellon, D. Ulcerative Dermatitis, Thrombocytopenia, and Neutropenia in Neonatal Foals. J. Vet. Intern. Med. 2005, 19, 211–216. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1939-1676.2005.tb02684.x (accessed on 13 January 2022). [CrossRef]
- Mendoza, F.; Pérez-Écija, R.; Monreal, L.; Estepa, J. Coagulation Profiles of Healthy Andalusian Donkeys are Different than Those of Healthy Horses. J. Vet. Intern. Med. 2011, 25, 967–970. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1939-1676.2011.0748.x (accessed on 13 January 2022). [CrossRef]
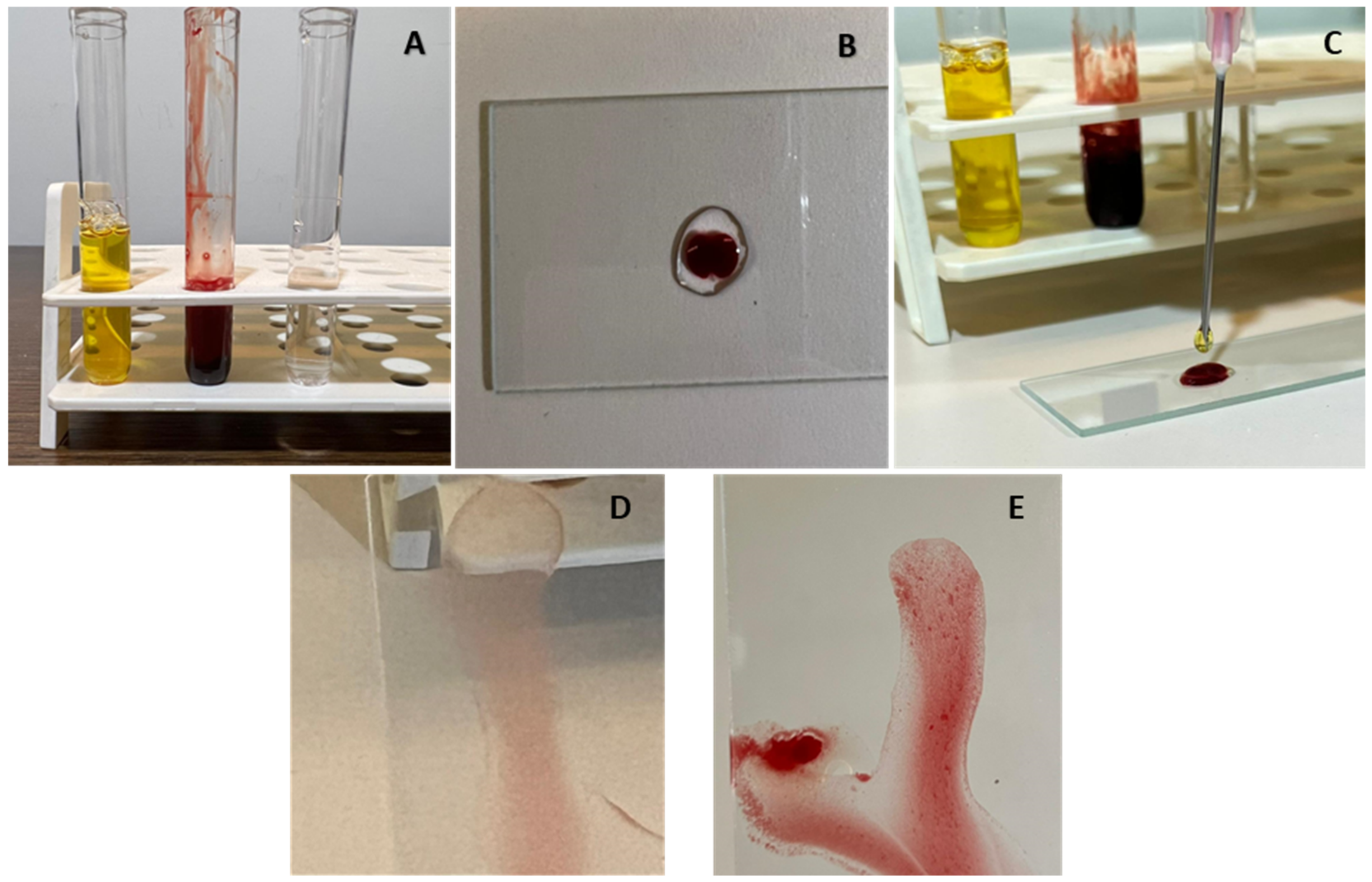
| • Tachycardia |
| ○ The higher the heart rate (HR) the more urgent |
| ○ Severe bleeding may be accompanied by HR > 100 beats/min |
| • Tachypnoea |
| ○ As above |
| • Decreased pulse quality |
| ○ Thready pulses |
| ○ Hard or impossible to palpate |
| • Cool extremities |
| • Pale mucus membranes |
| • Mentation changes |
| ○ Anxiety |
| ○ Distress |
| ○ Depression |
| ○ Compulsive thirst |
| • Hyperlactatemia |
| ○ Serial sampling most helpful |
| ○ Progressive increase indicates decreasing perfusion |
| • Decreased PCV |
| ○ Acute drop of 10% |
| ○ Absolute PCV < 12–15% usually requires |
| ○ ±Decreased TS |
| Alternate Calculations: |
| Blood Volume (BV)—8% BW (kg) |
| 20% of BV |
| Example: |
| 450 kg horse-circulating volume = 36 L |
| 20% of 36 L = 7.2 L |
| Alternately: |
| 10% BV every 4 weeks |
| 7.5% BV every 7 days |
| 1% BV every 24 h |
| Drug | Dose | Dose per 450 kg Horse |
|---|---|---|
| Dexamethasone 2 mg/mL | 0.02–0.1 mg/kg IV | 4–20 mL IV once a day |
| Prednisolone 50 mg/mL | 2–5 mg/kg IV | 18–45 mL IV once a day |
| Epinephrine/Norepinepherine 1:1000 (1 mg/mL) | 0.01–0.02 mg/kg IV-(anaphylaxis) up to 0.5 mg/kg for asystole | 4–9 mL IV repeated up to 3 times—can increase dose up to 225 mL for asystole |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamieson, C.A.; Baillie, S.L.; Johnson, J.P. Blood Transfusion in Equids—A Practical Approach and Review. Animals 2022, 12, 2162. https://doi.org/10.3390/ani12172162
Jamieson CA, Baillie SL, Johnson JP. Blood Transfusion in Equids—A Practical Approach and Review. Animals. 2022; 12(17):2162. https://doi.org/10.3390/ani12172162
Chicago/Turabian StyleJamieson, Camilla A., Sarah L. Baillie, and Jessica P. Johnson. 2022. "Blood Transfusion in Equids—A Practical Approach and Review" Animals 12, no. 17: 2162. https://doi.org/10.3390/ani12172162
APA StyleJamieson, C. A., Baillie, S. L., & Johnson, J. P. (2022). Blood Transfusion in Equids—A Practical Approach and Review. Animals, 12(17), 2162. https://doi.org/10.3390/ani12172162






