Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Evaluation of Russian sturgeon Semen Quality
2.1.1. Spermiation Induction and Semen Collection
2.1.2. Semen Quality Evaluation
2.2. Experiment 2: Cryopreservation of Russian sturgeon Semen
2.2.1. Preliminary Study for Testing the Activation Media
2.2.2. Semen Cryopreservation
2.2.3. Egg Fertilization
2.2.4. Statistical Analysis
3. Results
3.1. Semen Quality Evaluation
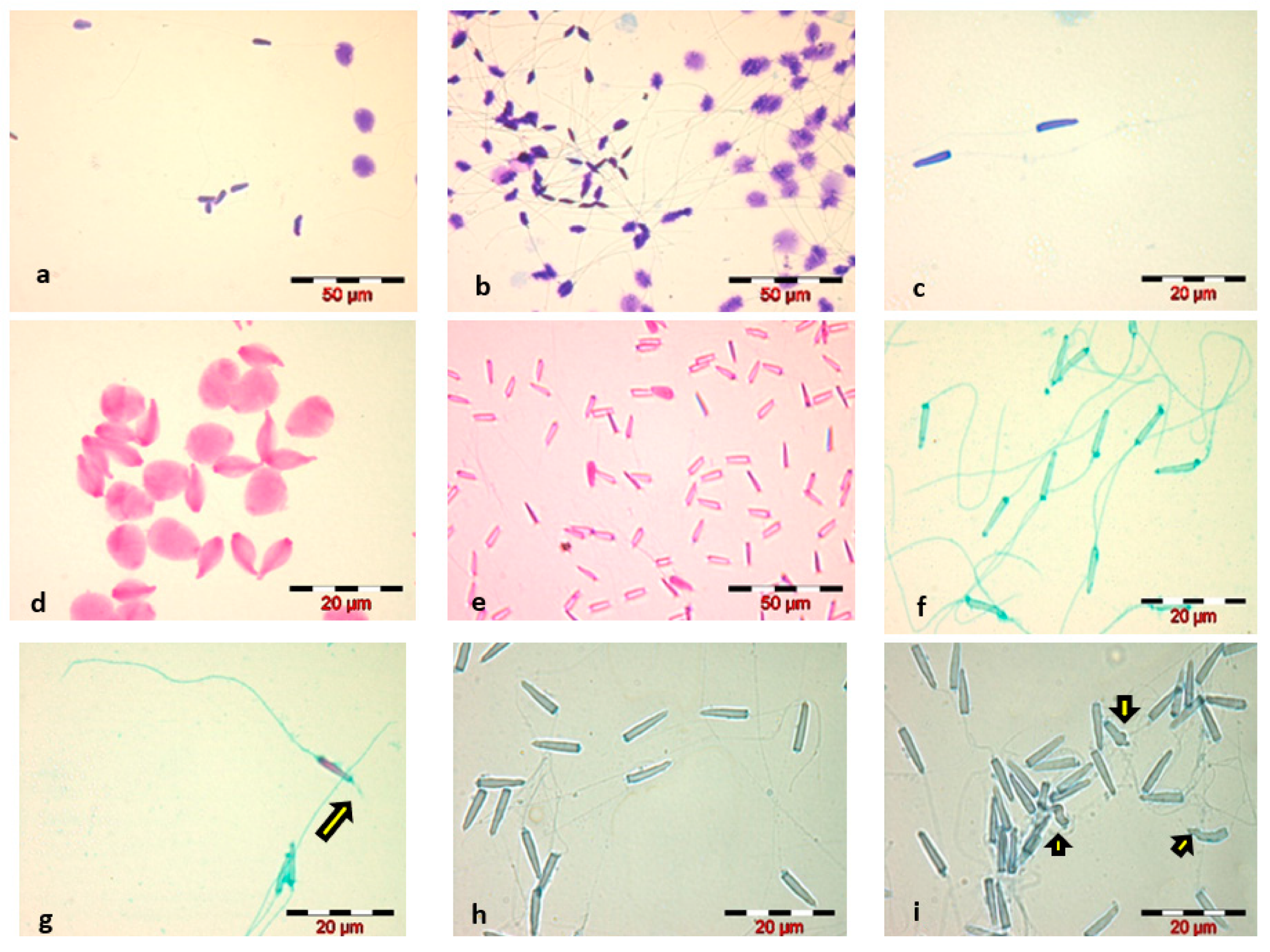
- Diff-Quik staining, WHO 2010 method, caused swelling of the sperm head and did not highlight the sperm tail—Figure 1a;Increasing the staining time to 1 min, for each component of the Diff-Quik kit, slightly stained the tail, but caused the sperm head to swell to a significant extent—Figure 1b;Reducing the immersion time to 5 s, for each of the three components of the Diff-Quik kit, resulted in good staining of the sperm head and avoidance of swelling, but very poor staining of the sperm tail—Figure 1c;
- Eosin staining, in both variants, led to spermatozoa head swelling and faded spermatozoa tail staining—Figure 1d;Decreasing the staining time with Eosin to 5 s eliminated the phenomenon of sperm head swelling that occurred in the classical staining variant, but we considered the staining of spermatozoa inadequate for a morphological and morphometric evaluation of the sperm cell due to poor visualization of the head outline and very poor staining of the spermatozoa tail—Figure 1e;
- Trypan Blue staining showed a proper visualization of the spermatozoa head—Figure 1h.
3.2. Cryopreservation of Russian sturgeon Semen
3.2.1. Testing the Activation Media
3.2.2. Semen Cryopreservation
3.2.3. Egg Fertilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chebanov, M.S.; Galich, E.V. Sturgeon Hatchery Manual; FAO: Ankara, Turkey, 2013. [Google Scholar]
- Hensel, K.; Holčík, J. Past and Current Status of Sturgeons in the Upper and Middle Danube River. Environ. Biol. Fishes 1997, 48, 184–200. [Google Scholar] [CrossRef]
- Gessner, J.; Freyhof, J.; Kottelat, M. Acipenser gueldenstaedtii. The IUCN Red List of Threatened Species 2022: E.T232A135063140. Available online: https://www.iucnredlist.org/species/232/135063140 (accessed on 6 July 2022).
- Skóra, M.E.; Arciszewski, B. New Reports on the Russian sturgeon, Acipenser gueldenstaedtii, in the Polish Baltic Sea. Fish. Aquat. Life 2013, 21, 363–366. [Google Scholar] [CrossRef]
- Kolyada, N.; Osipova, V.P.; Berberova, N.T.; Pimenov, Y.T. The Effect of Tin Compounds on the Lipid Peroxidation Level of Russian sturgeon Fresh and Cryopreserved Sperm. Environ. Res. Eng. Manag. 2020, 76, 34–42. [Google Scholar] [CrossRef]
- Yamaner, G.; Tunçelli, G.; Memiş, D. The Effect of Luteinizing Hormone-Releasing Hormone Analogue and Carp Pituitary Hormones on Russian sturgeon (Acipenser gueldenstaedtii) Sperm Characteristic. Aquac. Res. 2018, 49, 1127–1130. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Hatef, A.; Pšenička, M.; Kašpar, V.; Boryshpolets, S.; Dzyuba, B.; Cosson, J.; Bondarenko, V.; Rodina, M.; Gela, D.; et al. Sperm Biology and Control of Reproduction in Sturgeon: (II) Sperm Morphology, Acrosome Reaction, Motility and Cryopreservation. Rev. Fish Biol. Fish. 2012, 22, 861–886. [Google Scholar] [CrossRef]
- Wrobel, K.H.; Jouma, S. Morphology, Development and Comparative Anatomical Evaluation of the Testicular Excretory Pathway in Acipenser. Ann. Anat. 2004, 186, 99–113. [Google Scholar] [CrossRef]
- Dzyuba, B.; Boryshpolets, S.; Shaliutina, A.; Rodina, M.; Yamaner, G.; Gela, D.; Dzyuba, V.; Linhart, O. Spermatozoa Motility, Cryoresistance, and Fertilizing Ability in Sterlet Acipenser Ruthenus during Sequential Stripping. Aquaculture 2012, 356, 272–278. [Google Scholar] [CrossRef]
- Dzyuba, B.; Cosson, J.; Dzyuba, V.; Fedorov, P.; Bondarenko, O.; Rodina, M.; Linhart, O.; Shelton, W.L.; Boryshpolets, S. Sperm Maturation in Sturgeon (Actinopterygii, Acipenseriformes): A Review. Theriogenology 2017, 97, 134–138. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J.; Bondarenko, O.; Linhart, O. Sperm Motility in Fishes: (III) Diversity of Regulatory Signals from Membrane to the Axoneme. Theriogenology 2019, 136, 143–165. [Google Scholar] [CrossRef]
- Billard, R. Spermatogenesis and Spermatology of Some Teleost Fish Species. Reprod. Nutr. Dev. 1986, 26, 877–920. [Google Scholar] [CrossRef]
- Linhart, O.; Mims, S.D.; Shelton, W.L. Motility of Spermatozoa from Shovelnose Sturgeon and Paddlefish. J. Fish Biol. 1995, 47, 902–909. [Google Scholar] [CrossRef]
- Toth, G.P.; Ciereszko, A.; Christ, S.A.; Dabrowski, K. Objective Analysis of Sperm Motility in the Lake Sturgeon, Acipenser Fulvescens: Activation and Inhibition Conditions. Aquaculture 1997, 154, 337–348. [Google Scholar] [CrossRef]
- Linhart, O.; Cosson, J.; Mims, S.D.; Shelton, W.L.; Rodina, M. Effects of Ions on the Motility of Fresh and Demembranated Paddlefish (Polyodon spathula) Spermatozoa. Reproduction 2002, 124, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J. The Ionic and Osmotic Factors Controlling Motility of Fish Spermatozoa. Aquac. Int. 2004, 12, 69–85. [Google Scholar] [CrossRef]
- Boryshpolets, S.; Dzyuba, B.; Rodina, M.; Alavi, S.M.H.; Gela, D.; Linhart, O. Cryopreservation of Sterlet (Acipenser ruthenus) Spermatozoa Using Different Cryoprotectants. J. Appl. Ichthyol. 2011, 27, 1147–1149. [Google Scholar] [CrossRef]
- Prokopchuk, G.; Dzyuba, B.; Rodina, M.; Cosson, J. Control of Sturgeon Sperm Motility: Antagonism between K+ Ions Concentration and Osmolality. Anim. Reprod. Sci. 2016, 164, 82–89. [Google Scholar] [CrossRef]
- Hadi Alavi, S.M.; Cosson, J.; Karami, M.; Amiri, B.M.; Akhoundzadeh, M.A. Spermatozoa Motility in the Persian Sturgeon, Acipenser Persicus: Effects of PH, Dilution Rate, Ions and Osmolality. Reproduction 2004, 128, 819–828. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.H.; Rodina, M.; Linhart, O. Morphology and Fine Structure of the Russian sturgeon, Acipenser gueldenstaedtii (Acipenseridae, Chondrostei) Spermatozoa. J. Appl. Ichthyol. 2012, 28, 978–983. [Google Scholar] [CrossRef]
- Chenoweth, P.J.; Lorton, S.P. Animal Andrology: Theories and Applications; CABI International: Wallingford, UK, 2014; pp. 1–568. [Google Scholar] [CrossRef]
- Fabbrocini, A.; D’Adamo, R.; Del Prete, F.; Langellotti, A.L.; Barone, C.M.A.; Rinna, F.; Sessa, R.; Silvestri, F.; Villani, G.; Vitiello, V.; et al. Motility of Cryopreserved Spermatozoa for the Ecotoxicological Evaluation of Aquatic Environments. Chem. Ecol. 2013, 29, 660–667. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current Status of Sperm Cryopreservation: Why Isn’t It Better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Halimi, M.; Mohammadi, A.; Norousta, R.; Khara, H.; Karimi, M.R. Spermiation Time Affect the Milt Quality Indices of the Russian sturgeon, Acipenser Gueldenstaedtii, Brandt & Ratzeburg, 1833. Aquac. Res. 2015, 46, 2426–2430. [Google Scholar] [CrossRef]
- Rurangwa, E.; Kime, D.E.; Ollevier, F.; Nash, J.P. The Measurement of Sperm Motility and Factors Affecting Sperm Quality in Cultured Fish. Aquaculture 2004, 234, 1–28. [Google Scholar] [CrossRef]
- Bobe, J.; Labbé, C. Egg and Sperm Quality in Fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Fauvel, C.; Suquet, M.; Cosson, J. Evaluation of Fish Sperm Quality. J. Appl. Ichthyol. 2010, 26, 636–643. [Google Scholar] [CrossRef]
- Aramli, M.; Kalbassi, M.; Nazari, R. Study of Sperm Concentration, Seminal Plasma Composition and Their Physiological Correlation in the Persian Sturgeon, Acipenser Persicus. Reprod. Domest. Anim. 2013, 48, 1013–1018. [Google Scholar] [CrossRef]
- Kowalski, R.K.; Cejko, B.I. Sperm Quality in Fish: Determinants and Affecting Factors. Theriogenology 2019, 135, 94–108. [Google Scholar] [CrossRef]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.; Riesco, M.F.; Valcarce, D.G.; Sarasquete, C.; Herráez, M.P.; Robles, V. Factors Enhancing Fish Sperm Quality and Emerging Tools for Sperm Analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Shaliutina, A.; Hulak, M.; Gazo, I.; Linhartova, P.; Linhart, O. Effect of Short-Term Storage on Quality Parameters, DNA Integrity, and Oxidative Stress in Russian (Acipenser gueldenstaedtii) and Siberian (Acipenser baerii) Sturgeon Sperm. Anim. Reprod. Sci. 2013, 139, 127–135. [Google Scholar] [CrossRef]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of Fish Sperm: Applications and Perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Zemkov, G.V.; Pochevalova, T.I. Cytological Changes in Spermia of the Russian sturgeon (Acipenser gueldenstaedtii B.) after Cryopreservation Based on the Composition of Cryoprotective Medium. In News in Chemistry, Biochemistry and Biotechnology: State of the Art and Prospects of Development; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 257–262. [Google Scholar]
- Huang, X.R.; Zhuang, P.; Zhang, L.Z.; Liu, J.Y.; Zhang, T.; Feng, G.P.; Zhao, F. Effect of Cryopreservation on the Enzyme Activity of Russian sturgeon (Acipenser gueldenstaedtii Brandt & Ratzeburg, 1833) Semen. J. Appl. Ichthyol. 2014, 30, 1585–1589. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, T.; Zhao, F.; Feng, G.; Liu, J.; Yang, G.; Zhang, L.; Zhuang, P. Effects of Cryopreservation on Acrosin Activity and DNA Damage of Russian sturgeon (Acipenser gueldenstaedtii) Semen. Cryoletters 2021, 42, 129–136. [Google Scholar]
- Mirzoyan, A.V.; Nebesikhina, N.A.; Voynova, N.V.; Chistyakov, V.A. Preliminary Results on Ascorbic Acid and Lysine Suppression of Clastogenic Effect of Deep-Frozen Sperm of the Russian sturgeon (Acipenser gueldenstaedti). Int. J. Refrig. 2006, 29, 374–378. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, C.; Nam, Y.K. Ontogenetic Behavior of Farm-Bred Russian sturgeon (Acipenser gueldenstaedtii) Prelarvae in a Diel Photoperiodic Cycle: Behavioral Modifications in Response to Light Intensity. Fish. Aquat. Sci. 2019, 22, 4. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44261 (accessed on 18 November 2015).
- Jähnichen, H.; Warnecke, D.; Trölsch, E.; Kohlmann, K.; Bergler, H.; Pluta, H.J. Motility and Fertilizing Capability of Cryopreserved Acipenser Ruthenus L. Sperm. J. Appl. Ichthyol. 1999, 15, 204–206. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Horvath, A.; Urbanyi, B. Studies on the Semen Biology and Sperm Cryopreservation in the Sterlet, Acipenser Ruthenus L. Aquac. Res. 2004, 35, 519–528. [Google Scholar] [CrossRef]
- Shaliutina-Kolešová, A.; Gazo, I.; Cosson, J.; Llnhart, O. Comparison of Oxidant and Antioxidant Status of Seminal Plasma and Spermatozoa of Several Fish Species. Czech J. Anim. Sci. 2013, 58, 313–320. [Google Scholar] [CrossRef]
- Chebanov, M.; Rosenthal, H.; Gessner, J.; Van Anrooy, R.; Doukakis, P.; Pourkazemi, M.; Williot, P. Sturgeon Hatchery Practices and Management for Release: Guidelines. FAO Fish. Aquac. 2011, 570, 1–110. [Google Scholar]
- Siddique, M.A.M.; Psenicka, M.; Cosson, J.; Dzyuba, B.; Rodina, M.; Golpour, A.; Linhart, O. Egg Stickiness in Artificial Reproduction of Sturgeon: An Overview. Rev. Aquac. 2016, 8, 18–29. [Google Scholar] [CrossRef]
- Li, P.; Rodina, M.; Hulak, M.; Li, Z.H.; Linhart, O. Spermatozoa Concentration, Seminal Plasma Composition and Their Physiological Relationship in the Endangered Stellate Sturgeon (Acipenser stellatus) and Russian sturgeon (Acipenser gueldenstaedtii). Reprod. Domest. Anim. 2011, 46, 247–252. [Google Scholar] [CrossRef]
- Aksoy, E.; Aktan, T.M.; Duman, S.; Cuce, G. Assessment of Spermatozoa Morphology under Light Microscopy with Different Histologic Stains and Comparison of Morphometric Measurements. Int. J. Morphol. 2012, 30, 1544–1550. [Google Scholar] [CrossRef]
- Igna, V.N.; Mihailov, S.; Grozea, A. Staining Methods Suitable for Spermatozoa Morphology, Morphometry and Membrane Integrity Evaluation on Sterlet (Acipenser ruthenus). J. Biotechnol. 2018, 280, 33. [Google Scholar] [CrossRef]
- Cherr, G.N.; Clark, W.H. An Acrosome Reaction in Sperm from the White Sturgeon, Acipenser Transmontanus. J. Exp. Zool. 1984, 232, 129–139. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Rodina, M.; Cosson, J.; Psenicka, M.; Linhart, O. Roles of Extracellular Ca2+ and PH on Motility and Flagellar Waveform Parameters in Sturgeon Spermatozoa. Cybium 2008, 32, 124–126. [Google Scholar]
- Psenicka, M.; Dietrich, G.J.; Wojtczak, M.; Nynca, J.; Rodina, M.; Linhart, O.; Cosson, J.; Ciereszko, A. Acrosome Staining and Motility Characteristics of Sterlet Spermatozoa after Cryopreservation with Use of Methanol and DMSO. Cryobiology 2008, 56, 251–253. [Google Scholar] [CrossRef]
- Horokhovatskyi, Y.; Dietrich, M.A.; Lebeda, I.; Fedorov, P.; Rodina, M.; Dzyuba, B. Cryopreservation Effects on a Viable Sperm Sterlet (Acipenser ruthenus) Subpopulation Obtained by a Percoll Density Gradient Method. PLoS ONE 2018, 13, e0202514. [Google Scholar] [CrossRef]
- Rodríguez, M.; Nivia, A. Efecto de La Adición de Antioxidantes Sobre La Motilidad Espermática Post-Criopreservación y Fertilidad Del Semen de Peces. Rev. Vet. 2017, 28, 157–164. [Google Scholar] [CrossRef]
- Aramli, M.S.; Golshahi, K.; Nazari, R.M.; Aramli, S. Effect of Freezing Rate on Motility, Adenosine Triphosphate Content and Fertilizability in Beluga Sturgeon (Huso huso) Spermatozoa. Cryobiology 2015, 70, 170–174. [Google Scholar] [CrossRef]
- Osipova, V.P.; Berberova, N.T.; Gazzaeva, R.A.; Kudryavtsev, K.V. Application of New Phenolic Antioxidants for Cryopreservation of Sturgeon Sperm. Cryobiology 2016, 72, 112–118. [Google Scholar] [CrossRef]
- Nascimento, J.P.O. Influence of Sperm Density on Cryopreservation Outcomes in João Pedro Oliveira Nascimento Influence of Sperm Density on Cryopreservation Outcomes. PhD Thesis, University of the Algarve, Algarve, Portugal, 2018. [Google Scholar]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of Cryopreservation on Mitochondria of Fish Spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Horváth, Á.; Wayman, W.R.; Urbányi, B.; Ware, K.M.; Dean, J.C.; Tiersch, T.R. The Relationship of the Cryoprotectants Methanol and Dimethyl Sulfoxide and Hyperosmotic Extenders on Sperm Cryopreservation of Two North-American Sturgeon Species. Aquaculture 2005, 247, 243–251. [Google Scholar] [CrossRef]
- Yamaner, G.A. A Brief Overview on Cryopreservation Method of Sturgeon sperm. Istanbul Univ. J. Fish. Aquat. Sci. 2015, 30, 14–20. [Google Scholar] [CrossRef]
- Tsvetkova, L.I.; Cosson, J.; Linhart, O.; Billard, R. Motility and Fertilizing Capacity of Fresh and Frozen-Thawed Spermatozoa in Sturgeons Acipenser Baeri and A. Ruthenus. J. Appl. Ichthyol. 1996, 12, 107–112. [Google Scholar] [CrossRef]
- Glogowski, J.; Kolman, R.; Szczepkowski, M.; Horváth; Urbányi, B.; Sieczyński, P.; Rzemieniecki, A.; Domagała, J.; Demianowicz, W.; Kowalski, R.; et al. Fertilization Rate of Siberian Sturgeon (Acipenser baeri, Brandt) Milt Cryopreserved with Methanol. Aquaculture 2002, 211, 367–373. [Google Scholar] [CrossRef]
- Urbányi, B.; Horváth, Á.; Kovács, B. Successful Hybridization of Acipenser Species Using Cryopreserved Sperm. Aquac. Int. 2004, 12, 47–56. [Google Scholar] [CrossRef]
- Psenicka, M.; Kaspar, V.; Alavi, S.M.H.; Rodina, M.; Gela, D.; Li, P.; Borishpolets, S.; Cosson, J.; Linhart, O.; Ciereszko, A. Potential Role of the Acrosome of Sturgeon Spermatozoa in the Fertilization Process. J. Appl. Ichthyol. 2011, 27, 678–682. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. [Google Scholar] [CrossRef]
- Suquet, M.; Dreanno, C.; Fauvel, C.; Cosson, J.; Billard, R. Cryopreservation of Sperm in Marine Fish. Aquac. Res. 2000, 31, 231–243. [Google Scholar] [CrossRef]
 dead sperm cells,
dead sperm cells,  live sperm cells.
live sperm cells.
 dead sperm cells,
dead sperm cells,  live sperm cells.
live sperm cells.



| Activation Media | References | |
|---|---|---|
| AM0 | water from tank | [9] |
| AM1 | 10 mM Tris, 20 mM NaCl, 2 mM CaCl2, pH 8.5 | [39] |
| AM2 | 12.5 mM NaCl, pH 8.5 | [40] |
| AM3 | 12.5 mM NaCl + 1 mM MgSO4 | [40] |
| AM4 | 12.5 mM NaCl + 1 mM CaCl2 | [40] |
| AM5 | 10 mM NaCl, 1 mM CaCl2, 10 mM Tris–HCl, pH 8.5 | [41] |
| Male | Volume of Collected Semen (mL) | Concentration (nr.spz mL−1) | Total Number of Spermatozoa | Total Motility (TM%) | Velocity (VAP µm/s) |
|---|---|---|---|---|---|
| M1 | 22.8 | 4907.2 × 106 | 111.88 × 109 | 69 | 88.16 |
| M2 | 26.7 | 2870.2 × 106 | 76.63 × 109 | 79 | 88.05 |
| M3 | 49.0 | 2211.5 × 106 | 108.36 × 109 | 60 | 95.23 |
| M4 | 100.0 | 5779.1 × 106 | 577.9 × 109 | 72 | 42.75 |
| M5 | 87.0 | 4001.05 × 106 | 410.64 × 109 | 92 | 48.2 |
| Mean ± SD | 57.1 ± 35 | 3953.8 × 106 ± 1453.6 | 257.08 × 109 ± 224.8 | 74.4 ± 11.9 | 72.47 ± 24.8 |
| Parameter (Measuring Unit) | Measured Values (Mean ± Standard Deviation) | Values Cited in the Literature [20] |
|---|---|---|
| Head length (µm) | 8.01 ± 0. 40 | 8.02 (1.18/AL + 6.84/NL) * |
| Head width (µm) | 1.34 ± 0.20 | 1.48 (PNW) * |
| Tail length (µm) | 53.91 ± 2.07 | 51.06 (1.64/ML + 49.4/FL) * |
| Total sperm cell length (µm) | 61.88 ± 2.33 | 57.08 |
| Male Sturgeon | Activation Media | Total Motility (TM) (% ± SD) | Progressive Motility (PM) (% ± SD) | Path Velocity (V) (µm s−1 ± SD) |
|---|---|---|---|---|
| M1 | AM0 | 69.00 ± 14.00 | 12.33 ± 6.66 | 88.17 ± 31.39 |
| AM1 | 45.67 ± 17.79 | 10.00 ± 13.23 | 79.70 ± 44.64 | |
| AM2 | 43.33 ± 11.93 | 12.67 ± 8.08 | 106.20 ± 10.71 | |
| AM3 | 32.67 ± 9.02 | 11.33 ± 7.57 | 107.37 ± 39.89 | |
| AM4 | 57.67 ± 22.12 | 17.00 ± 16.64 | 91.10 ± 11.97 | |
| AM5 | 71.33 ± 18.01 | 16.00 ± 21.66 | 71.70 ± 23.59 | |
| Mean ± SD | 53.28 ± 19.74 a | 13.22 ± 11.60 a | 90.71 ± 28.48 a | |
| M2 | AM0 | 78.67 ± 12.01 | 28.67 ± 2.52 | 88.07 ± 14.15 |
| AM1 | 75.33 ± 8.02 | 5.67 ± 2.52 | 56.17 ± 6.69 | |
| AM2 | 81.00 ± 16.00 | 30.33 ± 4.04 | 92.60 ± 7.92 | |
| AM3 | 61.00 ± 25.06 | 17.33 ± 2.08 | 78.00 ±20.35 | |
| AM4 | 51.67 ± 2.52 | 11.67 ± 5.51 | 90.37 ± 7.64 | |
| AM5 | 70.67 ± 9.07 | 5.33 ± 1.15 | 62.57 ± 1.75 | |
| Mean ± SD | 69.72 ± 15.88 b | 16.50 ± 10.70 a | 77.96 ± 17.34 a,b | |
| M3 | AM0 | 60.33 ± 20.03 | 26.00 ± 12.00 | 95.23 ± 3.57 |
| AMI | 60.00 ± 22.65 | 1.00 ± 1.00 | 41.30 ± 8.40 | |
| AM2 | 91.33 ± 9.29 | 23.67 ± 13.01 | 69.67 ± 13.58 | |
| AM3 | 69.67 ± 28.38 | 11.00 ± 6.00 | 61.83 ± 31.17 | |
| AM4 | 63.33 ± 32.13 | 25.00 ± 11.53 | 86.80 ± 12.47 | |
| AM5 | 51.67 ± 14.57 | 2.33 ± 2.08 | 51.33 ± 15.70 | |
| Mean ± SD | 66.10 ± 22.89 b | 14.83 ± 13.24 a | 67.69 ± 23.84 b |
| Activation Media | Total Motility (TM) (% ± SD) | Progressive Motility (PM) (% ± SD) | Path Velocity (V) (µm s−1 ± SD) |
|---|---|---|---|
| AM0 | 69.33 ± 15.76 a,b | 22.33 ± 10.31 a | 90.49 ± 17.67 a |
| AM1 | 60.33 ± 19.71 a,b | 5.56 ± 7.80 b | 59.06 ± 28.43 b |
| AM2 | 71.89 ± 24.49 b | 22.22 ± 11.07 a | 89.49 ± 18.61 a |
| AM3 | 54.44 ± 25.68 a,c | 13.22 ± 5.83 b,c,d | 82.40 ± 33.82 a |
| AM4 | 57.56 ± 20.19 a,b | 17.89 ± 11.99 a,d | 89.42 ± 9.66 a |
| AM5 | 64.56 ± 15.76 a,b | 7.89 ± 12.54 b | 61.87 ± 16.72 b |
| Male | Volume of Collected Semen (mL) | Concentration (nr.spz mL−1) | Total Motility (%) | Velocity (VAP) (µm s−1) |
|---|---|---|---|---|
| M1 | 100 | 5779.1 × 106 | 72 | 42.7 |
| M2 | 87 | 4001.05 × 106 | 92 | 48.2 |
| M3 | 26.7 | 2870.2 × 106 | 91 | 73.9 |
| Mean ± SD | 72.23 ± 31.93 | 4216.78 × 106 ± 1202.09 | 85 ± 9.20 | 54.93 ± 13.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igna, V.; Telea, A.; Florea, T.; Popp, R.; Grozea, A. Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation. Animals 2022, 12, 2153. https://doi.org/10.3390/ani12162153
Igna V, Telea A, Florea T, Popp R, Grozea A. Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation. Animals. 2022; 12(16):2153. https://doi.org/10.3390/ani12162153
Chicago/Turabian StyleIgna, Violeta, Ada Telea, Tiana Florea, Roxana Popp, and Adrian Grozea. 2022. "Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation" Animals 12, no. 16: 2153. https://doi.org/10.3390/ani12162153
APA StyleIgna, V., Telea, A., Florea, T., Popp, R., & Grozea, A. (2022). Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation. Animals, 12(16), 2153. https://doi.org/10.3390/ani12162153






