Essential Oils and Melatonin as Functional Ingredients in Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
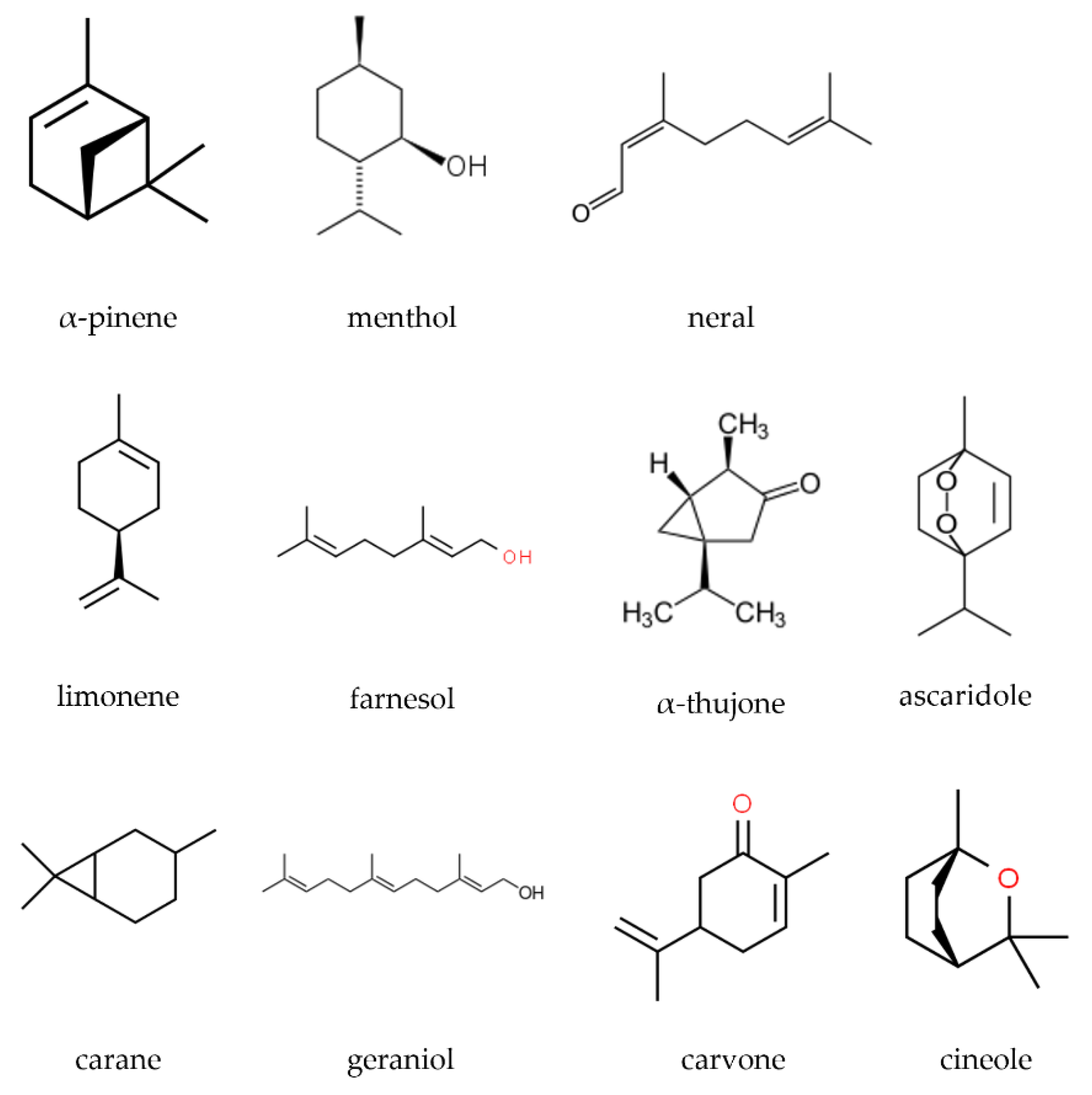
2. Essential Oils
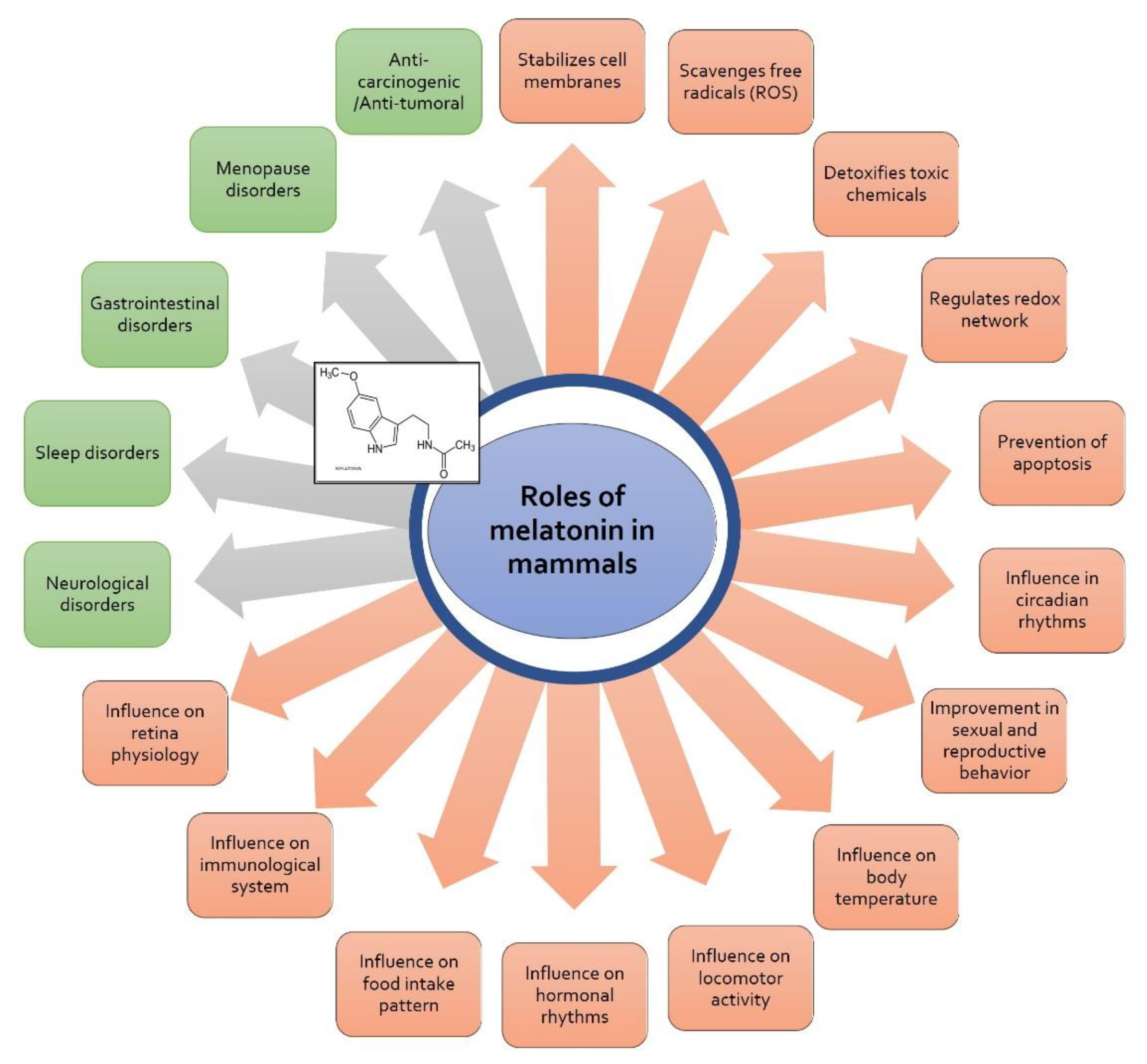
3. Melatonin
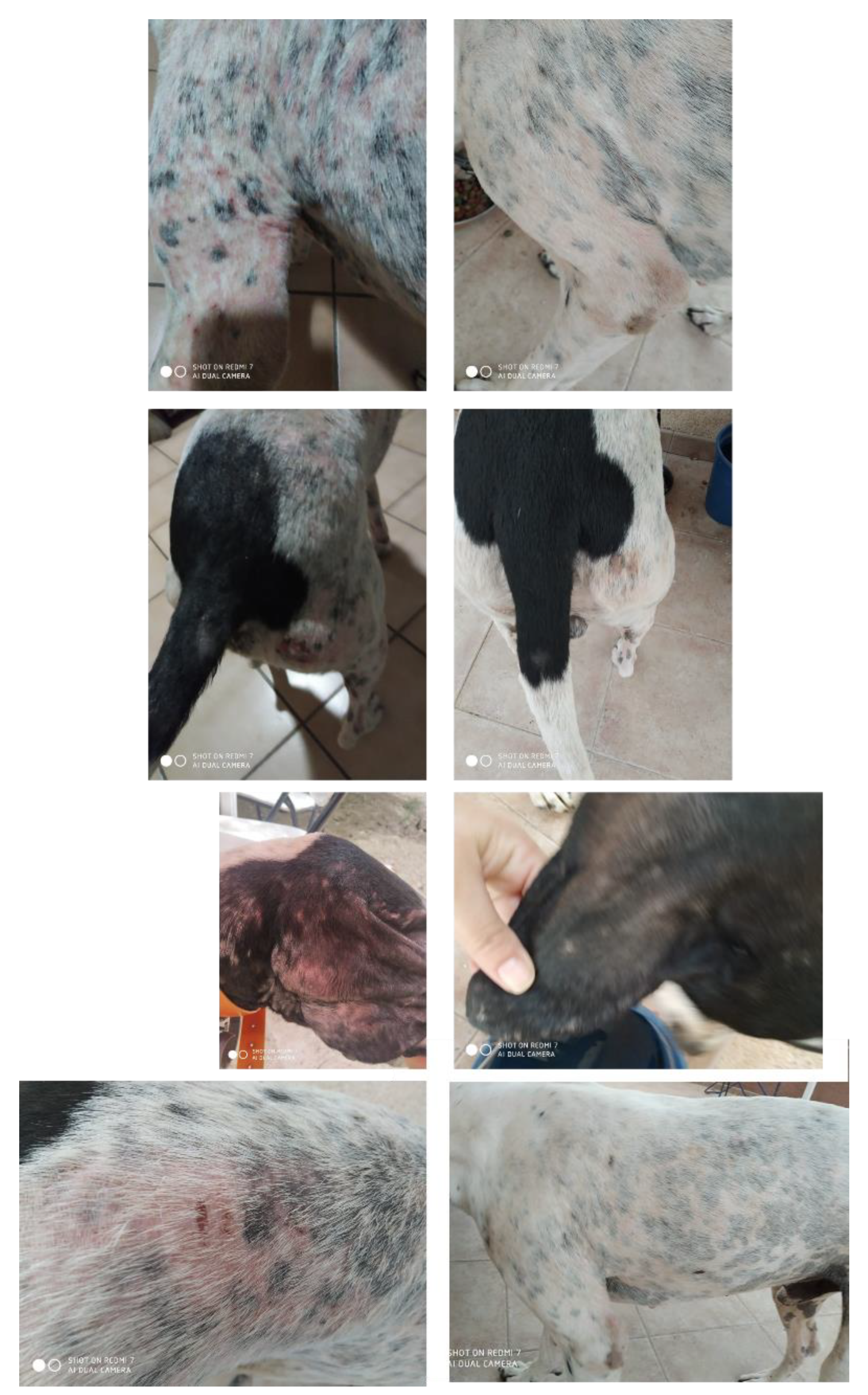
3.1. Seasonal (Recurrent) Alopecia of the Flanks
3.2. Anxiety, Phobia, and Nervousness Due to Noise or Disorientation
3.3. Sleep Disorders in Elderly Dogs
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeFelice, S.L. The Nutraceutical Revolution: Its Impact on Food Industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Pandey, M.; Verma, R.K.; Saraf, S.A. Nutraceuticals: New Era of Medicine and Health. Asian, J. Pharm. Clin. Res. 2010, 3, 11–15. [Google Scholar]
- Singh, J.; Sinha, S. Classification, Regulatory Acts And Applications Of Nutraceuticals for Health. Int. J. Pharma Biosci. 2012, 2, 177–187. [Google Scholar]
- da Costa, J.P. A Current Look at Nutraceuticals: Key Concepts and Future Prospects. Trends Food Sci. Technol. 2017, 62, 68–78. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Sánchez-Carrasco, G.; Arnao, M.B. Current Vision of Functional Foods in the Diet of Cats and Dogs. All Pet Food Mag. 2020, 3, 12–13. [Google Scholar]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M. Emerging Sulfated Flavonoids and Other Polyphenols as Drugs: Nature as an Inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects. A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Larrosa, M. Polyphenol-Rich Foods for Human Health and Disease. Nutrients 2020, 12, 400. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, Antihypertensive and Antimicrobial Properties of Phenolic Compounds Obtained from Native Plants by Different Extraction Methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.-N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and Biotechnological Processes To Enhance the Bioavailability of Dietary (Poly)Phenols in Humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, e9081686. [Google Scholar] [CrossRef]
- Pengelly, A. The Constituents of Medicinal Plants, 3rd ed.; CABI: Wallingford, UK, 2021; ISBN 978-1-78924-307-9. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Blackwell Pub.: New York, NY, USA, 2010; Volume 2, pp. 258–303. ISBN 978-1-4443-2050-3. [Google Scholar]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current Knowledge on Food Source, Metabolism, and Health Effects. Crit. Rev. Food Sci. Nutr. 2021, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L. Prevention and Therapy of Cancer by Dietary Monoterpenes. J. Nutr. 1999, 129, 775S–778S. [Google Scholar] [CrossRef]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; de Souza, D.P.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular Effects of Monoterpenes: A Review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant Terpenes: Defense Responses, Phylogenetic Analysis, Regulation and Clinical Applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 978-0-470-74276-1. [Google Scholar]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for Cancer Prevention and Therapy: Current Progress and Future Perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; de Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; et al. Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar] [CrossRef]
- Selmar, D. Biosynthesis of Cyanogenic Glycosides, Glucosinolates and Non-Protein Amino Acids. In Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Blackwell Pub.: New York, NY, USA, 2010; Volume 2, pp. 92–181. ISBN 978-1-4443-2050-3. [Google Scholar]
- Hernández-Ruiz, J.; Ruiz-Cano, D.; Giraldo-Acosta, M.; Cano, A.; Arnao, M. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules 2022, 27, 1523. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.P.W. Essential Oils. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 554–561. ISBN 978-0-12-369397-6. [Google Scholar]
- Kaewwongse, M.; Sanesuwan, K.; Pupa, P.; Bullangpoti, V. Essential Oil Compounds as Stress Reducing Agents in Rats. Commun. Agric. Appl. Biol. Sci. 2013, 78, 167–172. [Google Scholar] [PubMed]
- Baser, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-15566-6. [Google Scholar]
- Kowalski, R.; Kowalska, G.; Jamroz, J.; Nawrocka, A.; Metyk, D. Effect of the Ultrasound-Assisted Preliminary Maceration on the Efficiency of the Essential Oil Distillation from Selected Herbal Raw Materials. Ultrason. Sonochem. 2015, 24, 214–220. [Google Scholar] [CrossRef]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques For Extraction of Essential Oils From Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Essential Oils. In Food Additives and Human Health; Bentham Science Publisher: Singapore, 2020; Volume 1, pp. 104–119. ISBN 9789811446139. [Google Scholar]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S. Plant Essential Oil with Biological Activity. Plants 2022, 11, 980. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- EFSA. Available online: https://www.efsa.europa.eu/en/science/scientific-committee-and-panels/feedap#panel-members (accessed on 4 July 2022).
- EFSA2. Available online: https://webgate.ec.europa.eu/foods_system/main/?event=display (accessed on 4 July 2022).
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-0-429-15083-8. [Google Scholar]
- Kroes, R.; Galli, C.; Munro, I.; Schilter, B.; Tran, L.; Walker, R.; Würtzen, G. Threshold of Toxicological Concern for Chemical Substances Present in the Diet: A Practical Tool for Assessing the Need for Toxicity Testing. Food Chem. Toxicol. 2000, 38, 255–312. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications. In Hui: Food Science and Technology; Wiley: New York, NY, USA, 2018; ISBN 978-1-119-13535-7. [Google Scholar]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid)/Trolox-Equivalent Antioxidant Capacity) Radical Scavenging Mixed-Mode Assay. In Measurement of Antioxidant Activity & Capacity. Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons: Oxford, UK, 2018; Volume 1, pp. 117–139. ISBN 978-1-119-13538-8. [Google Scholar]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Campigotto, G.; Jaguezeski, A.M.; Alba, D.F.; Giombelli, L.C.D.; da Rosa, G.; Souza, C.F.; Baldissera, M.D.; Petrolli, T.G.; da Silva, A.S. Microencapsulated Phytogenic in Dog Feed Modulates Immune Responses, Oxidative Status and Reduces Bacterial (Salmonella and Escherichia Coli) Counts in Feces. Microb. Pathog. 2021, 159, 105113. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of Essential Oils for Poultry and Pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed]
- Meason-Smith, C.; Older, C.E.; Ocana, R.; Dominguez, B.; Lawhon, S.D.; Wu, J.; Patterson, A.P.; Rodrigues Hoffmann, A. Novel Association of Psychrobacter and Pseudomonas with Malodour in Bloodhound Dogs, and the Effects of a Topical Product Composed of Essential Oils and Plant-Derived Essential Fatty Acids in a Randomized, Blinded, Placebo-Controlled Study. Vet. Dermatol. 2018, 29, 465-e158. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; de Jesus, F.P.K.; Dutra, V.; Cândido, S.L.; Alves, S.H.; Santurio, J.M. Susceptibility Profile of Candida Rugosa (Diutina Rugosa) against Antifungals and Compounds of Essential Oils. J. Mycol. Med. 2019, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Bohmova, E.; Conkova, E.; Harcarova, M.; Sihelska, Z. Interactions between Clotrimazole and Selected Essential Oils against Malassezia Pachydermatis Clinical Isolates. Pol. J. Vet. Sci. 2019, 22, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial Activity of Thyme Oil, Oregano Oil, Thymol and Carvacrol against Sensitive and Resistant Microbial Isolates from Dogs with Otitis Externa. Vet. Dermatol. 2019, 30, 524-e159. [Google Scholar] [CrossRef]
- Komiya, M.; Sugiyama, A.; Tanabe, K.; Uchino, T.; Takeuchi, T. Evaluation of the Effect of Topical Application of Lavender Oil on Autonomic Nerve Activity in Dogs. Am. J. Vet. Res. 2009, 70, 764–769. [Google Scholar] [CrossRef]
- Godara, R.; Parveen, S.; Katoch, R.; Yadav, A.; Verma, P.K.; Katoch, M.; Kaur, D.; Ganai, A.; Raghuvanshi, P.; Singh, N.K. Acaricidal Activity of Extract of Artemisia Absinthium against Rhipicephalus Sanguineus of Dogs. Parasitol. Res. 2014, 113, 747–754. [Google Scholar] [CrossRef]
- Low, S.B.; Peak, R.M.; Smithson, C.W.; Perrone, J.; Gaddis, B.; Kontogiorgos, E. Evaluation of a Topical Gel Containing a Novel Combination of Essential Oils and Antioxidants for Reducing Oral Malodor in Dogs. Am. J. Vet. Res. 2014, 75, 653–657. [Google Scholar] [CrossRef]
- Monteiro, C.; Ferreira, L.L.; de Paula, L.G.F.; de Oliveira Filho, J.G.; de Oliveira Silva, F.; Muniz, E.R.; Menezes, K.M.F.; de Camargo, F.R.; de Oliveira Nonato, R.; Martins, D.B.; et al. Thymol and Eugenol Microemulsion for Rhiphicephalus Sanguineus Sensu Lato Control: Formulation Development, Field Efficacy, and Safety on Dogs. Vet. Parasitol. 2021, 296, 109501. [Google Scholar] [CrossRef]
- Goode, P.; Ellse, L.; Wall, R. Preventing Tick Attachment to Dogs Using Essential Oils. Ticks Tick Borne Dis. 2018, 9, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In Vitro and in Vivo Evaluation of a New Phytotherapic Blend to Treat Acute Externa Otitis in Dogs. J. Vet. Pharmacol. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.; Madrigal, I.; Puente, H.; Mencía-Ares, Ó.; Argüello, H.; Carvajal, A.; Fregeneda-Grandes, J.M. In Vitro Activity of Essential Oils against Microbial Isolates from Otitis Externa Cases in Dogs. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blaskovic, M.; Rosenkrantz, W.; Neuber, A.; Sauter-Louis, C.; Mueller, R.S. The Effect of a Spot-on Formulation Containing Polyunsaturated Fatty Acids and Essential Oils on Dogs with Atopic Dermatitis. Vet. J. 2014, 199, 39–43. [Google Scholar] [CrossRef]
- Catarino, M.; Combarros-Garcia, D.; Mimouni, P.; Pressanti, C.; Cadiergues, M.C. Control of Canine Idiopathic Nasal Hyperkeratosis with a Natural Skin Restorative Balm: A Randomized Double-Blind Placebo-Controlled Study. Vet. Dermatol. 2018, 29, 134-e53. [Google Scholar] [CrossRef]
- Schlieck, T.M.M.; Petrolli, T.G.; Bissacotti, B.F.; Copetti, P.M.; Bottari, N.B.; Morsch, V.M.; da Silva, A.S. Addition of a Blend of Essential Oils (Cloves, Rosemary and Oregano) and Vitamin E to Replace Conventional Chemical Antioxidants in Dog Feed: Effects on Food Quality and Health of Beagles. Arch. Anim. Nutr. 2021, 75, 389–403. [Google Scholar] [CrossRef]
- Michalczyk, A.; Ostrowska, P. Essential Oils and Their Components in Combating Fungal Pathogens of Animal and Human Skin. J. Mycol. Med. 2021, 31, 101118. [Google Scholar] [CrossRef]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial Activity of Some Essential Oils against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Pseudintermedius-Associated Pyoderma in Dogs. Animals 2020, 10, 1782. [Google Scholar] [CrossRef]
- Mondêgo-Oliveira, R.; de Sá Sousa, J.C.; Moragas-Tellis, C.J.; de Souza, P.V.R.; dos Santos Chagas, M.d.S.; Behrens, M.D.; de Jesús Hardoim, D.; Taniwaki, N.N.; Chometon, T.Q.; Bertho, A.L.; et al. Vernonia Brasiliana (L.) Druce Induces Ultrastructural Changes and Apoptosis-like Death of Leishmania Infantum Promastigotes. Biomed. Pharmacother. 2021, 133, 111025. [Google Scholar] [CrossRef]
- Rey-Valeirón, C.; Pérez, K.; Guzmán, L.; López-Vargas, J.; Valarezo, E. Acaricidal Effect of Schinus Molle (Anacardiaceae) Essential Oil on Unengorged Larvae and Engorged Adult Females of Rhipicephalus Sanguineus (Acari: Ixodidae). Exp. Appl. Acarol 2018, 76, 399–411. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.G.; Rodrigues, V.d.S.; Jorge, J.d.O.; Osava, C.F.; Szabó, M.P.J.; Garcia, M.V.; Andreotti, R. Efficacy of Tagetes Minuta (Asteraceae) Essential Oil against Rhipicephalus Sanguineus (Acari: Ixodidae) on Infested Dogs and in Vitro. Exp. Appl. Acarol. 2016, 70, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, V.d.Q.; Soares, M.J.C.; Matos, M.N.C.; Cavalcante, R.M.B.; Guerrero, J.A.P.; Soares Rodrigues, T.H.; Gomes, G.A.; de Medeiros Guedes, R.F.; Castelo-Branco, D.d.S.C.M.; Goes da Silva, I.N.; et al. Anti-Staphylococcal Activity of Cinnamomum Zeylanicum Essential Oil against Planktonic and Biofilm Cells Isolated from Canine Otological Infections. Antibiotics 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Bobeck, E.A. NUTRITION AND HEALTH: COMPANION ANIMAL APPLICATIONS: Functional Nutrition in Livestock and Companion Animals to Modulate the Immune Response. J. Anim. Sci. 2020, 98, skaa035. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Sánchez-Carrasco, G.; Arnao, M.B. Food Supplements in Pet Food: An Example in Dogs with Essential Oils and Melatonin as Functional Ingredients. All Pet Food Magazine 2022, 4, 8–12. [Google Scholar]
- Li, W.-J.; Zhang, L.; Wu, H.-X.; Li, M.; Wang, T.; Zhang, W.-B.; Du, Z.-Y.; Zhang, M.-L. Intestinal Microbiota Mediates Gossypol-Induced Intestinal Inflammation, Oxidative Stress, and Apoptosis in Fish. J. Agric. Food Chem. 2022, 70, 6688–6697. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Dai, L.; Liu, Y.; Cheng, M.; Chen, L. Isolation and Characterization of a Novel Gossypol-Degrading Bacteria Bacillus Subtilis Strain Rumen Bacillus Subtilis. Asian-Australas J. Anim. Sci. 2018, 31, 63–70. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Heinzelmann, R.V. Structure of Melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The Role of Melatonin, a Multitasking Molecule, in Retarding the Processes of Ageing. Ageing Res. Rev. 2018, 47, 198–213. [Google Scholar] [CrossRef]
- Socaciu, A.I.; Ionut, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an Ubiquitous Metabolic Regulator: Functions, Mechanisms and Effects on Circadian Disruption and Degenerative Diseases. Rev. Endocr. Metab. Disord. 2020, 21, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in Mitochondria: Mitigating Clear and Present Dangers. Physiology 2020, 35, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.G.; Reiter, R.J.; Agil, A. Melatonin Increases Brown Adipose Tissue Mass and Function in Zücker Diabetic Fatty Rats: Implications for Obesity Control. J. Pineal Res. 2018, 64, e12472. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Reilly, T.; Atkinson, G. Jet Lag. Lancet 1997, 350, 1611–1616. [Google Scholar] [CrossRef]
- Takahashi, T.; Sasaki, M.; Itoh, H.; Ozone, M.; Yamadera, W.; Hayshida, K.I.; Ushijima, S.; Matsunaga, N.; Obuchi, K.; Sano, H. Effect of 3 Mg Melatonin on Jet Lag Syndrome in an 8-h Eastward Flight. Psychiatry Clin. Neurosci. 2000, 54, 377–378. [Google Scholar] [CrossRef]
- Herxheimer, A. Jet Lag. Clin. Evid. 2005, 13, 2178–2183. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the Free Radical Scavenging Activities of Melatonin’s Metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; D’ Mello, J., Ed.; CAB Intern: Boston, MA, USA, 2015; pp. 390–435. ISBN 978-1-78064-263-5. [Google Scholar]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin Anticancer Effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural Plast. 2017, 2017, 1504507. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A Review of Sleep Disorders and Melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The Neuroprotective Role of Melatonin in Neurological Disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Angerer, M.; Raml, M.; del Giudice, R.; Santhi, N.; Pichler, G.; Kunz, A.B.; Scarpatetti, M.; Trinka, E.; Schabus, M. Healthier Rhythm, Healthier Brain? Integrity of Circadian Melatonin and Temperature Rhythms Relates to the Clinical State of Brain-Injured Patients. Eur. J. Neurol. 2019, 26, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Cardinali, D.; Reiter, R.; Brown, G. Low Melatonin as a Contributor to SARS-CoV-2 Disease. Melatonin Res. 2020, 3, 558–576. [Google Scholar] [CrossRef]
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin and Healthy Aging. In Vitamins and Hormones Hormones and Aging; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 115, pp. 67–88. ISBN 0083-6729. [Google Scholar]
- Santos-Ledo, A.; de Luxán-Delgado, B.; Caballero, B.; Potes, Y.; Rodríguez-González, S.; Boga, J.A.; Coto-Montes, A.; García-Macia, M. Melatonin Ameliorates Autophagy Impairment in a Metabolic Syndrome Model. Antioxidants 2021, 10, 796. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Nunes, B.P. Effects of Melatonin Supplementation on Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Clin. Nutr. 2021, 40, 4595–4605. [Google Scholar] [CrossRef]
- Lauritzen, E.S.; Kampmann, U.; Pedersen, M.G.B.; Christensen, L.-L.; Jessen, N.; Møller, N.; Støy, J. Three Months of Melatonin Treatment Reduces Insulin Sensitivity in Patients with Type 2 Diabetes—A Randomized Placebo-Controlled Crossover Trial. J. Pineal Res. 2022, 73, e12809. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ogugofor, M.O.; Nkwoemeka, N.E.; Nweze, E.J.; Okoye, C.O. Phytomelatonin: A Potential Phytotherapeutic Intervention on COVID-19-Exposed Individuals. Microbes Infect. 2022, 24, 104886. [Google Scholar] [CrossRef]
- Murayama, N.; Takahashi, S.; Hizume, T.; Nagata, M. Canine Recurrent Flank Alopecia with Hair Loss on the Nose Bridge and the Pinnae in a Family of Airedale Terrier. Jpn J. Vet. Dermatol. 2005, 11, 1–4. [Google Scholar] [CrossRef]
- Scott, D. Seasonal Flank Alopecia in Ovariohysterectomized Dogs. Cornell Vet. 1990, 80, 187–195. [Google Scholar] [PubMed]
- Waldman, L. Seasonal Flank Alopecia in Affenpinschers. J. Small Anim. Pract. 1995, 36, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.F.; Evans, H.; Lloyd, D.H. Investigation of the Reproductive and Growth Hormone Status of Dogs Affected by Idiopathic Recurrent Flank Alopecia. J. Small Anim. Pract. 1996, 37, 417–422. [Google Scholar] [CrossRef]
- Ando, J.; Nagata, M. Seasonal Flank Alopecia in a Boxer. Jpn. J. Vet. Dermatol. 2000, 6, 17–20. [Google Scholar] [CrossRef]
- Paradis, M. Melatonin Therapy in Canine Alopecia. In Kirk’s Current Veterinary Therapy XIII Small Animal Practice; WB Saunders Company: Philadelphia, PA, USA, 2000; pp. 546–549. [Google Scholar]
- van der Luer, R.; Bonestroo, J. A dog with an unusual case of alopecia; case report. Tijdschr. Diergeneeskd. 2010, 135, 492–494. [Google Scholar] [PubMed]
- Miller, W.H., Jr.; Griffin, C.E.; Campbell, K.L. Muller and Kirk’s Small Animal Dermatology; Elsevier Health Sciences: St Louis, MO, USA, 2012; ISBN 1-4160-0028-3. [Google Scholar]
- Rachid, M.A.; Demaula, C.D.; Scott, D.W.; Miller, W.H.; Senter, D.A.; Myers, S. Concurrent Follicular Dysplasia and Interface Dermatitis in Boxer Dogs. Vet. Dermatol. 2003, 14, 159–166. [Google Scholar] [CrossRef]
- Diaz, S.F.; Torres, S.M.F.; Nogueira, S.a.F.; Gilbert, S.; Jessen, C.R. The Impact of Body Site, Topical Melatonin and Brushing on Hair Regrowth after Clipping Normal Siberian Husky Dogs. Vet. Dermatol. 2006, 17, 45–50. [Google Scholar] [CrossRef]
- Perego, R.; Proverbio, D.; Roccabianca, P.; Spada, E. Color Dilution Alopecia in a Blue Doberman Pinscher Crossbreed. Can. Vet. J. 2009, 50, 511–514. [Google Scholar]
- Rees, C.A. New Drugs in Veterinary Dermatology. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1449–1460. [Google Scholar] [CrossRef]
- Behrend, E.N.; Kennis, R. Atypical Cushing’s Syndrome in Dogs: Arguments For and Against. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 285–296. [Google Scholar] [CrossRef]
- Frank, L.A.; Hnilica, K.A.; Oliver, J.W. Adrenal Steroid Hormone Concentrations in Dogs with Hair Cycle Arrest (Alopecia X) before and during Treatment with Melatonin and Mitotane. Vet. Dermatol. 2004, 15, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Donnell, R.L.; Kania, S.A. Oestrogen Receptor Evaluation in Pomeranian Dogs with Hair Cycle Arrest (Alopecia X) on Melatonin Supplementation. Vet. Dermatol. 2006, 17, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the Role of Melatonin in Skin Physiology and Pathology. Endocrine 2005, 27, 137–147. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the Skin: Synthesis, Metabolism and Functions. Trends Endocrinol. Metabol. 2008, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. Antiaging Efficacy of Melatonin-Based Day and Night Creams: A Randomized, Split-Face, Assessor-Blinded Proof-of-Concept Trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the Hair Follicle. J. Pineal Res. 2008, 44, 1–15. [Google Scholar] [CrossRef]
- Fischer, T.W.; Burmeister, G.; Schmidt, H.W.; Elsner, P. Melatonin Increases Anagen Hair Rate in Women with Androgenetic Alopecia or Diffuse Alopecia: Results of a Pilot Randomized Controlled Trial. Br. J. Dermatol. 2004, 150, 341–345. [Google Scholar] [CrossRef]
- Sladden, M.J.; Hutchinson, P.E. Is Melatonin Useful in Alopecia: Critical Appraisal of a Randomized Trial? Br. J. Dermatol. 2005, 153, 859–860. [Google Scholar] [CrossRef]
- Fischer, T.W.; Trüeb, R.M.; Hänggi, G.; Innocenti, M.; Elsner, P. Topical Melatonin for Treatment of Androgenetic Alopecia. Int. J. Trichology 2012, 4, 236. [Google Scholar] [CrossRef]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the Role of Melatonin in Human Melanocyte Physiology: A Skin Context Perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef]
- Dahlitz, M.; Alvarez, B.; Vignau, J.; English, J.; Arendt, J.; Parkes, J. Delayed Sleep Phase Syndrome Response to Melatonin. Lancet 1991, 337, 1121–1124. [Google Scholar] [CrossRef]
- Jan, J.; Hamilton, D.; Seward, N.; Fast, D.; Freeman, R.; Laudon, M. Clinical Trial of Controlled-Release Melatonin in Children with Sleep-Wake Disorders. J. Pineal Res. 2000, 29, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Rodríguez, E.B.; Osornio, A.O.; Hernández-Avitia, E.; Mendoza-Fernández, V.; Escobar, A. Anxiolytic-like Actions of Melatonin, 5-Metoxytryptophol, 5-Hydroxytryptophol and Benzodiazepines on a Conflict Procedure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2000, 24, 117–129. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-Dependent Regulation of ROS Signaling Is Required for Melatonin-Induced Salinity Tolerance in Arabidopsis. Free. Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Yang, Z.; Li, C.-Q. The Melatonergic System in Anxiety Disorders and the Role of Melatonin in Conditional Fear. Vitam. Horm. 2017, 103, 281–294. [Google Scholar]
- Li, C.; Ma, D.; Li, M.; Wei, T.; Zhao, X.; Heng, Y.; Ma, D.; Anto, E.O.; Zhang, Y.; Niu, M.; et al. The Therapeutic Effect of Exogenous Melatonin on Depressive Symptoms: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 737972. [Google Scholar] [CrossRef]
- Kholghi, G.; Eskandari, M.; Shokouhi Qare Saadlou, M.-S.; Zarrindast, M.-R.; Vaseghi, S. Night Shift Hormone: How Does Melatonin Affect Depression? Physiol. Behav. 2022, 252, 113835. [Google Scholar] [CrossRef]
- Tandon, V.R.; Sharma, S.; Mahajan, A.; Mahajan, A.; Tandon, A. Menopause and Sleep Disorders. J. Midlife Health 2022, 13, 26–33. [Google Scholar] [CrossRef]
- Niggemann, J.R.; Tichy, A.; Eberspächer-Schweda, M.C.; Eberspächer-Schweda, E. Preoperative Calming Effect of Melatonin and Its Influence on Propofol Dose for Anesthesia Induction in Healthy Dogs. Vet. Anaesth. Analg. 2019, 46, 560–567. [Google Scholar] [CrossRef]
- Rosas-Luna, L.E.; Castelán-Martínez, O.D.; Mora-Magaña, I.; Ángeles-Castellanos, M.; Ubaldo-Reyes, L.M. Midazolam Reduction with Pre-Operative Melatonin in Abdominal Hysterectomy: Double-Blind Randomized Clinical Trial. Cir. Cir. 2022, 90, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Losada, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Phytomelatonin Content in Valeriana Officinalis L. and Some Related Phytotherapeutic Supplements. Int. J. Plant Based Pharm. 2022, 2, 176–181. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A. Melatonin Prolonged Release: In the Treatment of Insomnia in Patients Aged >= 55 Years. Drugs Aging 2012, 29, 911–923. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.-W.; Wicht, H. Synchronizing Effects of Melatonin on Diurnal and Circadian Rhythms. Gen. Comp. Endocrinol. 2018, 258, 215–221. [Google Scholar] [CrossRef]
- Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Suppression of Melatonin Secretion in Totally Visually Blind People by Ocular Exposure to White Light: Clinical Characteristics. Ophthalmology 2018, 125, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Bologna, C.; Madonna, P.; Pone, E. Efficacy of Prolonged-Release Melatonin 2 Mg (PRM 2 Mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study. J. Clin. Med. 2021, 10, 5857. [Google Scholar] [CrossRef]
- Simpson, B.S.; Papich, M.G. Pharmacologic Management in Veterinary Behavioral Medicine. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 365–404. [Google Scholar] [CrossRef]
- Mandelker, L.; Wynn, S. Cellular Effects of Common Nutraceuticals and Natural Food Substances. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 339–353. [Google Scholar] [CrossRef]
- Aronson, L. Animal Behavior Case of the Month. A Dog Was Evaluated Because of Extreme Fear. J. Am. Vet. Med. Assoc. 1999, 215, 22–24. [Google Scholar]
- Fourtillan, J.B. Role of Melatonin in the Induction and Maintenance of Sleep. Dialogues Clin. Neurosci. 2002, 4, 395–401. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive Dysfunction Syndrome: A Disease of Canine and Feline Brain Aging. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.M.; DePorter, T.; Araujo, J.A. Clinical Signs and Management of Anxiety, Sleeplessness, and Cognitive Dysfunction in the Senior Pet. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 565–590. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G. Therapeutic Options for Cognitive Decline in Senior Pets. J. Am. Anim. Hosp. Assoc. 2006, 42, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.A.; Chidester, R.M.; Chrisman, C.L. Clinical Characteristics, Management and Long-Term Outcome of Suspected Rapid Eye Movement Sleep Behaviour Disorder in 14 Dogs. J. Small Anim. Pract. 2011, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zanghi, B.M.; Gardner, C.; Araujo, J.; Milgram, N.W. Diurnal Changes in Core Body Temperature, Day/Night Locomotor Activity Patterns, and Actigraphy-Generated Behavioral Sleep in Aged Canines with Varying Levels of Cognitive Dysfunction. Neurobiol. Sleep Circadian Rhythm. 2016, 1, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Lefman, S.H.; Prittie, J.E. Psychogenic Stress in Hospitalized Veterinary Patients: Causation, Implications, and Therapies. J. Vet. Emerg. Crit. Care 2019, 29, 107–120. [Google Scholar] [CrossRef]
- Bódizs, R.; Kis, A.; Gácsi, M.; Topál, J. Sleep in the Dog: Comparative, Behavioral and Translational Relevance. Curr. Opin. Behav. Sci. 2020, 33, 25. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Sweatman, T.W.; Slominski, A.T. The Melatonin-Producing System Is Fully Functional in Retinal Pigment Epithelium. Mol. Cell Endocrinol. 2009, 307, 211–216. [Google Scholar] [CrossRef]
- Agorastos, A.; Huber, C.G. The Role of Melatonin in Glaucoma: Implications Concerning Pathophysiological Relevance and Therapeutic Potential. J. Pineal Res. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Bubenik, G. Gastrointestinal Melatonin: Localization, Function, and Clinical Revelance. Diges Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Sommansson, A.; Yamskova, O.; Schiöth, H.B.; Nylander, O.; Sjöblom, M. Long-Term Oral Melatonin Administration Reduces Ethanol-Induced Increases in Duodenal Mucosal Permeability and Motility in Rats. Acta Physiol. 2014, 212, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.; Weber, A.P.M.; Wallenfang, M.; Hoffmann, T.; Mettler-Altmann, T.; Truse, R.; Bauer, I.; Picker, O.; Mathes, A.M. Melatonin Pretreatment Improves Gastric Mucosal Blood Flow and Maintains Intestinal Barrier Function during Hemorrhagic Shock in Dogs. Microcirculation 2017, 24, e12345. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; López-Peña, M.; Miño, N.; Gómez-Moreno, G.; Guardia, J.; Cutando, A. Topical Application of Melatonin and Growth Hormone Accelerates Bone Healing around Dental Implants in Dogs. Clin. Implant Dent. Relat. Res. 2012, 14, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Boquete-Castro, A.; Guardia, J.; Piattelli, A.; Perrotti, V.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L. Outcomes of Topical Applications of Melatonin in Implant Dentistry: A Systematic Review. Implant Dent. 2015, 24, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Ivanovski, S. Melatonin as a Pro-Osteogenic Agent in Oral Implantology: A Systematic Review of Histomorphometric Outcomes in Animals and Quality Evaluation Using ARRIVE Guidelines. J. Periodontal Res. 2017, 52, 151–161. [Google Scholar] [CrossRef]
- Lopes, J.R.; Maschio, L.B.; Jardim-Perassi, B.V.; Moschetta, M.G.; Ferreira, L.C.; Martins, G.R.; Gelaleti, G.B.; De Campos Zuccari, D.A.P. Evaluation of Melatonin Treatment in Primary Culture of Canine Mammary Tumors. Oncol. Rep. 2015, 33, 311–319. [Google Scholar] [CrossRef]
- Gonçalves, N.d.N.; Colombo, J.; Lopes, J.R.; Gelaleti, G.B.; Moschetta, M.G.; Sonehara, N.M.; Hellmén, E.; de Zanon, C.F.; Oliani, S.M.; Zuccari, D.A.P. de C. Effect of Melatonin in Epithelial Mesenchymal Transition Markers and Invasive Properties of Breast Cancer Stem Cells of Canine and Human Cell Lines. PLoS ONE 2016, 11, e0150407. [Google Scholar] [CrossRef]
- Custódio, P.R.; Colombo, J.; Ventura, F.V.; Castro, T.B.; Zuccari, D.A.P.C. Melatonin Treatment Combined with TGF-β Silencing Inhibits Epithelial- Mesenchymal Transition in CF41 Canine Mammary Cancer Cell Line. Anticancer Agents Med. Chem. 2020, 20, 989–997. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Molecular Targets for the Management of Gastrointestinal Cancer Using Melatonin, a Natural Endogenous Body Hormone. Biomed. Pharmacother. 2021, 140, 111782. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Arnao, M.B. Free Radical-Scavenging Activity of Indolic Compounds in Aqueous and Ethanolic Media. Anal. Bioanal. Chem. 2003, 376, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin, Natural Melatonin from Plants as a Novel Dietary Supplement: Sources, Activities and World Market. J. Funct. Foods 2018, 48, 37–42. [Google Scholar] [CrossRef]
- Bonomini, F.; Borsani, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Dietary Melatonin Supplementation Could Be a Promising Preventing/Therapeutic Approach for a Variety of Liver Diseases. Nutrients 2018, 10, 1135. [Google Scholar] [CrossRef]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef]
| Chemical Category/Class | Chemical Name/Subclass | Example of Compounds | Potential Benefits |
|---|---|---|---|
| Phenylpropanoids | |||
| Simple phenols | Arbutin, tyrosol | Antiseptic, diuretic, anti-tumoral | |
| Hydroxycinnamic acids Free forms Esters Alcohols, Aldehydes & Glycosides | Ferulic, caffeic, cinnamic Chlorogenic, rosmarinic, cynarin, cichoric, caftaric Coniferyl, caffeoyl, feruloyl, vanillin, eugenol | Antioxidant, chemoprotector, immunomodulatory, neuroprotector, dyspepsia, hypercholesterolemia | |
| Acetophenones | Apocynin, androsin, piceol, picein | Antiasthmatic, anti-inflammatory, neuroprotective, sedative | |
| Salicylates | Salicin, salicortin, populin | Analgesic, febrifuges, sciatica, myalgia | |
| Curcuminoids | Curcumin, dimethoxy- and bisdemethoxy-curcumin, and breakdown metabolites | Anti-inflammatory, anti-tumoral, cardioprotective, wound healing, anti-arthritis, antioxidant, anti-depressive | |
| Lignans & Neolignans | Pinoresinol, masoprocol, silybin, schizandrin, podophyllotoxin, enterodiol | Hypoglycemic, chemoprotector, antioxidant, keratosis, antifungal, anti-inflammatory, anti-tumoral, phytoestrogen precursors | |
| Coumarins & Furanocoumarins | Coumarin, aesculetin, xanthotoxin, umbelliferone, psoralen, angelican, bergapten, khellin | Photosensitizer, anti-vitiligo, psoriasis, tinea hypopigmentation, spasmolytic, bronchodilator, asthma, anti-hypertensive, renal calculi, hay fever, rhinitis | |
| Betalains Betacyanins Betaxanthins | Betanin, (iso-, pro-, neo-) Vulga-xanthin (mira-, portula-, indica-) | Antioxidant, antimicrobial, anti-tumoral | |
| Polyketide-derived | |||
| Stilbenes | Resveratrol, pinosylvin, piceatannol, piceid, pallidol, viniferin, pterostylbene | Anti-inflammatory, neuroprotective, anti-tumoral, cardioprotective, anti-aging, antioxidant, antifungal, hypoglycemic | |
| Quinones Naphthoquinones, Naphthodiantrones, Anthraquinones & Kavalactones | Ubiquinol (Q10), menaquinone (vit K), plastoquinone, phylloquinone Juglone, lapachol, plumbagone, shikonin, hypericin, sennosides, carmine, fagopyrin, emodins, rhein, kavain, yangonin, methysticin | Anti-tumoral, anti-leukemic, antimicrobial, antiparasitic, antifungal, antiviral, anti-inflammatory, cardioprotective, laxative, hypnotic, sedative, anesthetic | |
| Flavonoids | |||
| Flavones | Apigenin, luteolin, baicalein | ||
| Isoflavones | Genistein, diadzein, biochanin | ||
| Flavonones | Naringenin, eriodictyol, hesperetin, liquiritin | Antioxidant, anti-tumoral, anti-microbial, antiviral, anti-atheromatous, anti-hypertensive, anti-inflammatory, hepatoprotective, endothelial protection, cardioprotective, neuroprotective, chemoprotective, immunoprotective, estrogen-mediated responses, anti-aging | |
| Flavonols | Quercetin, kaempferol, myricetin, isorhamnetin | ||
| Flavanols | Catechin, epicatechin | ||
| Flavan-3-ol (OPC)1 | Epicatechin-3-gallate, epigallocatechin-3-gallate | ||
| Anthocyanidins | Malvadin, cyanidin, delphinidin, europinidin, pelargonidin, peonidin, rosinidin, aurantinidin | ||
| Tannins Gallo- & Ellagitannins Condensed tannins (Proanthocyanidins) | Galloyl derivatives, ellagic acid, punicalagin, rugosin-D, oenthein-B, sanguiin, geraniin, agrimoniin, puncialin, corilagin Procyanidins (OPC), propelargonidins, prodelphinidins, profisetinidins, proteracacinidins, theaflavins | Anti-tumoral, anti-inflammatory, antioxidant, antidiarrhoeic, anti-hemorrhagic, antimicrobial, hypolipidaemic, astringent, sclerosis, cardioprotective, endothelial function, platelet function, anti-hypertensive, anti-atherosclerotic, oral health |
| Chemical Category/Class | Chemical Name/Subclass | Example of Compounds | Potential Benefits |
|---|---|---|---|
| Monoterpenes/oids (main constituent of essential oils) | Regular Monocyclics Acyclics Bicyclics Irregular Iridoids Pyrethrins Cannabinoids | Limonene, terpineol, menthol, thymol, p-cymene, carvacrol Linalool, citronelle, geranial Camphor, α-pinene, thujone Nepetalactone, valtrate, harpagide, oleuropein Pyrethrin, chrysanthemic acid, cinerin, jazmolone Δ9-tetrahydrocannabinol, cannabidiol, cannabicylol | Antifungal, antibacterial, antioxidant, anticancer, anti-spasmodic, analgesic, vasodilator, cardiovascular protector, anti-inflammatory, antidiabetic, anti-obesity, gut microbiota modulator, sedative, hepatoprotector, chloleretic, laxative, antiviral, insecticidal Euphoriant, analgesic, neuroprotective, antiemetic, anxiolytic, anti-tumoral, anti-inflammatory, bronchodilator |
| Sesquiterpenes/oids | In EOs Lactones | Bisabolol and its oxides, matricin, chamazulene, gossypol, zingerbene Germacrene, achillin, artemisin, cnicin, parthenolide, tanacetin, helenalin | Anti-inflammatory, wound-healing, contraceptive, anesthetic, antibacterial, antifungal, anti-protozoal, analgesic, anti-tumoral |
| Diterpenes/oids | Acyclic, mono-, bi-, tri-, and tetracyclic | Forskolin, marrubiin, paclitaxel, andrographolide, ginkgolides, bilobide, stevioside, rebaudioside, abietic acid, hautriwaic acid | Antihypertensive, vasodilatory, bronchodilatory, platelet aggregation inhibition, anti-tumoral, intraocular pressure regulator, hepatoprotector, immunomodulatory, neuroprotection, anti-diabetic, sweetener |
| Triterpenes/oids | Free Phytosterols Limonoids | Lanosterol, ganosterol, lupeol Sitosterol, campesterol, gugusterol, stigmasterol, brassicasterol, avenasterol, cycloartenol Azadirachtin, limonin, nomilin | Blood cholesterol and LDL level regulator, hypocholesterolemic, hypolipidemic, anti-obesity, cardio-, neuro-, thyroid-protective, anti-tumoral Antifeedant, insecticidal |
| Saponins Non-steroidal Steroidal (so-called Cardenolides/Bufanolides, including some cardiac glycosides *) | Glycyrrhicin, ginsenosides, jujubosides, asiatoside, betulin Diosgenin, sarasapogenin, ruscogenins, withaferin-A Digitoxin *, digoxin *, convallatoxin *, cimarin *, proscillaridin * | Many systemic effects: antiallergic, anti-tumoral, immunomodulatory, anti-(bacterial, fungal, viral), cardio-, hepato-, neuro-protective, hypoglycemic, estrogenic-, digestive-regulator, hypocholesterolaemic, hearth arrhythmia & failure *, angiogenesis inhibitor *, apoptotic *, autophagic *, neuroprotective * | |
| Tetraterpenes/oids | Carotenes Xanthophylls Gukulenins | α-, β-, γ-, and δ-Carotene, lycopene, phytoene Lutein, xanthins (viola-, luteo-, zea-, β-crypto-, astha-, anthera, cantha-), crocetins, and crocins Gukulenin A and B | Antitumoral, pro-vitamin A, hypocholesterolemic, cardiovascular protection, neuroprotector, immunoactivator, skin protection Antitumoral |
| Meroterpenes | Terpenophenols | Bakuchiol, ferruginol, totarol, epiconicol | Antioxidant, antibacterial, anti-inflammatory, ocular protection |
| Chemical Category/Class | Chemical Name/Subclass | Example of Compounds | Potential Benefits |
|---|---|---|---|
| Alkaloids | From lysine From ornithine From tryptophan From phenylalanine/tyrosine Steroidal (alkaloid saponins) | Lupanine, cytosine, sedamine Cocaine, hyoscyamine, nicotine Vincamine, yohimbine, physostigmine, ergotamine, quinine, camptothecin Ephedrine, berberine, emetine, morphine, capsaicin, eserpine, ergotamine, caffeine Solanine, veratrine, solasodine | Analgesic, stimulant, narcotic, hyper-, hypotensive, bronchodilator, antimicrobial, anti-tumoral, vermicide, antimalarial, anticholinergic, cholagogue, emetic, cardiotonic, sympathetic, vasoconstrictor, antiasthmatic, anthelmintic |
| Glucosinolates & derivatives | Aliphatic Aromatic Indolic Sulfur-derivatives | Glucoraphanin, sinigrin Gluconasturtiin, glucotropaelin Glucobrassicins Isothiocyanates (allyl, benzyl), sulforaphane | Cancer prevention, anti-tumoral, antibacterial, antifungal, antioxidant, bronchodilator, skin irritation shooting |
| Common Name | Scientific Name | Compounds * |
|---|---|---|
| Anise | Pimpinella anisum | trans-Anethole, γ-himachalene, estragole, 2-methyl-isoeugenol, anisaldehyde |
| Basil | Ocimum basilicum | Linalool, 1,8-cineole, methyl eugenol, estragole, myrcene |
| Bergamot | Citrus bergamia | Limonene, linalyl acetate,γ-terpinene, linalool, β-pinene, β-bisabolene |
| Cinnamon | Cinnamomum zeylanicum | Eugenol, β-caryophyllene, benzyl benzoate, cinnamyl acetate, α-phellandrene |
| Chinese tea tree | Malaleuca alternifolia | Terpinen-4-ol, γ-terpinene, α-terpinene, 1,8-cineole, α-terpineol, p-cymene, terpinolene, α-pinene |
| Clove | Syzygium aromaticum | Eugenol, β-caryophyllene, α-humulene, δ-cadinene |
| Eucalypt | Eucalyptus globulus | 1,8-Cineole, α-pinene, limonene, p-cymene |
| Fennel | Foeniculum vulgare | Anethole, fenchone, α-pinene, limonene, estragole, anisaldehyde, β-phellandrene |
| Ginger | Zingiber officinale | Geranial, neral, geraniol, limonene |
| Hypericum | Hypericum perforatum | α-Pinene, β-caryophyllene, methyl-2-octane, dodecanol, myrcene |
| Lavender | Lavandula angustifolia | Linalyl acetate, linalool, terpinen-4-ol, ocimene, 1,8-cineole, limonene, camphor |
| Lemongrass | Cymbopogon citratus | Geranial, neral, geraniol, geranyl acetate, β-caryophyllene |
| Marjoram | Thymus mastichina | 1,8-Cineole, linalool, α-terpineol, α-pinene, limonene, linalyle acetate |
| Peppermint | Mentha piperita | Menthol, menthone, 1,8-cineole, menthylacetate, isomenthone, neomenthol, menthofurane, limonene, β-caryophyllene |
| Rosemary | Rosmarinus officinalis | α-Thuyone, α-pinene, camphene, camphor, limonene, myrcene, borneol |
| Sagebrush | Artemisia vulgaris | α-Thuyone, lyratol, 1,8-cineole, camphor, β-thuyone, artemisinin |
| Salvia | Salvia officinalis | α-Thuyone, camphor, 1,8-cineole, α-humulene, β-thuyone, α-pinene, bornyle acetate, limonene |
| Savory | Satureja montana | Carvacrol,p-cymene, γ-terpinene, thymol |
| Thyme | Thymus vulgaris | 1,8-cineole, β-phellandrene, camphor, α-pinene, myrcene, borneol, limonene, neral |
| Plant/EOs/Dose/App Form | Animals | Benefits | Refs. |
|---|---|---|---|
| Lavender/0.18 mL/inner pinnas of both ears | Beagles | ↓ sympathovagal activity ↑ relax and calming | [51] |
| Artemisia absinthium in vitro bioassay | Dogs | ↑ acaricidal activity ↓ egg and larvae of Rhipicephalus sanguineus dog tick | [52] |
| Menthol and thymol oils applied as gel | Adult dogs | ↓ buccal halitosis | [53] |
| Thymol and eugenol EOs 10 mL/kg, applied all over the skin and hair | English cocker spaniel dogs | ↓ larvae of Rhipicephalus sanguineus dog tick | [54] |
| Turmeric oil at 2.5% in spray | Dogs with tick infestation | ↓ number of tick bites In vitro effectivity: turmeric > DEET > PMD | [55] |
| Otogen®, EOs (tea tree, thyme, sage, eucalyptus, rosemary, lavender), and vegetable oil (macadamia and sunflower) 7 days applied | Dogs of different breeds and ages | ↓ external otitis ↓ head shaking, erythema, and scraping | [56] |
| Thymol, cinnamaldehyde, and carvacrol; also clove and oregano EOs In vitro assay | Dogs (bacterial and Malassezia pachydermatis isolates) | ↑ bactericidal and fungicidal activity ↑ Gram-positive bactericidal activity ↓ canine otitis | [57] |
| Dermoscent BIO BALM® Neem, rosemary, lavender, clove, tea tree, oregano, peppermint EOs, cedar bark extract, and PUFAs Topical administration (0.6–2.4 mL weekly) | Dogs with low, medium, and severe atopic dermatitis | ↓ canine atopic dermatitis and pruritus score ↑ beneficial in ameliorating the clinical signs of atopic dermatitis | [58] |
| Dermoscent BIO BALM® Topical administration | Dogs of different breeds | ↓ canine idiopathic noncomplicated nasal hyperkeratosis | [59] |
| Dog food containing EOs (clove, rosemary, and oregano; also, vit. E) vs. synthetic antioxidant BHT | Dogs of different breeds and ages | ↓ lymphocytes, fecal bacterial count, oxidative stress (ROS), ↑ NPSH and glutathione S-transferases, feed conservation | [60] |
| Microencapsulated thymol, carvacrol, and cinnamaldehyde300 mg/kg of feed | Beagle dogs | neutrophils, lymphocytes, globulins, nitrogen oxide, GSH-POX ↓ ROS, fecal bacterial count, Salmonella, Escherichia coli | [44] |
| Cinnamon, thyme, clove, geranium, and tea tree EOs; also, eugenol, geraniol, cinnamaldehyde, thymol, and carvacrol individual components In vitro assay | Dog and human skin fungal dermatosis | ↑ fungicidal activity, higher in dermatomycetes ↑ anti-mycosis therapy | [61] |
| Citrus, basilic, eucalyptus, cinnamon, lemon balm, lemongrass, lemon verbena, tea tree, savory, myrrh, and cannabis EOs | Possible application to dogs with pyoderma | ↑ bactericidal activity against methicillin-resistant Staphylococcus ↑ pyoderma therapy | [62] |
| Vernonia brasiliana EO (Asteraceae) | Antileishmanial activity against L. infantum promastigotes and cytotoxicity on canine DH82 cells | ↑ Antiparasitic effect, ROS, cell death by apoptosis ↓ mitochondrial membrane potential Antagonistic interaction with miltefosine drug | [63] |
| Schinus molle EO (Anacardiaceae) | Acaricidal effect on females and larval stages of R. sanguineus | EO (2%) caused larval mortality (99.3%) Inhibition of oviposition, egg hatching, and reproductive efficiency | [64] |
| Tagetes minuta EO (Asteraceae) | Acaricidal effect in vitro and on dogs of R. sanguineus | 100% efficacy against larvae, nymphs, and adults of the tick on all tested conditions | [65] |
| Thyme and oregano EOs | Bacterial and fungal isolates from canine otitis externa | EOs showed good in vitro bactericidal and fungicidal activity against 100 isolates from dogs with otitis externa, including some highly drug-resistant isolates | [50] |
| Cinnamon EO | Staphylococcus strains from canine otitis | Effective antimicrobial and antibiofilm activity Potential alternative to treat ear infections in canines | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Cano, D.; Sánchez-Carrasco, G.; El-Mihyaoui, A.; B. Arnao, M. Essential Oils and Melatonin as Functional Ingredients in Dogs. Animals 2022, 12, 2089. https://doi.org/10.3390/ani12162089
Ruiz-Cano D, Sánchez-Carrasco G, El-Mihyaoui A, B. Arnao M. Essential Oils and Melatonin as Functional Ingredients in Dogs. Animals. 2022; 12(16):2089. https://doi.org/10.3390/ani12162089
Chicago/Turabian StyleRuiz-Cano, Domingo, Ginés Sánchez-Carrasco, Amina El-Mihyaoui, and Marino B. Arnao. 2022. "Essential Oils and Melatonin as Functional Ingredients in Dogs" Animals 12, no. 16: 2089. https://doi.org/10.3390/ani12162089
APA StyleRuiz-Cano, D., Sánchez-Carrasco, G., El-Mihyaoui, A., & B. Arnao, M. (2022). Essential Oils and Melatonin as Functional Ingredients in Dogs. Animals, 12(16), 2089. https://doi.org/10.3390/ani12162089







