Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
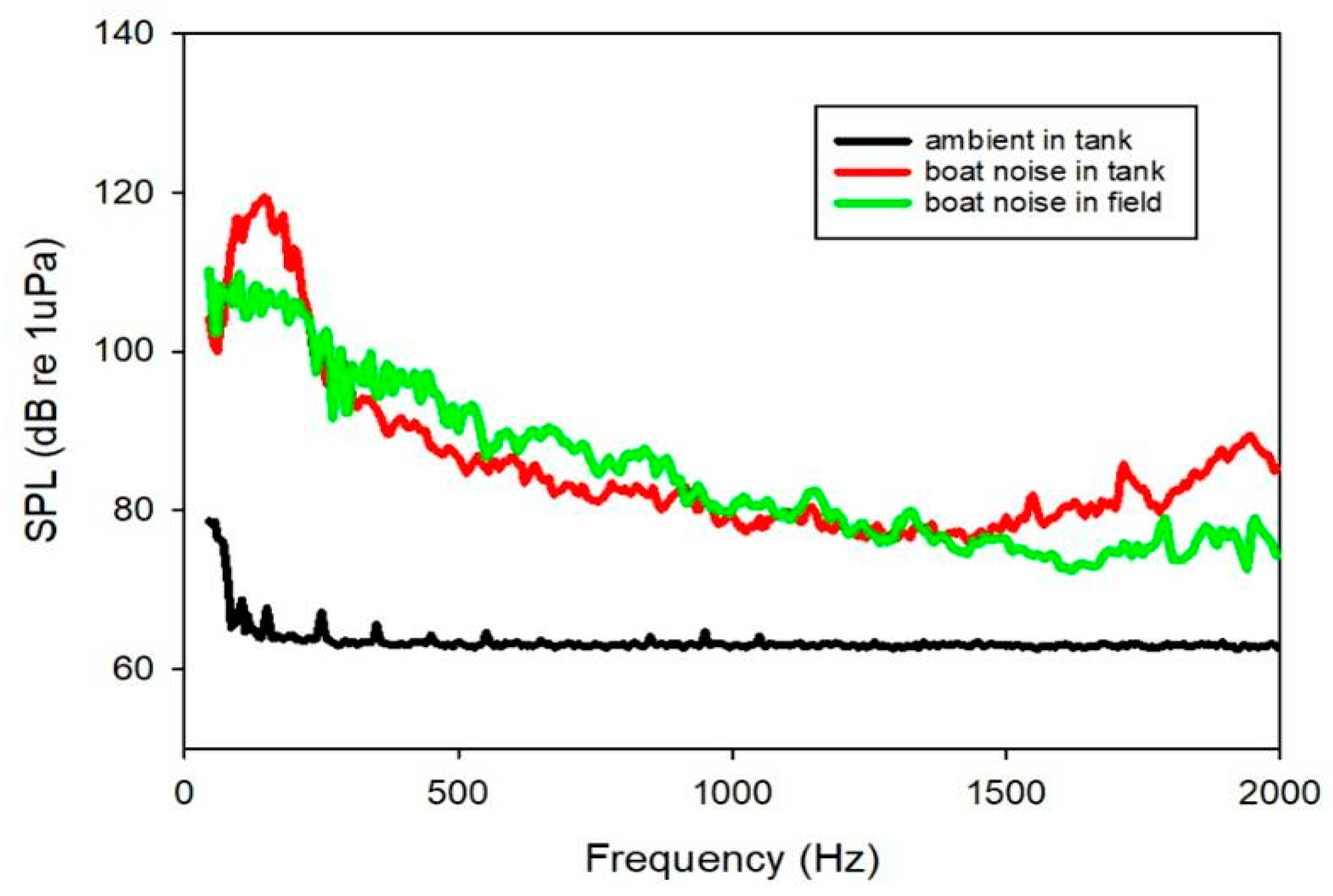
2.2. Sound Exposure and Behavior Experiment
2.3. Total RNA Isolation and Illumina Sequencing
2.4. Alignment of Transcriptomic Data
2.5. GO and KEGG Pathway Enrichment Analysis
2.6. Quantitative Real-Time PCR
3. Results
3.1. Transcriptome Overview
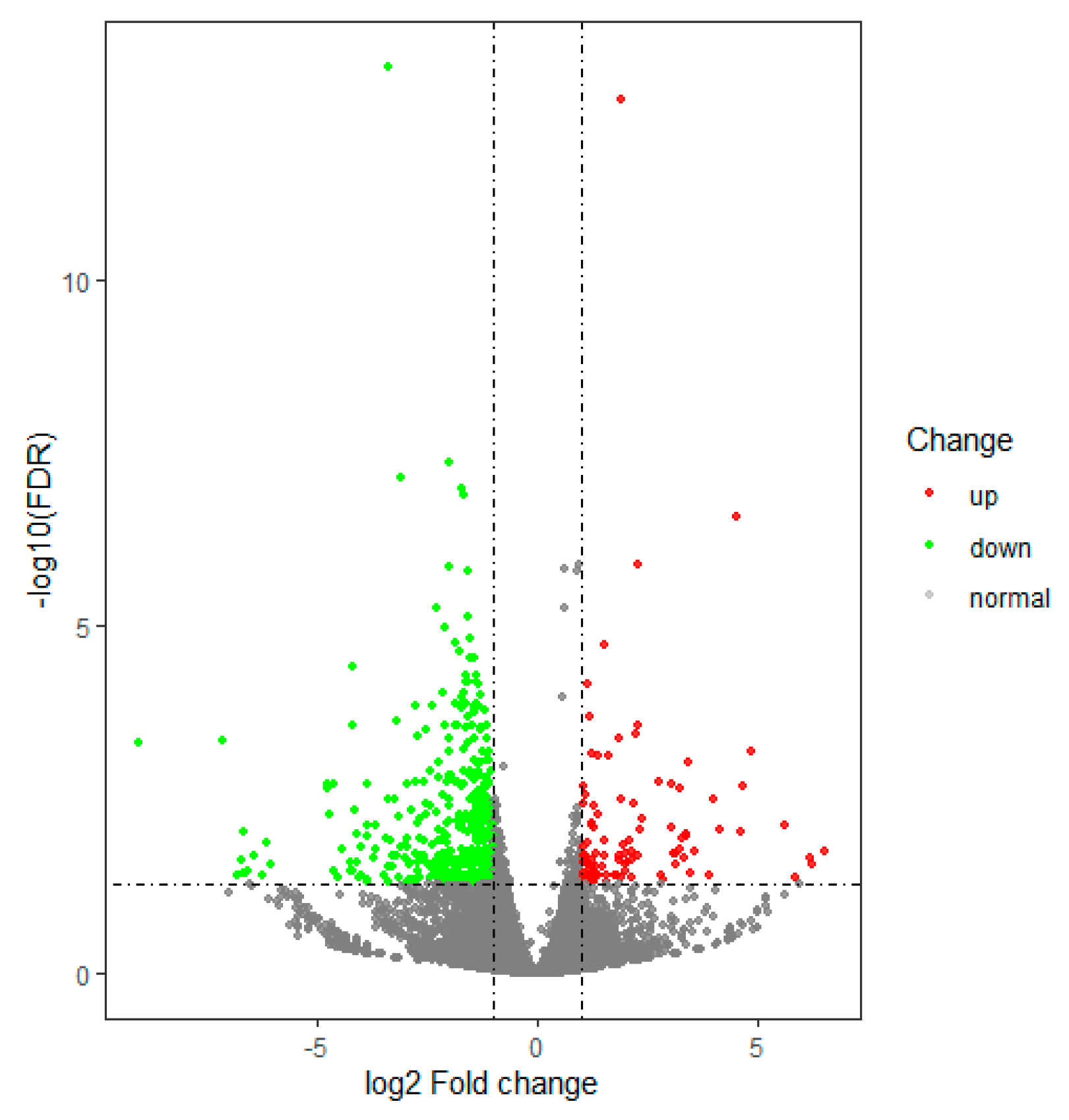
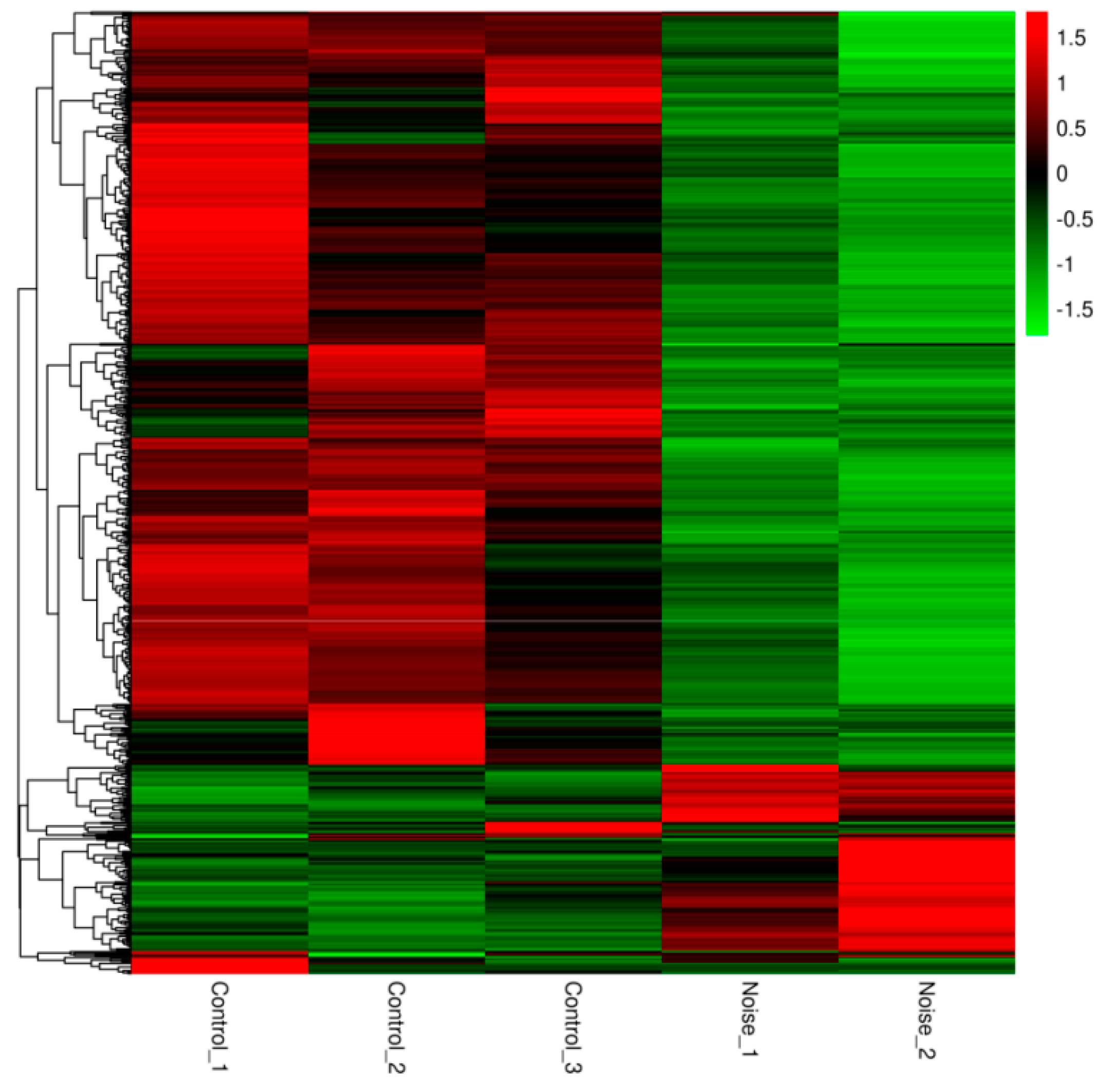
3.2. Gene Differential Expression Analysis
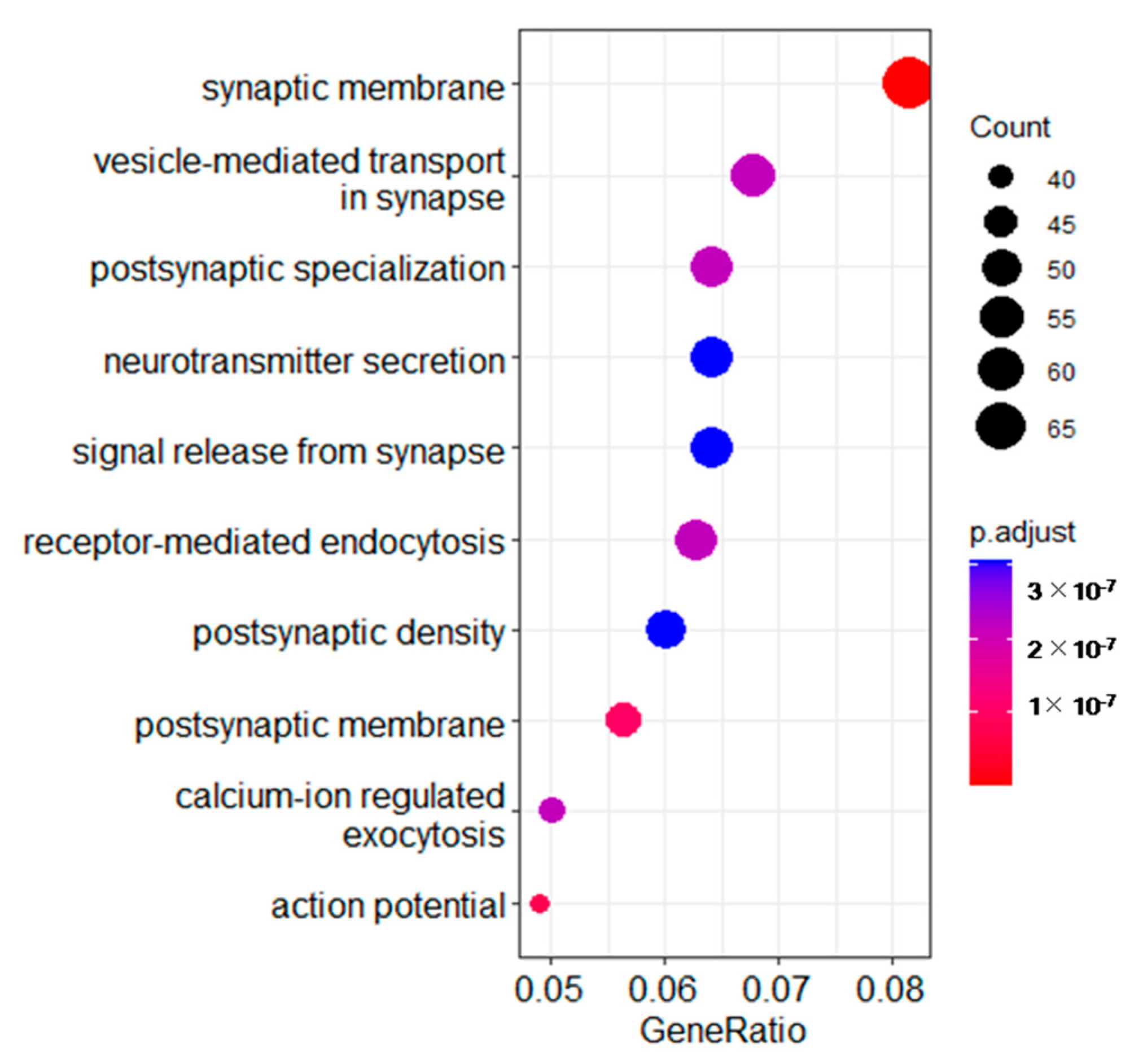
3.3. Gene Ontology (GO) Analysis of Different Expressed Transcripts between the Two Groups
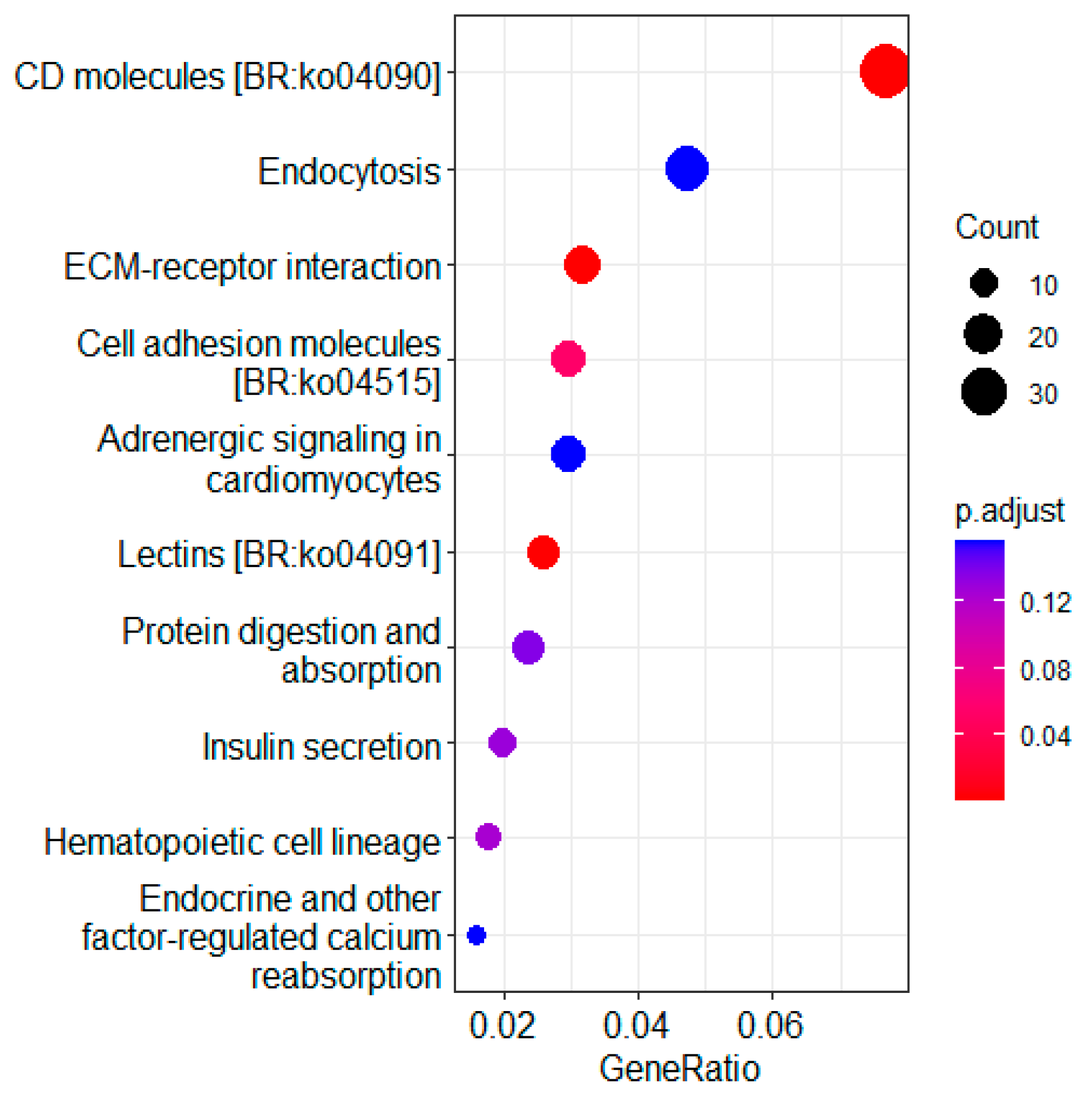
3.4. KEGG Pathway Enrichment Analysis of DEGs
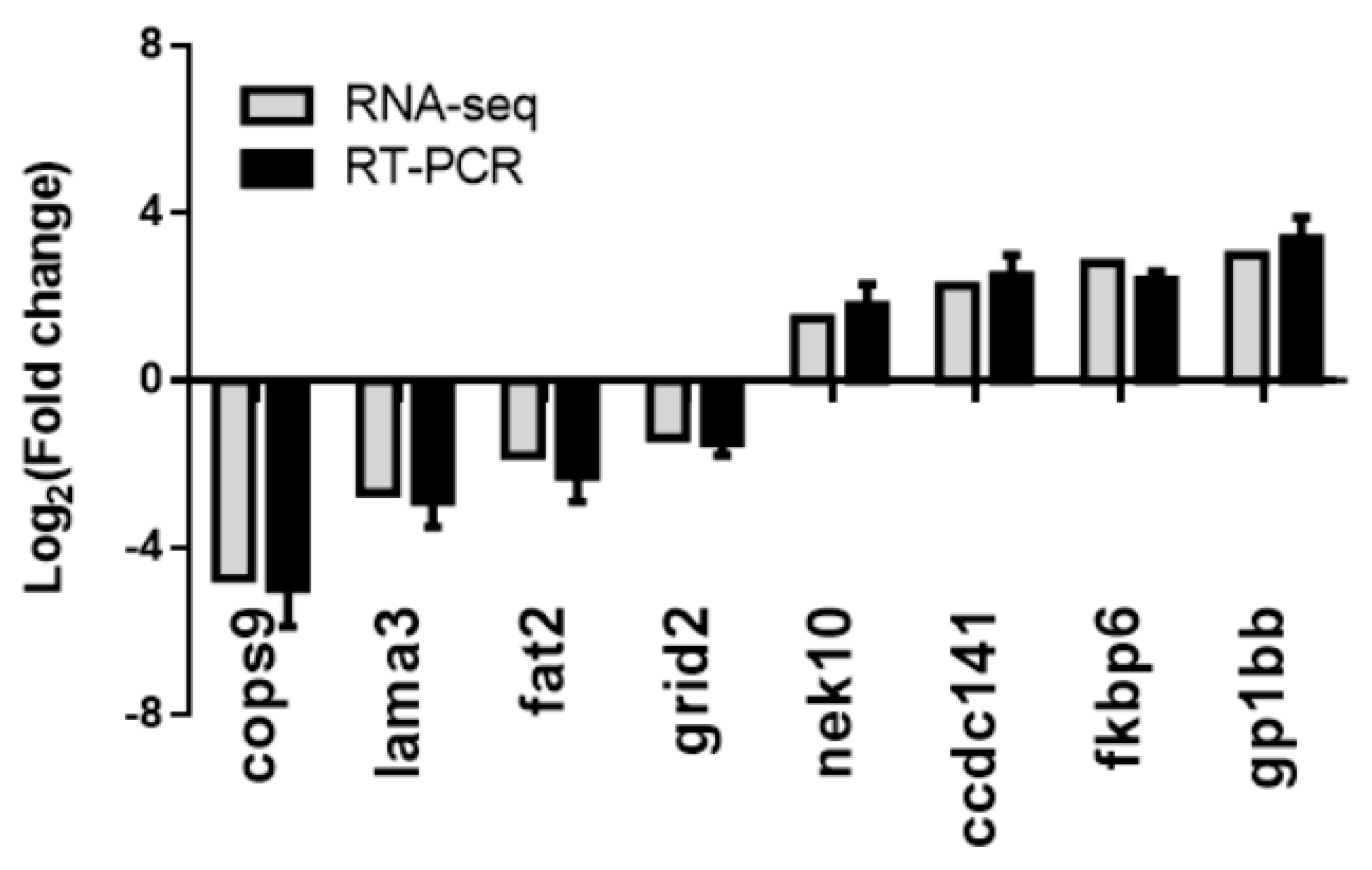
3.5. Validation of the RNA-seq Results Using RT–PCR Methods
3.6. Locomotor Behavior of L. polyactis after Noise Exposure
4. Discussion
4.1. Synaptic Dysfunction
4.2. Neurotransmitters and Synaptic Transmission
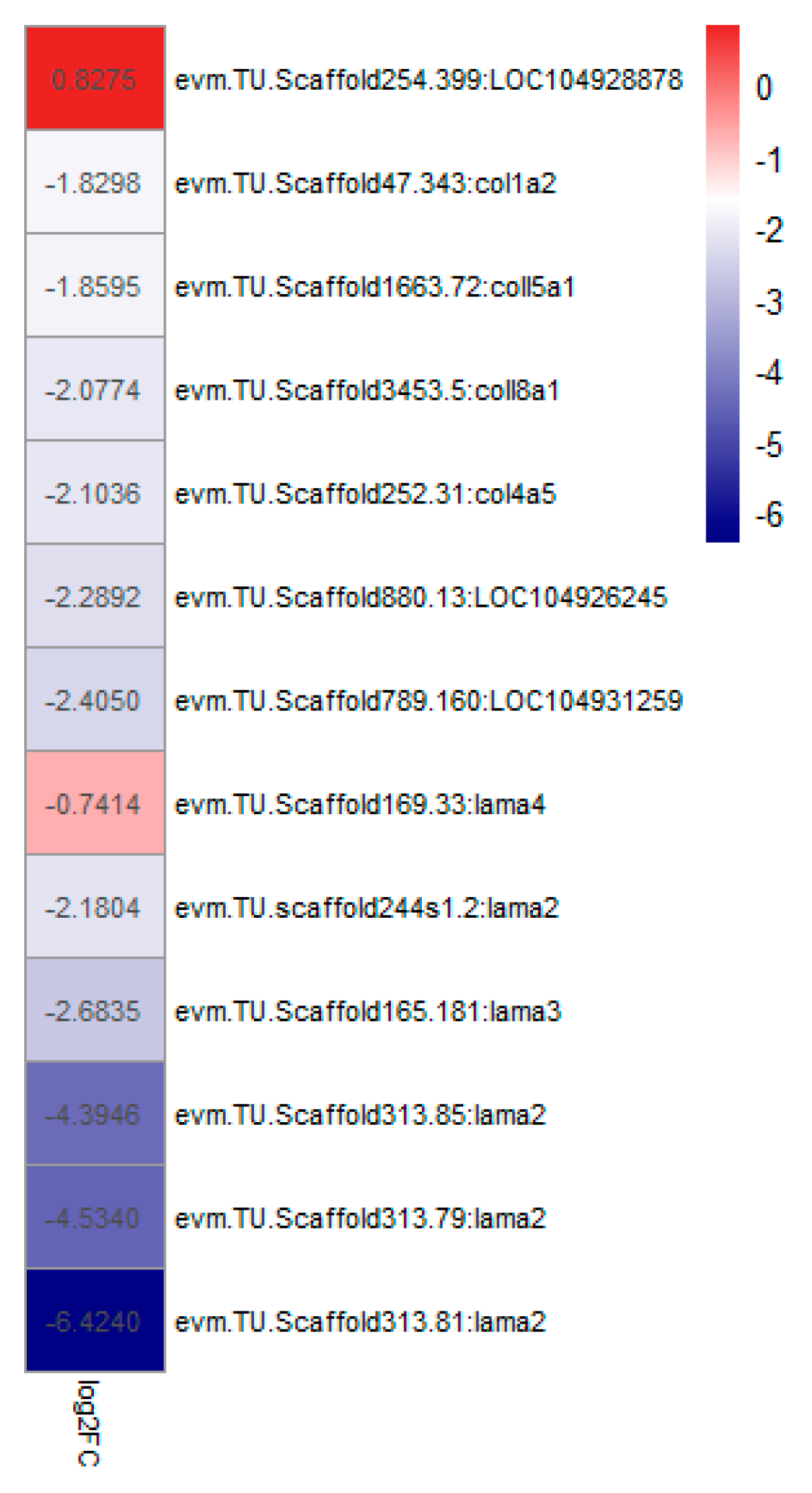
4.3. The Composition of the Extracellular Matrix (ECM)
4.4. Linkages between Behavioral Responses and Neurotransmitters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieretti, N.; Lo Martire, M.; Corinaldesi, C.; Musco, L.; Dell’Anno, A.; Danovaro, R. Anthropogenic noise and biological sounds in a heavily industrialized coastal area (Gulf of Naples, Mediterranean Sea). Mar. Environ. Res. 2020, 159, 105002. [Google Scholar] [CrossRef]
- Voellmy, I.K.; Purser, J.; Simpson, S.D.; Radford, A.N. Increased noise levels have different impacts on the anti-predator behaviour of two sympatric fish species. PLoS ONE 2014, 9, e102946. [Google Scholar] [CrossRef]
- McCormick, M.I.; Allan, B.; Harding, H.; Simpson, S.D. Boat noise impacts risk assessment in a coral reef fish but effects depend on engine type. Sci. Rep. 2018, 8, 3847. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.; Hastings, M. The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 2009, 75, 455–489. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.I.; Wilkens, S.L.; Stanley, J.A.; Jeffs, A.G. Vessel generator noise as a settlement cue for marine biofouling species. Biofouling 2014, 30, 741–749. [Google Scholar] [CrossRef]
- Popper, A.N.; Fay, R.R. Sound Detection and Processing by Fish: Critical Review and Major Research Questions (Part 2 of 2). Brain Behav. Evol. 1993, 41, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.N.; Emma, K.; Simpson, S.D. Acoustic communication in a noisy world: Can fish compete with anthropogenic noise? Behav. Ecol. 2014, 5, 1022–1030. [Google Scholar] [CrossRef]
- McCormick, M.I.; Fakan, E.P.; Nedelec, S.L.; Allan, B.J.M. Effects of boat noise on fish fast start escape response depend on engine type. Sci. Rep. 2019, 9, 6554. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.D.; Radford, A.N.; Nedelec, S.L.; Ferrari, M.C.O.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Anthropogenic noise increases fish mortality by predation. Nat. Commun. 2015, 7, 10544. [Google Scholar] [CrossRef]
- Davidson, J.; Bebak, J.; Mazik, P. The effects of aquaculture production noise on the growth, condition factor, feed conversion, and survival of rainbow trout, Oncorhynchus mykiss. Aquaculture 2009, 288, 337–343. [Google Scholar] [CrossRef]
- Hang, S.; Zhao, J.; Ji, B.; Li, H.; Zhang, Y.; Peng, Z.; Zhou, F.; Ding, X.; Ye, Z. Impact of underwater noise on the growth, physiology and behavior of Micropterus salmoides in industrial recirculating aquaculture systems. Environ. Pollut. 2021, 291, 118152. [Google Scholar] [CrossRef]
- Zahra, J.; Kolb, B.E.; Mohajerani, M.H. Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp. Neurol. 2018, 308, 1–12. [Google Scholar]
- Wang, X.; Lu, G.; Zhao, L.; Yang, Q.; Gao, T. Assessment of fishery resources using environmental DNA: Small yellow croaker (Larimichthys polyactis) in East China Sea. PLoS ONE 2020, 15, e0244495. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Chen, J.; Song, J.; Xu, K.; Lin, J.; Zhang, S. Potential effects of underwater noise from wind turbines on the marbled rockfish (Sebasticus marmoratus). J. Appl. Ichthyol. 2021, 37, 514–522. [Google Scholar] [CrossRef]
- Xie, Q.P.; Zhan, W.; Shi, J.; Liu, F.; Niu, B.-L.; He, X.; Liu, M.; Liang, Q.; Xie, Y.; Xu, P.; et al. Whole-genome assembly and annotation of little yellow croaker (Larimichthys polyactis) provide insights into the evolution of hermaphroditism and gonochorism. Authorea Prepr. 2021. [Google Scholar]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Huson, D.H.; Buchfink, B. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method 2013, 25, 402–408. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Ladich, F.; Riesch, R.; Plath, M. Otolith morphology and hearing abilities in cave- and surface-dwelling ecotypes of the Atlantic molly, Poecilia mexicana (Teleostei: Poeciliidae). Hear. Res. 2010, 267, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A. Interactions between cell adhesion and the synaptic vesicle cycle in Parkinson’s disease. Med. Hypotheses 2014, 83, 203–207. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after ‘‘temporary’’ noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Narinobu, J.; Muroyama, A.; Hiasa, M.; Omote, H.; Moriyama, Y. Vesicular Inhibitory Amino Acid Transporter Is a Cl−/γ-Aminobutyrate Co-transporter. J. Biol. Chem. 2009, 284, 35073–35078. [Google Scholar]
- Marrone, D.F.; Petit, T.L. Marrone, D.F.; Petit, T.L. The role of synaptic morphology in neural plasticity: Structural interactions underlying synaptic power. Brain Res. Brain Res. Rev. 2002, 38, 291–308. [Google Scholar] [CrossRef]
- Rabenstein, R.L.; Addy, N.A.; Caldarone, B.J.; Asaka, Y.; Gruenbaum, L.M.; Peters, L.L.; Gilligan, D.M.; Fitzsimonds, R.M.; Picciotto, M.R. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J. Neurosci. 2005, 25, 2138–2145. [Google Scholar] [CrossRef]
- Scott, D.A.; Tabarean, I.; Tang, Y.; Cartier, A.; Masliah, E.; Roy, S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J. Neurosci. 2010, 30, 8083–8095. [Google Scholar] [CrossRef]
- Nakano-Kobayashi, A.; Kasri, N.N.; Newey, S.E.; Van Aelst, L. The Rho-Linked Mental Retardation Protein OPHN1 Controls Synaptic Vesicle Endocytosis via Endophilin A1. Curr. Biol. 2009, 19, 1133–1139. [Google Scholar] [CrossRef]
- Bo, C.; Wu, M.; She, X.; Liu, H. Impulse noise exposure in rats causes cognitive deficits and changes in hippocampal neurotransmitter signaling and tau phosphorylation. Brain Res. 2012, 1427, 35–43. [Google Scholar]
- Manikandan, S.; Padma, M.K.; Srikumar, R.; Jeya Parthasarathy, N.; Muthuvel, A.; Sheela Devi, R. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci. Lett. 2006, 399, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, F.; Haider, S.; Batool, Z.; Perveen, T.; Haleem, D.J. Subchronic exposure to noise affects locomotor activity and produces anxiogenic and depressive like behavior in rats. Pharmacol. Rep. 2012, 64, 64–69. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated Stress Causes Cognitive Impairment by Suppressing Glutamate Receptor Expression and Function in Prefrontal Cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef]
- Singewald, N.; Kouvelas, D.; Mostafa, A.; Sinner, C.; Philippu, A. Release of glutamate and GABA in the amygdala of conscious rats by acute stress and baroreceptor activation: Differences between SHR and WKY rats. Brain Res. 2000, 864, 138–141. [Google Scholar] [CrossRef]
- Kazi, A.I.; Oommen, A. Chronic noise stress-induced alterations of glutamate and gamma-aminobutyric acid and their metabolism in the rat brain. Noise Health 2014, 16, 343–349. [Google Scholar] [CrossRef]
- Schil, K.V.; Meire, F.; Karlstetter, M.; Bauwens, M.; Verdin, H.; Coppieters, F.; Scheiffert, E.; Van Nechel, C.; Langmann, T.; Deconinck, N.; et al. Early-onset autosomal recessive cerebellar ataxia associated with retinal dystrophy: New human hotfoot phenotype caused by homozygous GRID2 deletion. Genet. Med. 2015, 17, 291–299. [Google Scholar] [CrossRef]
- Frischknecht, R.; Gundelfinger, E.D. The brain’s extracellular matrix and its role in synaptic plasticity. Adv. Exp. Med. Biol. 2012, 970, 153–171. [Google Scholar]
- Chen, Z.L.; Strickland, S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 1997, 91, 917–925. [Google Scholar] [CrossRef]
- Tsirka, S.E.; Rogove, A.D.; Bugge, T.H.; Degen, J.L.; Strickland, S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 1997, 17, 543–552. [Google Scholar] [CrossRef]
- Monavarfeshani, A.; Knill, C.N.; Sabbagh, U.; Su, J.; Fox, M.A. Region- and cell-specific expression of transmembrane collagens in mouse brain. Front. Integr. Neurosci. 2017, 11, 20. [Google Scholar] [CrossRef]
- Jongkamonwiwat, N.; Ramirez, M.A.; Pak, K.; Ryan, A.F.; Savas, J.N. Noise Exposures Causing Hearing Loss Generate Proteotoxic Stress and Activate the Proteostasis Network. Cell Rep. 2020, 33, 108431. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Rutherford, M.A.; Green, S.H. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. Proc. Natl. Acad. Sci. USA. 2020, 117, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Mzia, Z.; Nina, G.; Yousef, T.; Nadezhda, J.; Nino, P.; Fuad, R.; Eldar, G. Behavioral and neuroanatomical effects on exposure to White noise in rats. Neurosci. Lett. 2020, 728, 134898. [Google Scholar]
- Hakuba, N.; Koga, K.; Gyo, K.; Usami, S.I.; Tanaka, K. Exacerbation of Noise-Induced Hearing Loss in Mice Lacking the Glutamate Transporter GLAST. J. Neurosci. 2001, 20, 8750–8753. [Google Scholar] [CrossRef]
- Wankhar, W.; Srinivasan, S.; Sundareswaran, L.; Wankhar, D.; Rajan, R.; Sheeladevi, R. Role of Scoparia dulcis linn on noise-induced nitric oxide synthase (NOS) expression and neurotransmitter assessment on motor function in Wistar albino rats. Biomed. Pharmacother. 2017, 86, 475–481. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, J.; Xu, W.; Zhan, W.; Zou, H.; Lin, J. Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure. Animals 2022, 12, 2061. https://doi.org/10.3390/ani12162061
Zhang X, Zhou J, Xu W, Zhan W, Zou H, Lin J. Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure. Animals. 2022; 12(16):2061. https://doi.org/10.3390/ani12162061
Chicago/Turabian StyleZhang, Xuguang, Jun Zhou, Wengang Xu, Wei Zhan, Huafeng Zou, and Jun Lin. 2022. "Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure" Animals 12, no. 16: 2061. https://doi.org/10.3390/ani12162061
APA StyleZhang, X., Zhou, J., Xu, W., Zhan, W., Zou, H., & Lin, J. (2022). Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure. Animals, 12(16), 2061. https://doi.org/10.3390/ani12162061





