Age Evolution of Lipid Accretion Rate in Boars Selected for Lean Meat and Duroc Barrows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection and Dissection Process
2.3. Analytical Procedures
2.3.1. Apparent Ileal Digestibility
2.3.2. Tissue Fatty Acids Analysis
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Production Data and Allometric Tissue Growth
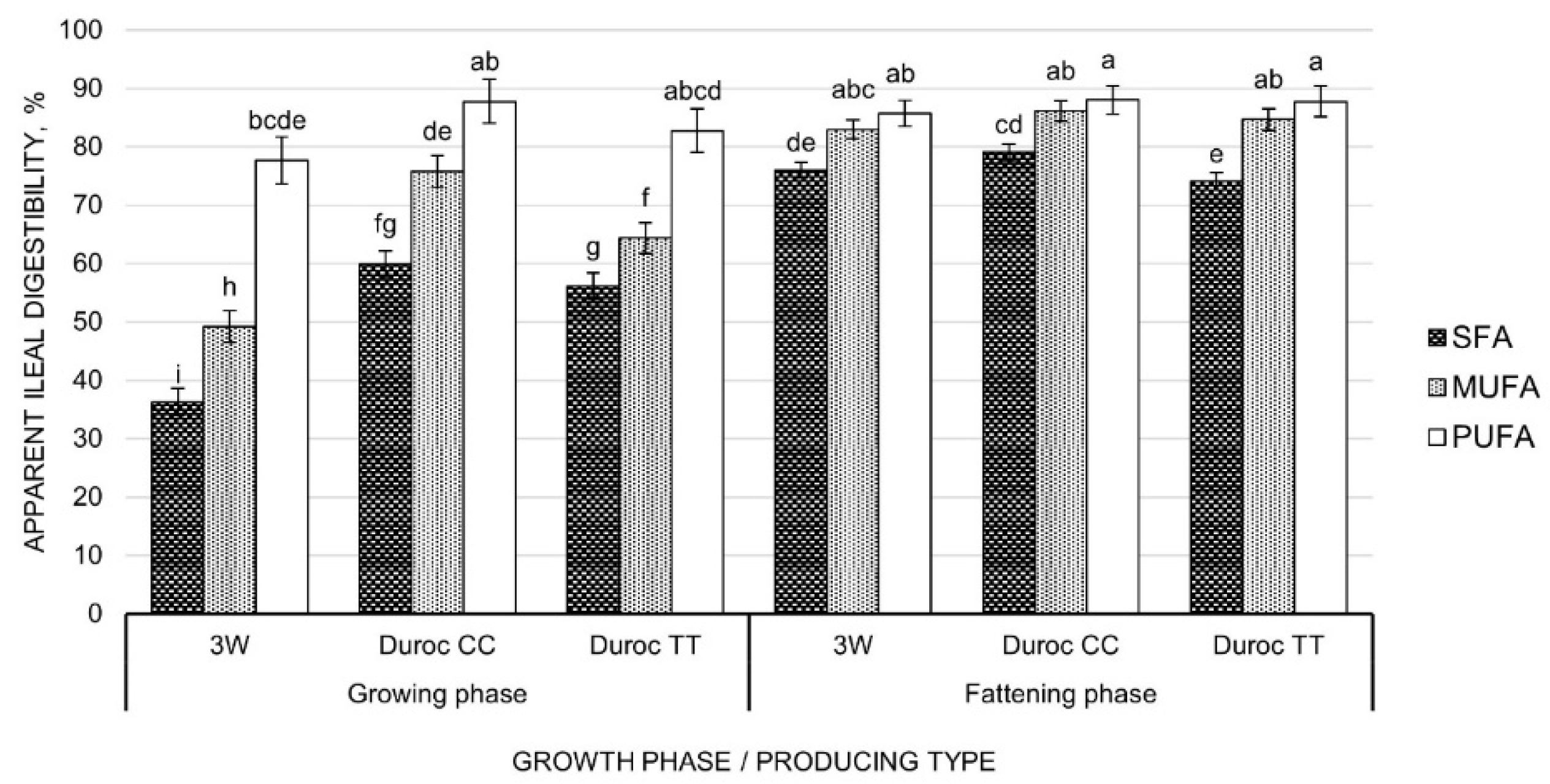
3.2. Apparent Ileal Digestibility
3.3. Fractional Incorporation Rate (FIR)
3.4. Oleic Acid De Novo Synthesis: Δ9-Desaturase Activity
4. Discussion
4.1. Production Data and Differential Growth Intensity
4.2. Apparent Ileal Digestibility
4.3. Fractional Incorporation Rate of Stearic Acid
4.4. Endogenous Oleic Acid Synthesis: Oleic/Stearic Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Gudiño, J.; Blanco-Penedo, I.; Gispert, M.; Brun, A.; Perea, J.; Font-i-Furnols, M. Understanding Consumers’ Perceptions towards Iberian Pig Production and Animal Welfare. Meat Sci. 2021, 172, 108317. [Google Scholar] [CrossRef] [PubMed]
- Gol, S.; González-Prendes, R.; Bosch, L.; Tor, M.; Reixach, J.; Pena, R.N.; Estany, J. Linoleic Acid Metabolic Pathway Allows for an Efficient Increase of Intramuscular Fat Content in Pigs. J. Anim. Sci. Biotechnol. 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Estany, J.; Ros-Freixedes, R.; Tor, M.; Pena, R.N. Triennial Growth and Development Symposium: Genetics and Breeding for Intramuscular Fat and Oleic Acid Content in Pigs. J. Anim. Sci. 2017, 95, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ciria, L.; Miana-Mena, F.J.; Álvarez-Rodríguez, J.; Latorre, M.A. Effect of Castration Type and Diet on Growth Performance, Serum Sex Hormones and Metabolites, and Carcass Quality of Heavy Male Pigs. Animals 2022, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.A.; Medel, P.; Fuentetaja, A.; Lázaro, R.; Mateos, G.G. Effect of Gender, Terminal Sire Line and Age at Slaughter on Performance, Carcass Characteristics and Meat Quality of Heavy Pigs. Anim. Sci. 2003, 77, 33–45. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Carcass Composition and Meat Quality of Three Different Iberian × Duroc Genotype Pigs. Meat Sci. 2007, 75, 388–396. [Google Scholar] [CrossRef]
- Alonso, V.; Muela, E.; Gutiérrez, B.; Calanche, J.B.; Roncalés, P.; Beltrán, J.A. The Inclusion of Duroc Breed in Maternal Line Affects Pork Quality and Fatty Acid Profile. Meat Sci. 2015, 107, 49–56. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Wu, H.; Xu, L.; Ballantyne, C.M. Dietary and Pharmacological Fatty Acids and Cardiovascular Health. J. Clin. Endocrinol. Metab. 2020, 105, 1030–1045. [Google Scholar] [CrossRef]
- O’Hea, E.K.; Leveille, G.A. Significance of Adipose Tissue and Liver as Sites of Fatty Acid Synthesis in the Pig and the Efficiency of Utilization of Various Substrates for Lipogenesis. J. Nutr. 1969, 99, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; D’Souza, D.N. A Review—Fat Deposition and Metabolism in the Pig. In Manipulating Pig Production IX; Australian Pig Science Association (APSA): Fremantle, Australia, 2003; pp. 126–150. [Google Scholar]
- Kloareg, M.; Noblet, J.; van Milgen, J. Deposition of Dietary Fatty Acids, de Novo Synthesis and Anatomical Partitioning of Fatty Acids in Finishing Pigs. Br. J. Nutr. 2007, 97, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Batorek-Lukač, N.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 10, 424. [Google Scholar] [CrossRef]
- Estany, J.; Ros-Freixedes, R.; Tor, M.; Pena, R.N. A Functional Variant in the Stearoyl-CoA Desaturase Gene Promoter Enhances Fatty Acid Desaturation in Pork. PLoS ONE 2014, 9, e86177. [Google Scholar] [CrossRef] [PubMed]
- Kloareg, M.; Le Bellego, L.; Mourot, J.; Noblet, J.; van Milgen, J. Deposition of Dietary Fatty Acids and of de Novo Synthesised Fatty Acids in Growing Pigs: Effects of High Ambient Temperature and Feeding Restriction. Br. J. Nutr. 2005, 93, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Dugan, M.E.R.; López-Campos, Ó.; Prieto, N.; Uttaro, B.; Gariépy, C.; Aalhus, J.L. Relative Contribution of Breed, Slaughter Weight, Sex, and Diet to the Fatty Acid Composition of Differentiated Pork. Can. J. Anim. Sci. 2017, 97, 395–405. [Google Scholar] [CrossRef]
- Sarri, L.; Balcells, J.; de la Fuente, G.; Tor, M.; Gómez-Arrue, J.; Seradj, A.R. Evolution of Viscera and Muscle Fractional Protein Synthesis Rate in Lean Meat Selected Hybrids and Castrated Duroc Pigs Fed under Moderate Crude Protein Restriction. Animal 2021, 15, 100220. [Google Scholar] [CrossRef]
- Walstra, P.; Merkus, G.S.M. Procedure for Assessment of the Lean Meat Percentage as a Consequence of the New EU Reference Dissection Method in Pig Carcass Classification; ID-DLO: Lelystad, The Netherlands, 1996. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- ISO. Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content, 1st ed.; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- Tor, M.; Vilaró, F.; Ros-Freixedes, R.; Álvarez-Rodríguez, J.; Bosch, L.; Gol, S.; Pena, R.N.; Reixach, J.; Estany, J. Circulating Non-Esterified Fatty Acids as Biomarkers for Fat Content and Composition in Pigs. Animals 2021, 11, 386. [Google Scholar] [CrossRef]
- Darambazar, E. Determination of Titanium Dioxide as a Livestock Digestibility Marker; Assay Protocol; University of Saskatchewan: Saskatoon, SK, Canada, 2019. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Aldai, N.; Osoro, K.; Barrón, L.J.R.; Nájera, A.I. Gas-Liquid Chromatographic Method for Analysing Complex Mixtures of Fatty Acids Including Conjugated Linoleic Acids (Cis9trans11 and Trans10cis12 Isomers) and Long-Chain (n-3 or n-6) Polyunsaturated Fatty Acids. Application to the Intramuscular Fat of Bee. J. Chromatogr. A 2006, 1110, 133–139. [Google Scholar] [CrossRef]
- Huxley, J.S. Problems of Relative Growth, 6th ed.; Methuen and Co: London, UK, 1932. [Google Scholar]
- Huber, L.; Squires, E.J.; Mandell, I.B.; De Lange, C.F.M. Age at Castration (Surgical or Immunological) Impacts Carcass Characteristics and Meat Quality of Male Pigs. Animal 2018, 12, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Enser, M.; Whittington, F.M.; Nute, G.R.; Wood, J.D. Effect of a High-Linolenic Acid Diet on Lipogenic Enzyme Activities, Fatty Acid Composition, and Meat Quality in the Growing Pig. J. Anim. Sci. 2003, 81, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Mourot, J.; Peiniau, P. Stearoyl-CoA Desaturase Activity in Adipose Tissues and Liver of Growing Large White and Meishan Pigs. Comp. Biochem. Physiol. 1997, 118, 509–514. [Google Scholar] [CrossRef]
- Guillevic, M.; Kouba, M.; Mourot, J. Effect of a Linseed Diet or a Sunflower Diet on Performances, Fatty Acid Composition, Lipogenic Enzyme Activities and Stearoyl-CoA-Desaturase Activity in the Pig. Livest. Sci. 2009, 124, 288–294. [Google Scholar] [CrossRef]
- Solé, E.; Ros-Freixedes, R.; Gol, S.; Bosch, L.; Tor, M.; Pena, R.N.; Reixach, J.; Estany, J. The Effect of the SCD Genotype on Litter Size and Weight at Weaning. Livest. Sci. 2021, 254, 104763. [Google Scholar] [CrossRef]
- Kouba, M.; Sellier, P. A Review of the Factors Influencing the Development of Intermuscular Adipose Tissue in the Growing Pig. Meat Sci. 2011, 88, 213–220. [Google Scholar] [CrossRef]
- Henriquez-Rodriguez, E.; Bosch, L.; Tor, M.; Pena, R.N.; Estany, J. The Effect of SCD and LEPR Genetic Polymorphisms on Fat Content and Composition Is Maintained throughout Fattening in Duroc Pigs. Meat Sci. 2016, 121, 33–39. [Google Scholar] [CrossRef]
- Landgraf, S.; Susenbeth, A.; Knap, P.W.; Looft, H.; Plastow, G.S.; Kalm, E.; Roehe, R. Developments of Carcass Cuts, Organs, Body Tissues and Chemical Body Composition during Growth of Pigs. Anim. Sci. 2006, 82, 889–899. [Google Scholar] [CrossRef]
- Bosch, L.; Tor, M.; Reixach, J.; Estany, J. Age-Related Changes in Intramuscular and Subcutaneous Fat Content and Fatty Acid Composition in Growing Pigs Using Longitudinal Data. Meat Sci. 2012, 91, 358–363. [Google Scholar] [CrossRef]
- Powles, J.; Wiseman, J.; Cole, D.J.A.; Jagger, S. Prediction of the Apparent Digestible Energy Value of Fats given to Pigs. Anim. Sci. 1995, 61, 149–154. [Google Scholar] [CrossRef]
- Ndou, S.P.; Kiarie, E.; Walsh, M.C.; Ames, N.; De Lange, C.F.M.; Nyachoti, C.M. Interactive Effects of Dietary Fibre and Lipid Types Modulate Gastrointestinal Flows and Apparent Digestibility of Fatty Acids in Growing Pigs. Br. J. Nutr. 2019, 421, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Duran-Montgé, P.; Lizardo, R.; Torrallardona, D.; Esteve-Garcia, E. Fat and Fatty Acid Digestibility of Different Fat Sources in Growing Pigs. Livest. Sci. 2007, 109, 66–69. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Lyu, Z.; Huang, B.; Hu, Q.; Lai, C. Endogenous Losses of Fat and Fatty Acids in Growing Pigs Are Not Affected by Vegetable Oil Sources but by the Method of Estimation. Animals 2020, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.P.B.; Peiniau, J.; Cunha, L.F.; Almeida, J.A.A.; Aumaitre, A. Comparative Effects of Dietary Fat and Fibre in Alentejano and Large White Piglets: Digestibility, Digestive Enzymes and Metabolic Data. Livest. Prod. Sci. 1998, 53, 37–47. [Google Scholar] [CrossRef]
- Len, N.T.; Hong, T.T.T.; Ogle, B.; Lindberg, J.E. Comparison of Total Tract Digestibility, Development of Visceral Organs and Digestive Tract of Mong Cai and Yorkshire x Landrace Piglets Fed Diets with Different Fibre Sources. J. Anim. Physiol. Anim. Nutr. 2009, 93, 181–191. [Google Scholar] [CrossRef]
- Duran-Montgé, P.; Theil, P.K.; Lauridsen, C.; Esteve-Garcia, E. Fat Metabolism Is Regulated by Altered Gene Expression of Lipogenic Enzymes and Regulatory Factors in Liver and Adipose Tissue but Not in Semimembranosus Muscle of Pigs during the Fattening Period. Animal 2009, 3, 1580–1590. [Google Scholar] [CrossRef]
- Scott, R.A.; Cornelius, S.G.; Mersmann, H.J. Effects of Age on Lipogenesis and Lipolysis in Lean and Obese Swine. J. Anim. Sci. 1981, 52, 505–511. [Google Scholar] [CrossRef]
- Palma-Granados, P.; Seiquer, I.; Benítez, R.; Óvilo, C.; Nieto, R. Effects of Lysine Deficiency on Carcass Composition and Activity and Gene Expression of Lipogenic Enzymes in Muscles and Backfat Adipose Tissue of Fatty and Lean Piglets. Animal 2019, 13, 2406–2418. [Google Scholar] [CrossRef]
- Zhang, G.H.; Lu, J.X.; Chen, Y.; Zhao, Y.Q.; Guo, P.H.; Yang, J.T.; Zang, R.X. Comparison of the Adipogenesis in Intramuscular and Subcutaneous Adipocytes from Bamei and Landrace Pigs. Biochem. Cell Biol. 2014, 92, 259–267. [Google Scholar] [CrossRef]
- Tao, X.; Liang, Y.; Yang, X.; Pang, J.; Zhong, Z.; Chen, X.; Yang, Y.; Zeng, K.; Kang, R.; Lei, Y.; et al. Transcriptomic Profiling in Muscle and Adipose Tissue Identifies Genes Related to Growth and Lipid Deposition. PLoS ONE 2017, 12, e0184120. [Google Scholar] [CrossRef]
- Bartz, M.; Szydlowski, M.; Kociucka, B.; Salamon, S.; Jeleń, H.H.; Switonski, M. Transcript Abundance of the Pig Stearoyl-CoA Desaturase Gene Has No Effect on Fatty Acid Composition in Muscle and Fat Tissues, but Its Polymorphism within the Putative MicroRNA Target Site Is Associated with Daily Body Weight Gain and Feed Conversion Rat. J. Anim. Sci. 2013, 91, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.; Airoldi, E.; Slavov, N. Post-Transcriptional Regulation across Human Tissues. PLoS Comput. Biol. 2017, 13, e1005535. [Google Scholar] [CrossRef] [PubMed]
- Skiba, G.; Polawska, E.; Raj, S.; Weremko, D.; Czauderna, M.; Wojtasik, M. The Influence of Dietary Fatty Acids on Their Metabolism in Liver and Subcutaneous Fat in Growing Pigs. J. Anim. Feed Sci. 2011, 20, 379–388. [Google Scholar] [CrossRef][Green Version]
| Fatty Acids, % | Growing | Fattening | ||
|---|---|---|---|---|
| LP | SP | LP | SP | |
| Myristic (C14:0) | 0.14 | 0.14 | 0.26 | 0.27 |
| Palmitic (C16:0) | 14.27 | 14.17 | 17.07 | 17.44 |
| Palmitoleic (C16:1) | 0.12 | 0.12 | 0.31 | 0.30 |
| Margaric (C17:0) | 0.14 | 0.15 | 0.16 | 0.16 |
| Stearic (C18:0) | 2.62 | 3.06 | 2.91 | 3.27 |
| Oleic (C18:1 c9) | 18.76 | 18.04 | 20.14 | 19.77 |
| Vaccenic (C18:1 c11) | 0.96 | 1.00 | 1.19 | 1.21 |
| Linoleic (C18:2 c9, c12) | 56.22 | 55.33 | 51.73 | 50.72 |
| Linolenic (C18:3 c9, c12, c15) | 5.73 | 6.96 | 4.99 | 5.54 |
| Arachidic (C20:0) | 0.33 | 0.34 | 0.38 | 0.39 |
| Eicosenoic (C20:1 c11) | 0.28 | 0.27 | 0.36 | 0.37 |
| Behenic (C22:0) | 0.25 | 0.28 | 0.31 | 0.36 |
| Lignoceric (C24:0) | 0.18 | 0.17 | 0.21 | 0.20 |
| Items | Producing Type (PT) | Growth Phase (GP) | p-Value | |||
|---|---|---|---|---|---|---|
| Growing | Fattening | GP | PT | GP × PT | ||
| Animals (n) | 24 | 24 | ||||
| ADFI, g/day | 3W | 966.84 ± 37.673 c | 2131.38 ± 110.492 ab | <0.001 | 0.159 | 0.032 |
| Duroc CC | 867.25 ± 37.673 c | 2441.48 ± 127.578 a | ||||
| Duroc TT | 916.78 ± 37.673 c | 2045.23 ± 110.492 b | ||||
| Performance data | ||||||
| ADG, g/day | 3W | 418.63 ± 31.640 cd | 1053.53 ± 99.324 a | <0.001 | 0.013 | 0.045 |
| Duroc CC | 440.67 ± 31.640 cd | 816.41 ± 106.182 ab | ||||
| Duroc TT | 374.37 ± 29.596 d | 625.69 ± 106.182 bc | ||||
| Feed:gain | 3W | 2.27 ± 0.215 ab | 1.97 ± 0.294 b | 0.090 | 0.228 | 0.095 |
| Duroc CC | 1.94 ± 0.201 b | 2.64 ± 0.314 ab | ||||
| Duroc TT | 2.22 ± 0.201 ab | 2.96 ± 0.340 a | ||||
| Items | Producing Type (PT) | p-Value | |
|---|---|---|---|
| Duroc (k ± SE) | 3W (k ± SE) | PT | |
| Animals (n) | 20 | 12 | |
| Whole parts | |||
| Liver | 0.73 ± 0.051 * | 0.77 ± 0.069 * | 0.689 |
| Loin | 1.01 ± 0.033 | 1.03 ± 0.048 | 0.705 |
| Ham | 1.08 ± 0.024 * | 1.05 ± 0.027 | 0.455 |
| Ham components | |||
| Skin and subcutaneous fat | 1.37 ± 0.072 * | 1.27 ± 0.079 * | 0.332 |
| Intermuscular fat | 1.02 ± 0.098 | 1.40 ± 0.111 * | 0.015 |
| Intramuscular fat (GM 1) | 1.42 ± 0.118 * | 1.38 ± 0.144 * | 0.848 |
| Intramuscular fat (SM 1) | 0.56 ± 0.062 * | 0.76 ± 0.069 * | 0.058 |
| Ham muscles | 1.08 ± 0.041 * | 1.03 ± 0.051 | 0.345 |
| Biceps femoris | 1.11 ± 0.032 * | 1.05 ± 0.047 | 0.884 |
| Gluteus medius | 1.09 ± 0.068 | 1.07 ± 0.081 | 0.825 |
| Semimembranous | 0.81 ± 0.049 * | 0.80 ± 0.058 * | 0.917 |
| Bones | 0.80 ± 0.035 * | 0.77 ± 0.047 * | 0.666 |
| Sacrum | 0.52 ± 0.133 * | 0.51 ± 0.150 * | 0.955 |
| Coxae | 0.85 ± 0.057 * | 0.87 ± 0.074 | 0.828 |
| Femur | 0.87 ± 0.041 * | 0.82 ± 0.050 * | 0.379 |
| AID | Producing Type (PT) | Growth Phase (GP) | p-Value | |||
|---|---|---|---|---|---|---|
| Growing | Fattening | GP | PT | GP × PT | ||
| Animals (n) | 24 | 24 | ||||
| DM | 3W | 71.95 ± 2.101 c | 78.38 ± 2.667 bc | 0.724 | 0.002 | 0.053 |
| Duroc CC | 86.52 ± 2.101 a | 82.04 ± 2.851 ab | ||||
| Duroc TT | 83.82± 2.246 ab | 79.66 ± 3.080 ab | ||||
| CP | 3W | 77.59 ± 1.625 c | 83.75 ± 2.099 ab | 0.547 | 0.012 | 0.010 |
| Duroc CC | 88.40 ± 1.737 a | 84.56 ± 2.099 ab | ||||
| Duroc TT | 84.57 ± 1.737 ab | 79.34 ± 2.267 bc | ||||
| EE | 3W | 61.08 ± 3.936 c | 82.25 ± 2.778 ab | < 0.001 | 0.011 | 0.045 |
| Duroc CC | 80.59 ± 3.936 ab | 84.24 ± 2.970 a | ||||
| Duroc TT | 74.10 ± 3.936 b | 83.49 ± 3.208 ab | ||||
| FIR | Producing Type (PT) | Growth Phase (GP) | p-Value | |||
|---|---|---|---|---|---|---|
| Growing | Fattening | GP | PT | GP × PT | ||
| Animals (n) | 24 | 24 | ||||
| LIVER | 3W | 11.87 ± 1.746 b | 52.50 ± 6.005 a | <0.001 | 0.242 | 0.139 |
| Duroc CC | 13.61 ± 1.746 b | 37.95 ± 6.487 a | ||||
| Duroc TT | 12.69 ± 1.746 b | 37.98 ± 6.005 a | ||||
| SC | 3W | 18.90 ± 2.717 b | 31.76 ± 3.876 a | 0.415 | <0.001 | 0.037 |
| Duroc CC | 11.78 ± 2.717 bc | 7.38 ± 4.475 c | ||||
| Duroc TT | 13.62 ± 2.905 bc | 12.22 ± 3.876 bc | ||||
| LD | 3W | 14.45 ± 1.618 a | 4.31 ± 0.464 b | <0.001 | <0.001 | 0.051 |
| Duroc CC | 7.54 ± 1.618 b | 1.57 ± 0.536 c | ||||
| Duroc TT | 6.62 ± 1.618 b | 2.33 ± 0.464 c | ||||
| SM | 3W | 1.39 ± 0.176 a | Under LOQ * | 0.019 | 0.187 | 0.040 |
| Duroc CC | 1.31 ± 0.176 a | 0.63 ± 0.120 b | ||||
| Duroc TT | 1.26 ± 0.176 a | 1.22 ± 0.111 a | ||||
| FUR | Producing Type (PT) | Phase (GP) | p-Value | |||
|---|---|---|---|---|---|---|
| Growing | Fattening | GP | PT | GP × PT | ||
| Animals (n) | 24 | 24 | ||||
| Apparent | 3W | 5.47 ± 0.231 a | 2.03 ± 0.121 d | <0.001 | <0.001 | <0.001 |
| Duroc CC | 2.84 ± 0.216 c | 1.60 ± 0.121 e | ||||
| Duroc TT | 4.25 ± 0.216 b | 1.99 ± 0.121 d | ||||
| Real | 3W | 2.16 ±0.300 c | 7.23 ± 0.617 a | <0.001 | 0.107 | 0.016 |
| Duroc CC | 2.14 ± 0.300 c | 5.28 ± 0.660 b | ||||
| Duroc TT | 1.56 ± 0.300 c | 7.68 ± 0.617 a | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarri, L.; Balcells, J.; Seradj, A.R.; Pena, R.N.; Ramírez, G.A.; Tor, M.; de la Fuente, G. Age Evolution of Lipid Accretion Rate in Boars Selected for Lean Meat and Duroc Barrows. Animals 2022, 12, 1868. https://doi.org/10.3390/ani12141868
Sarri L, Balcells J, Seradj AR, Pena RN, Ramírez GA, Tor M, de la Fuente G. Age Evolution of Lipid Accretion Rate in Boars Selected for Lean Meat and Duroc Barrows. Animals. 2022; 12(14):1868. https://doi.org/10.3390/ani12141868
Chicago/Turabian StyleSarri, Laura, Joaquim Balcells, Ahmad Reza Seradj, Ramona N. Pena, Gustavo A. Ramírez, Marc Tor, and Gabriel de la Fuente. 2022. "Age Evolution of Lipid Accretion Rate in Boars Selected for Lean Meat and Duroc Barrows" Animals 12, no. 14: 1868. https://doi.org/10.3390/ani12141868
APA StyleSarri, L., Balcells, J., Seradj, A. R., Pena, R. N., Ramírez, G. A., Tor, M., & de la Fuente, G. (2022). Age Evolution of Lipid Accretion Rate in Boars Selected for Lean Meat and Duroc Barrows. Animals, 12(14), 1868. https://doi.org/10.3390/ani12141868








