Administration of Altrenogest to Maintain Pregnancy in Asian Elephants (Elephas maximus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
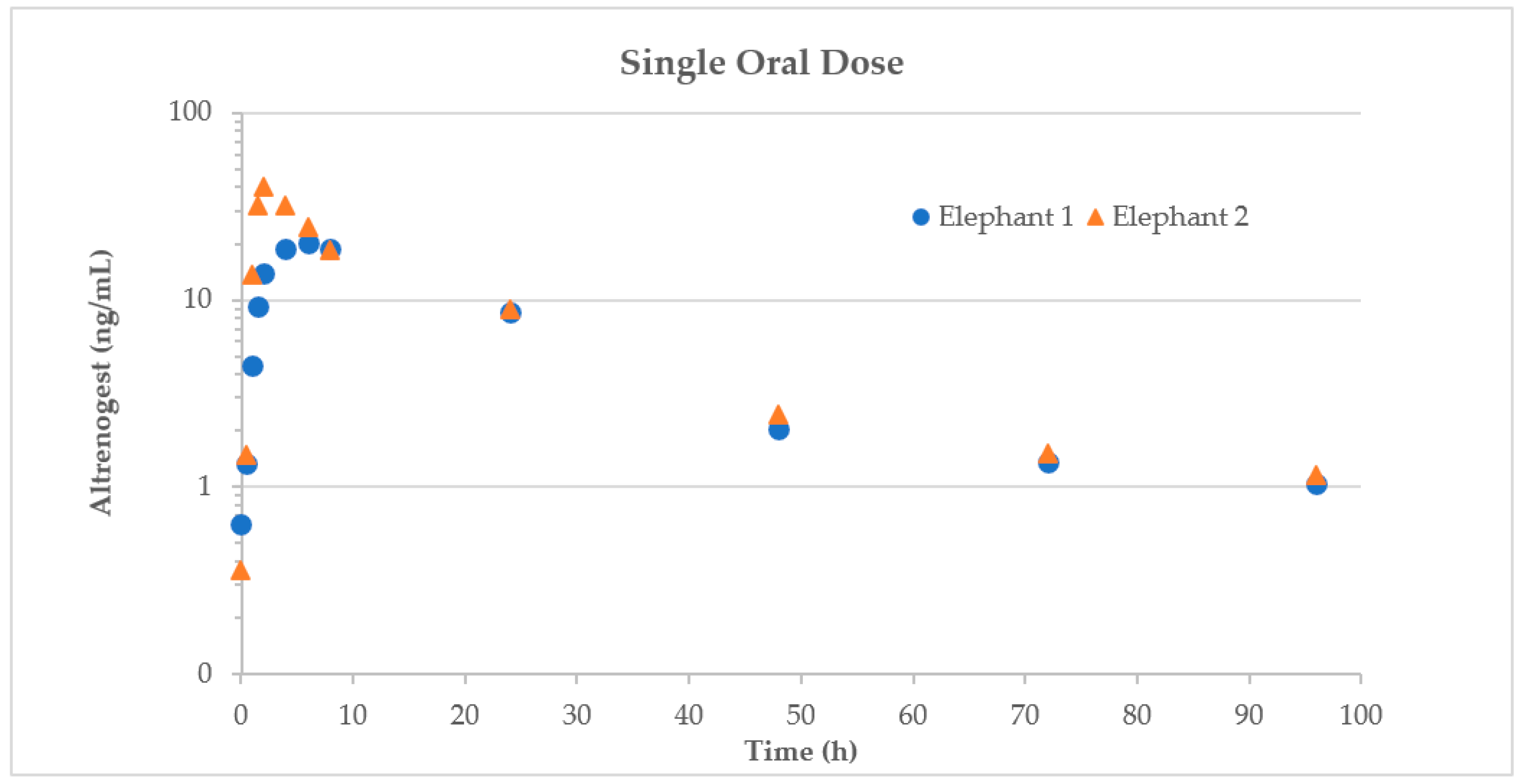
2.1. Pilot Study in Non-Pregnant, Non-Breeding Animals
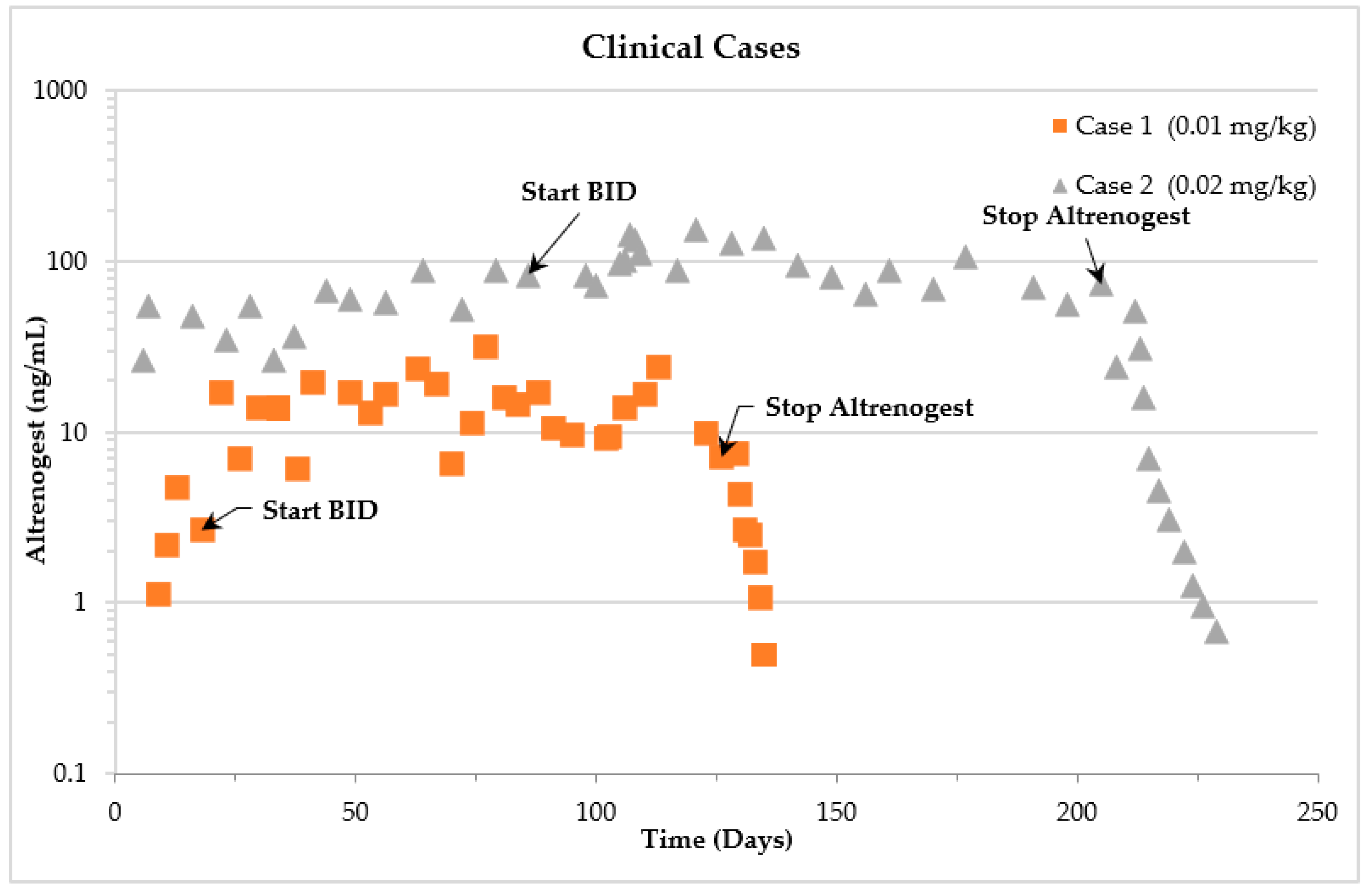
2.2. Clinical Cases
2.3. Case Descriptions
2.3.1. Case 1
2.3.2. Case 2
2.3.3. Case 3
3. Sample Analysis
3.1. Serum Sample Analysis
3.2. Pharmacokinetic Analysis
3.2.1. Pilot Study
3.2.2. Pregnant Animals
4. Results
4.1. Pilot Study Elephants
4.2. Clinical Cases
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hildebrandt, T.; Göritz, F.; Hermes, R.; Reid, C.; Dehnhard, M.; Brown, J. Aspects of the reproductive biology and breeding management of Asian and African elephants Elephas maximus and Loxodonta africana. Int. Zoo Yearb. 2006, 40, 20–40. [Google Scholar] [CrossRef]
- Brown, J.L. Update on Comparative Biology of Elephants: Factors Affecting Reproduction, Health and Welfare. Adv. Exp. Med. Biol. 2019, 1200, 243–273. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.J. Asian elephants are not self-sustaining in North America. Zoo Biol. 2000, 19, 299–309. [Google Scholar] [CrossRef]
- Spencer, T.E.; Bazer, F.W. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front. Biosci. 2002, 7, 1879–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csapo, A.I.; Wiest, W.G. An Examination of the Quantitative Relationship between Progesterone and the Maintenance of Pregnancy. Endocrinology 1969, 85, 735–746. [Google Scholar] [CrossRef]
- Dehnhard, M.; Heistermann, M.; Goritz, F.; Hermes, R.; Hildebrandt, T.; Haber, H. Demonstration of 2-unsaturated C19-steroids in the urine of female Asian elephants, Elephas maximus, and their dependence on ovarian activity. Reproduction 2001, 121, 475–484. [Google Scholar] [CrossRef]
- Dehnhard, M.; Hatt, J.M.; Eulenberger, K.; Ochs, A.; Strauss, G. Headspace solid-phase microextraction (SPME) and gas chromatography-mass spectrometry (GC-MS) for the determination of 5alpha-androst-2-en-17-one and -17beta-ol in the female Asian elephant: Application for reproductive monitoring and prediction of parturition. J. Steroid Biochem. Mol. Biol. 2003, 84, 383–391. [Google Scholar] [CrossRef]
- Stansfield, F.J.; Allen, W.R. Luteal maintenance of pregnancy in the African elephant (Loxodonta africana). Reproduction 2012, 143, 845–854. [Google Scholar] [CrossRef] [Green Version]
- REGU-MATE®. Available online: https://merckusa.cvpservice.com/product/basic/view/1047378 (accessed on 19 March 2022).
- SWINEMATE®. Available online: https://aurorapharmaceutical.com/wp-content/uploads/2020/07/SWINEMATE.pdf (accessed on 20 March 2022).
- Shoemaker, C.F.; Squires, E.L.; Shideler, R.K. Safety of altrenogest in pregnant mares and on health and development of offspring. J. Equine Vet. Sci. 1989, 9, 69–72. [Google Scholar] [CrossRef]
- Parry-Weeks, L.C.; Holtan, D.W. Effect of altrenogest on pregnancy maintenance in unsynchronized equine embryo recipients. J. Reprod. Fertil. Suppl. 1987, 35, 433–438. [Google Scholar]
- Palm, F.M.; Schenk, I.; Neuhauser, S.; Schubert, D.; Machnik, M.; Schanzer, W.; Aurich, C. Concentrations of altrenogest in plasma of mares and foals and in allantoic and amniotic fluid at parturition. Theriogenology 2010, 74, 229–235. [Google Scholar] [CrossRef]
- Gaggini, T.S.; Perin, J.; Arend, L.S.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Altrenogest treatment associated with a farrowing induction protocol to avoid early parturition in sows. Reprod. Domest. Anim. 2013, 48, 390–395. [Google Scholar] [CrossRef]
- Foisnet, A.; Farmer, C.; David, C.; Quesnel, H. Altrenogest treatment during late pregnancy did not reduce colostrum yield in primiparous sows. J. Anim. Sci. 2010, 88, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Canisso, I.F.; Beltaire, K.A.; Bedford-Guaus, S.J. Premature luteal regression in a pregnant mare and subsequent pregnancy maintenance with the use of oral altrenogest. Equine Vet. J. 2013, 45, 97–100. [Google Scholar] [CrossRef]
- Drews, B.; Hermes, R.; Goritz, F.; Gray, C.; Kurz, J.; Lueders, I.; Hildebrandt, T.B. Early embryo development in the elephant assessed by serial ultrasound examinations. Theriogenology 2008, 69, 1120–1128. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Drews, B.; Gaeth, A.P.; Goeritz, F.; Hermes, R.; Schmitt, D.; Gray, C.; Rich, P.; Streich, W.J.; Short, R.V.; et al. Foetal age determination and development in elephants. Proc. Biol. Sci. 2007, 274, 323–331. [Google Scholar] [CrossRef]
- Hildebrandt, T.B.; Goritz, F.; Pratt, N.C.; Brown, J.L.; Montali, R.J.; Schmitt, D.; Fritsch, G.; Hermes, R. Ultrasonography of the urogenital tract in elephants Loxodonta africana and Elephas maximus. Zoo Biol. 2000, 19, 321–332. [Google Scholar] [CrossRef]
- Lampinen-Salomonsson, M.; Beckman, E.; Bondesson, U.; Hedeland, M. Detection of altrenogest and its metabolites in post administration horse urine using liquid chromatography tandem mass spectrometry—Increased sensitivity by chemical derivatization of the glucuronic acid conjugate. J. Chromatogr. B 2006, 833, 245–256. [Google Scholar] [CrossRef]
- Machnik, M.; Hegger, I.; Kietzmann, M.; Thevis, M.; Guddat, S.; Schanzer, W. Pharmacokinetics of altrenogest in horses. J. Vet. Pharmacol. Ther. 2007, 30, 86–90. [Google Scholar] [CrossRef]
- Bechert, U.S.; Brown, J.L.; Dierenfeld, E.S.; Ling, P.D.; Molter, C.M.; Schulte, B.A. Zoo elephant research: Contributions to conservation of captive and free-ranging species. Int. Zoo Yearb. 2019, 53, 89–115. [Google Scholar] [CrossRef]
- Taylor, V.J.; Poole, T.B. Captive breeding and infant mortality in Asian elephants: A comparison between twenty western zoos and three eastern elephant centers. Zoo Biol. 1998, 17, 311–332. [Google Scholar] [CrossRef]
- Robeck, T.R.; Gill, C.; Doescher, B.M.; Sweeney, J.; De Laender, P.; Van Elk, C.E.; O’Brien, J.K. Altrenogest and progesterone therapy during pregnancy in bottlenose dolphins (Tursiops truncatus) with progesterone insufficiency. J. Zoo Wildl. Med. 2012, 43, 296–308. [Google Scholar] [CrossRef]
- Stoops, M.A.; West, G.D.; Roth, T.L.; Lung, N.P. Use of Urinary Biomarkers of Ovarian Function and Altrenogest Supplementation to Enhance Captive Breeding Success in the Indian Rhinoceros (Rhinoceros unicornis). Zoo Biol. 2014, 33, 83–88. [Google Scholar] [CrossRef]
- Roth, T.L.; Bateman, H.L.; Kroll, J.L.; Steinetz, B.G.; Reinhart, P.R. Endocrine and ultrasonographic characterization of a successful pregnancy in a Sumatran rhinoceros (Dicerorhinus sumatrensis) supplemented with a synthetic progestin. Zoo Biol. 2004, 23, 219–238. [Google Scholar] [CrossRef]
- Berkeley, E.V.; Kirkpatrick, J.F.; Schaffer, N.E.; Bryant, W.M.; Threlfall, W.R. Serum and Fecal Steroid Analysis of Ovulation, Pregnancy, and Parturition in the Black Rhinoceros (Diceros bicornis). Zoo Biol. 1997, 16, 121–132. [Google Scholar] [CrossRef]
- Schwarzenberger, F.; Rietschel, W.; Matern, B.; Schaftenaar, W.; Bircher, P.; Van Puijenbroeck, B.; Leus, K. Noninvasive reproductive monitoring in the okapi (Okapia johnstoni). J. Zoo Wildl. Med. 1999, 30, 497–503. [Google Scholar]
- Schaffer, N.E.; Bryant, W.M. Progesterone Supplementation and pregnancy in a black rhinoceros (Diceros bicornis): A case report. In Proceedings of the International Elephant and Rhino Research Symposium, Vienna, Austria, 7–11 June 2001; p. 76. [Google Scholar]
- Brown, J.L.; Walker, S.L.; Moeller, T. Comparative endocrinology of cycling and non-cycling Asian (Elephas maximus) and African (Loxodonta africana) elephants. Gen. Comp. Endocrinol. 2004, 136, 360–370. [Google Scholar] [CrossRef]
- Brown, J.L. Reproductive endocrine monitoring of elephants: An essential tool for assisting captive management. Zoo Biol. 2000, 19, 347–367. [Google Scholar] [CrossRef]
- Hildebrandt, T.B.; Lueders, I.; Hermes, R.; Goeritz, F.; Saragusty, J. Reproductive cycle of the elephant. Anim. Reprod. Sci. 2011, 124, 176–183. [Google Scholar] [CrossRef]
- Lueders, I.; Niemuller, C.; Gray, C.; Rich, P.; Hildebrandt, T.B. Luteogenesis during the estrous cycle in Asian elephants (Elephas maximus). Reproduction 2010, 140, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Lehnhardt, J. Serum and urinary hormones during pregnancy and the peri- and postpartum period in an Asian elephants. Zoo Biol. 1995, 14, 555–564. [Google Scholar] [CrossRef]
- Dale, R.H. Birth statistics for African (Loxodonta africana) and Asian (Elephas maximus) elephants in human care: History and implications for elephant welfare. Zoo Biol. 2010, 29, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.M.; Walker, S.L.; Freeman, E.W.; Steinetz, B.G.; Brown, J.L. Species and fetal gender effects on the endocrinology of pregnancy in elephants. Gen. Comp. Endocrinol. 2004, 138, 263–270. [Google Scholar] [CrossRef]
- Kiso, W.K.; Wiedner, E.; Isaza, R.; Lindsay, W.; Aria, J.; Jacobson, G.; Jacobson, K.; Schmitt, D. Reproductive Parameters and Birth Statistics for a Herd of Asian Elephants (Elephas Maximus) in North America over a 20-Year Period. J. Zoo Wildl. Med. 2017, 48, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sun, P.; Sun, F.; Qiu, J.; Wang, J.; Wang, J.; Lin, Y.; Gong, X.; Zhang, L.; Zhang, S.; et al. Pharmacokinetics of altrenogest in gilts. J. Vet. Pharmacol. Ther. 2019, 42, 660–664. [Google Scholar] [CrossRef]
- Greene, W.; Dierenfeld, E.S.; Minkota, S. A review of Asian and African elephant gastrointestinal anatomy, physiology and pharmacology. J. Zoo Aquar. Res. 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Hagey, L.; Schteingart, C.D.; Tonnu, H.T.; Rossi, S.S.; Hofmann, A.F. Unique bile alcohols (3, 6, 7, 25, 27-pentahydroxy cholestanes) and absence of bile-acids are a common feature of 3 ancient mammals. Hepatology 1993, 18, A177. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef] [Green Version]
- Bechert, U.; Christensen, J.M.; Kottwitz, J.; Boothe, D.; Alshahrani, S.; Mohammed, S. Pharmacokinetics of Orally Administered Flunixin Meglumine in African (Loxodonta africana) and Asian (Elephas maximus) Elephants. J. Zoo Wildl. Med. 2021, 51, 905–914. [Google Scholar] [CrossRef]
- Bechert, U.; Christensen, J.M.; Nguyen, C.; Neelkant, R.; Bendas, E. Pharmacokinetics of orally administered phenylbutazone in African and Asian elephants (Loxodonta africana and Elephas maximus). J. Zoo Wildl. Med. 2008, 39, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Ousey, J.C. Effects of progesterone administration to mares during late gestation. Theriogenology 2002, 58, 793–795. [Google Scholar]
- Guthrie, H.D.; Meckley, P.E.; Young, E.P.; Hartsock, T.G. Effect of altrenogest and Lutalyse on parturition control, plasma progesterone, unconjugated estrogen and 13,14-dihydro-15-keto-prostaglandin F2 alpha in sows. J. Anim. Sci. 1987, 65, 203–211. [Google Scholar] [CrossRef] [PubMed]
| Case Number | Initial Altrenogest Dose | Duration of Initial Dose (Days) | Number of Samples Analyzed | Mean Serum Altrenogest Concentration (ng/mL) | Increased Altrenogest Dose | Duration Higher Dose was Administered (Days) | Number of Samples Analyzed | Mean Serum Altrenogest Concentration (ng/mL) | Duration Altrenogest Was Detectable in Maternal Serum after Final Dose (Days) |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 0.01 mg/kg once per day | 20 | 4 | 2.7 ± 1.5 | 0.01 mg/kg twice per day | 131 | 31 | 13.0 ± 6.9 | 4 |
| Case 2 | 0.02 mg/kg once per day | 95 | 15 | 51 ± 20.7 | 0.02 mg/kg twice per day | 210 | 26 | 86.2 ± 33.9 | 15 |
| Case Number | Age at Conception | Time of Gestation that Progesterone Decreased to Baseline (<0.20 ng/mL) | Time of Gestation Altrenogest Began | Time of Gestation Altrenogest Stopped | Length of Altrenogest Therapy | Pregnancy Length | Time of Parturition after Stopping Altrenogest |
|---|---|---|---|---|---|---|---|
| Case 1 | 14 years | 489 days | 490 days | 621 days | 131 days | 637 days | 16 days |
| Case 2 | ~48 years | 351 days | 351 days | 561 days | 210 days | 592 days | 31 days |
| Case 3 | 10 years | 568 days | 568 days | 621 days | 53 days | 636 days | 15 days |
| Pharmacokinetic Parameter | Mean | ± Standard Deviation |
|---|---|---|
| AUCext (%) | 11.99 | 1.6 |
| AUC∞ (ng·h/mL) | 635.4 | 73.8 |
| Cmax (ng/mL) | 30.2 | 14.4 |
| Clss/F | 18.0 | 2.4 |
| Terminal T½ (h)2 | 47.5 | 4.2 |
| Disappearance λ (1/h) | 0.01 | 0.001 |
| MRT (h) | 36.0 | 3.4 |
| Tmax (h) | 4 | 2.8 |
| Vd/F (L/kg) | 1243.8 | 275.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kottwitz, J.J.; Kiso, W.; Boothe, D.M.; Schmitt, D. Administration of Altrenogest to Maintain Pregnancy in Asian Elephants (Elephas maximus). Animals 2022, 12, 1852. https://doi.org/10.3390/ani12141852
Kottwitz JJ, Kiso W, Boothe DM, Schmitt D. Administration of Altrenogest to Maintain Pregnancy in Asian Elephants (Elephas maximus). Animals. 2022; 12(14):1852. https://doi.org/10.3390/ani12141852
Chicago/Turabian StyleKottwitz, Jack J., Wendy Kiso, Dawn M. Boothe, and Dennis Schmitt. 2022. "Administration of Altrenogest to Maintain Pregnancy in Asian Elephants (Elephas maximus)" Animals 12, no. 14: 1852. https://doi.org/10.3390/ani12141852
APA StyleKottwitz, J. J., Kiso, W., Boothe, D. M., & Schmitt, D. (2022). Administration of Altrenogest to Maintain Pregnancy in Asian Elephants (Elephas maximus). Animals, 12(14), 1852. https://doi.org/10.3390/ani12141852






