Routine Decontamination of Surfaces Relevant to Working Dogs: Neutralization of Superficial Coronavirus Contamination
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Production and Enumeration:
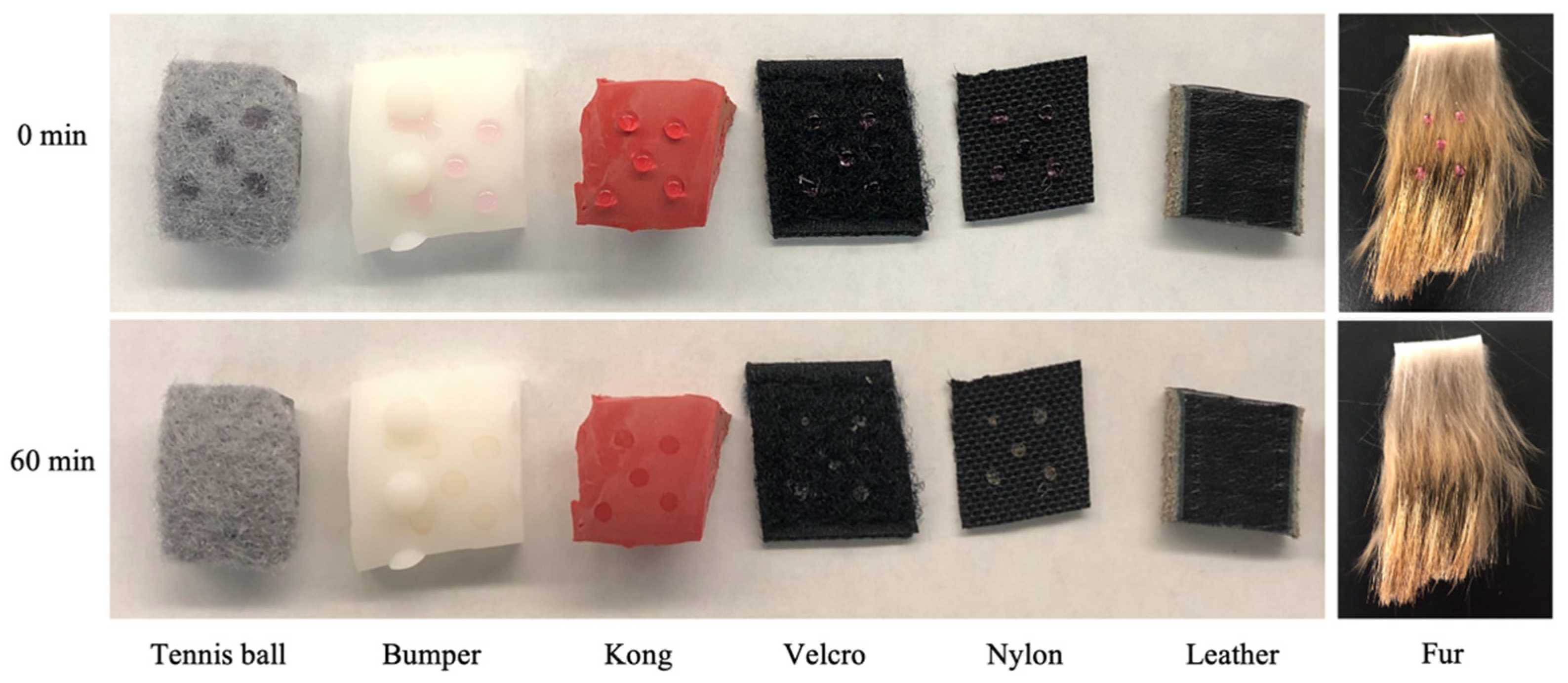
2.2. Tested Surfaces
2.3. Neutralization Solutions and Wipes
2.4. Cytotoxicity of Neutralization Solutions
2.5. Dye Recovery Experiments
2.6. Virus Neutralization on Surface Coupons
2.7. Virus Neutralization Following Direct Contact
2.8. Statistical Analysis
3. Results
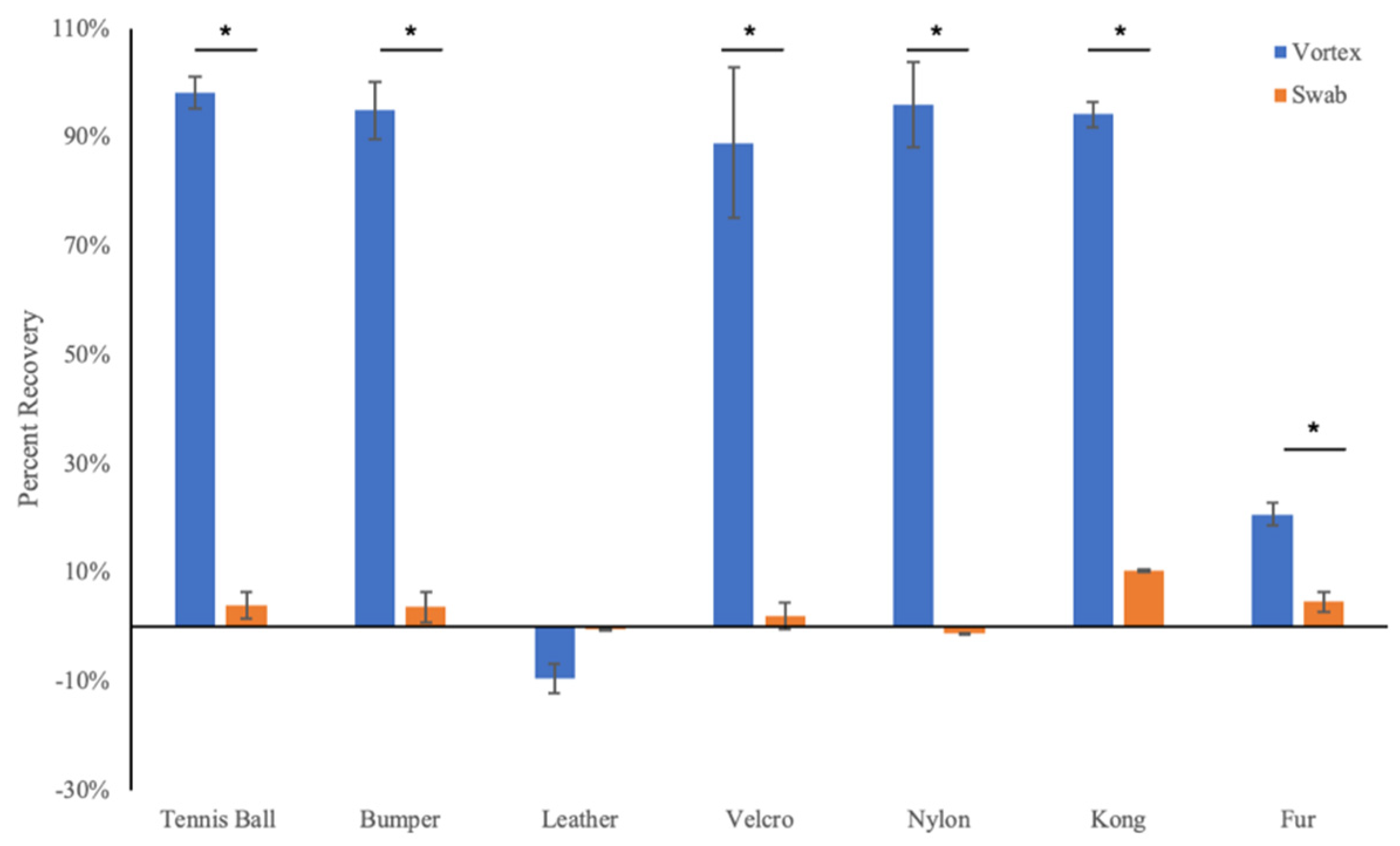
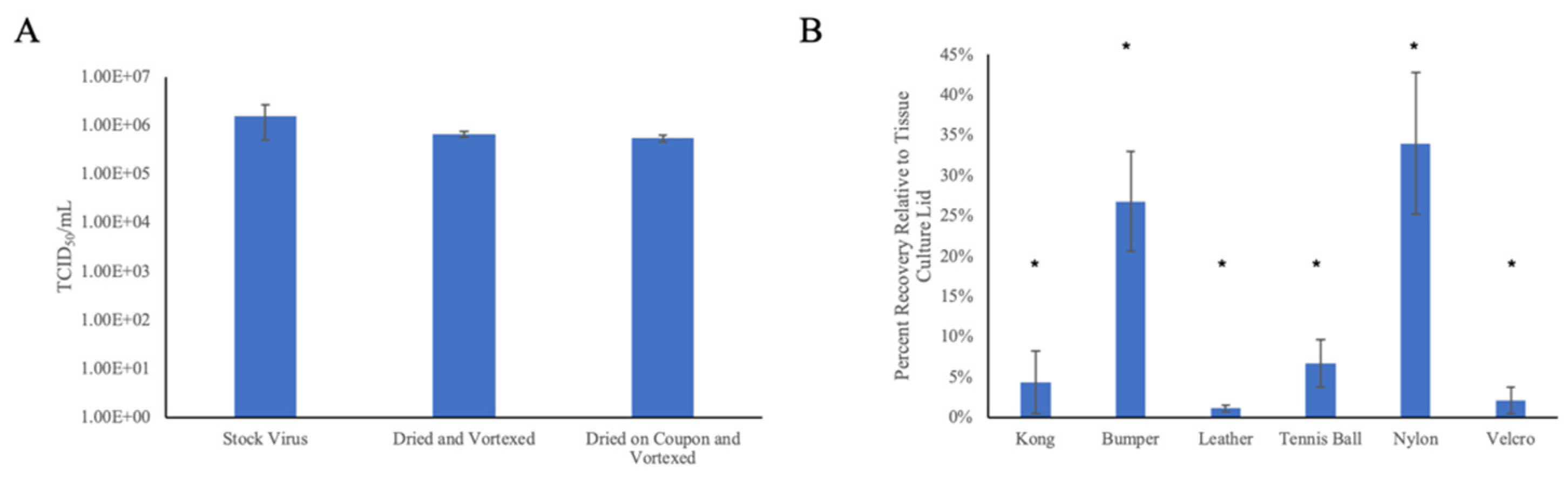
3.1. Droplet Drying Rates and Recovery Strategy
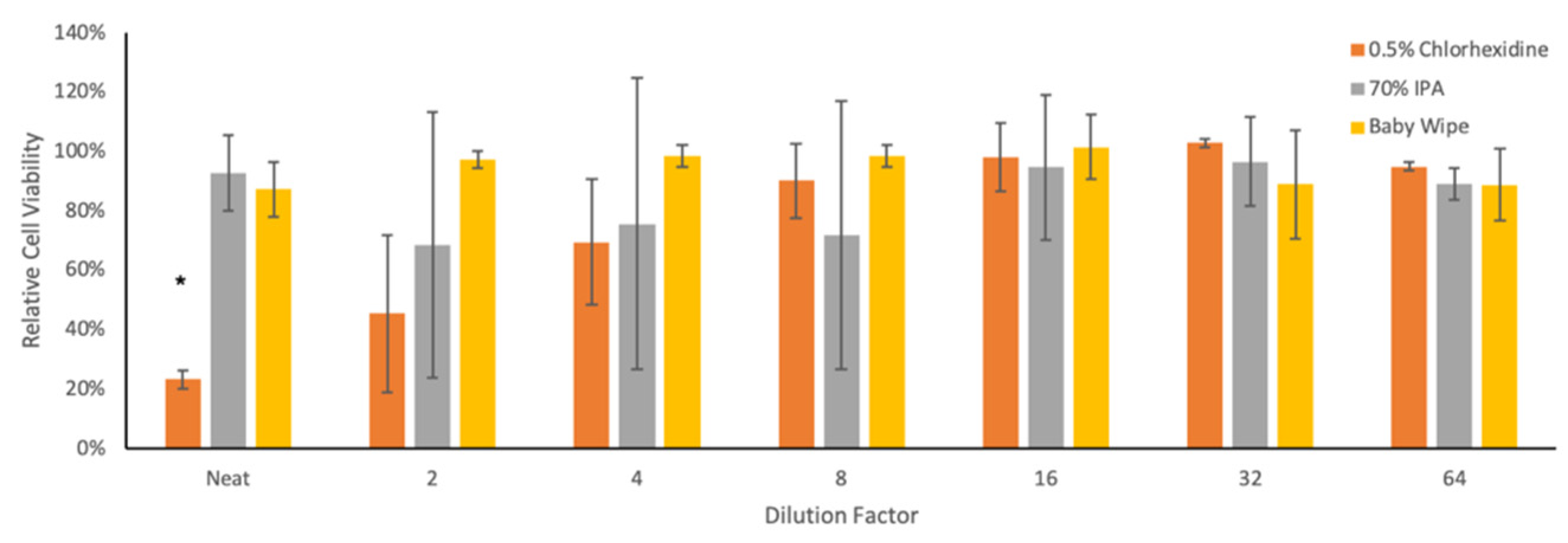
3.2. Cytotoxicity of Neutralization Solutions
3.3. Virus Neutralization by Wiping
3.4. Virus Neutralization by Direct Contact
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bessling, S.L.; Grady, S.L.; Corson, E.C.; Schilling, V.A.; Sebeck, N.M.; Therkorn, J.H.; Brensinger, B.R.; Meidenbauer, K.L. Routine Decontamination of Working Canines: A Study on the Removal of Superficial Gross Contamination. Health Secur. 2021, 19, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Gonder-Frederick, L.; Rice, P.; Warren, D.; Vajda, K.; Shepard, J. Diabetic alert dogs: A preliminary survey of current users. Diabetes Care 2013, 36, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.; Morant, S.; Kane, S.; Pesterfield, C.; Guest, C.; Rooney, N.J. An Owner-Independent Investigation of Diabetes Alert Dog Performance. Front. Vet. Sci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Cambau, E.; Poljak, M. Sniffing animals as a diagnostic tool in infectious diseases. Clin. Microbiol. Infect. 2020, 26, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Angle, T.C.; Passler, T.; Waggoner, P.L.; Fischer, T.D.; Rogers, B.; Galik, P.K.; Maxwell, H.S. Real-Time Detection of a Virus Using Detection Dogs. Front. Vet. Sci 2015, 2, 79. [Google Scholar] [CrossRef] [Green Version]
- Jendrny, P.; Schulz, C.; Twele, F.; Meller, S.; von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, J.; Pilchová, V.; Pink, I.; Welte, T.; et al. Scent dog identification of samples from COVID-19 patients—A pilot study. BMC Infect. Dis 2020, 20, 536. [Google Scholar] [CrossRef]
- Jendrny, P.; Twele, F.; Meller, S.; Schulz, C.; von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, H.; Ebbers, J.; Pilchová, V.; Pink, I.; et al. Scent dog identification of SARS-CoV-2 infections in different body fluids. BMC Infect. Dis 2021, 21, 707. [Google Scholar] [CrossRef] [PubMed]
- Sakr, R.; Ghsoub, C.; Rbeiz, C.; Lattouf, V.; Riachy, R.; Haddad, C.; Zoghbi, M. COVID-19 detection by dogs: From physiology to field application—A review article. Postgrad Med. J. 2022, 98, 212–218. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Contamination of inert surfaces by SARS-CoV-2: Persistence, stability and infectivity. A review. Environ. Res. 2021, 193, 110559. [Google Scholar] [CrossRef]
- Marzoli, F.; Bortolami, A.; Pezzuto, A.; Mazzetto, E.; Piro, R.; Terregino, C.; Bonfante, F.; Belluco, S. A systematic review of human coronaviruses survival on environmental surfaces. Sci. Total Environ. 2021, 778, 146191. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Spencer, E.A.; Brassey, J.; Plüddemann, A.; Evans, D.H.; Conly, J.M.; Jefferson, T. SARS-CoV-2 and the role of fomite transmission: A systematic review. F1000Res 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Balboni, A.; Bertolotti, L.; Martino, P.A.; Mazzei, M.; Mira, F.; Pagnini, U. SARS-CoV-2 Infection in Dogs and Cats: Facts and Speculations. Front. Vet. Sci 2021, 8, 619207. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, N.; Mitchell, E.; Rohm, E.; Rothe, M.; Kelly, C.; String, G.; Lantagne, D. A Systematic Review of Surface Contamination, Stability, and Disinfection Data on SARS-CoV-2 (Through July 10, 2020). Environ. Sci. Technol. 2021, 55, 4162–4173. [Google Scholar] [CrossRef]
- Gerlach, M.; Wolff, S.; Ludwig, S.; Schäfer, W.; Keiner, B.; Roth, N.J.; Widmer, E. Rapid SARS-CoV-2 inactivation by commonly available chemicals on inanimate surfaces. J. Hosp. Infect. 2020, 106, 633–634. [Google Scholar] [CrossRef]
- Jarrett, C.L.; Brathwaite, M.; Gogal, R.M.; Holladay, S.D. Working Dog Service, Harmful Agent Exposure and Decontamination. Front. Vet. Sci 2022, 9, 892998. [Google Scholar] [CrossRef]
- Powell, E.B.; Apgar, G.A.; Jenkins, E.K.; Liang, S.Y.; Perry, E.B. Handler training improves decontamination of working canines with oil-based exposure in field conditions using disposable kits. J. Vet. Behav. 2019, 29, 4–10. [Google Scholar] [CrossRef]
- Perry, E.B.; Discepolo, D.R.; Liang, S.Y.; Jenkins, E.K. Removal of Aerosolized Contaminants from Working Canines via a Field Wipe-Down Procedure. Animals 2021, 11, 120. [Google Scholar] [CrossRef]
- LaBarre, D.D.; Lowy, R.J. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J. Virol. Methods 2001, 96, 107–126. [Google Scholar] [CrossRef]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. North Am. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef]
- Byrd-Leotis, L.; Lasanajak, Y.; Bowen, T.; Baker, K.; Song, X.; Suthar, M.S.; Cummings, R.D.; Steinhauer, D.A. SARS-CoV-2 and other coronaviruses bind to phosphorylated glycans from the human lung. Virology 2021, 562, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Saif, L.J. Bovine Coronavirus and the Associated Diseases. Front. Vet. Sci. 2021, 8, 643220. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.; Owczarek, K.; Bzowska, M.; Gula, K.; Drebot, I.; Ochman, M.; Maksym, B.; Rajfur, Z.; Mitchell, J.A.; Pyrc, K. Canine Respiratory Coronavirus, Bovine Coronavirus, and Human Coronavirus OC43: Receptors and Attachment Factors. Viruses 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios, M.E.; Díaz, S.M.; Torres, C.; Costamagna, D.M.; Blanco Fernández, M.D.; Mbayed, V.A. Dynamics of SARS-CoV-2 in wastewater in three districts of the Buenos Aires metropolitan region, Argentina, throughout nine months of surveillance: A pilot study. Sci. Total Environ. 2021, 800, 149578. [Google Scholar] [CrossRef] [PubMed]
- Firquet, S.; Beaujard, S.; Lobert, P.E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Venable, E.; Discepolo, D.; Powell, E.; Liang, S.Y. An evaluation of current working canine decontamination procedures and methods for improvement. J. Vet. Behav. 2017, 21, 53–58. [Google Scholar] [CrossRef]
- Gordon, L.E. Injuries and illnesses among Federal Emergency Management Agency-certified search-and-recovery and search-and-rescue dogs deployed to Oso, Washington, following the March 22, 2014, State Route 530 landslide. J. Am. Vet. Med. Assoc. 2015, 247, 901–908. [Google Scholar] [CrossRef]
- Fox, P.R.; Puschner, B.; Ebel, J.G. Assessment of acute injuries, exposure to environmental toxins, and five-year health surveillance of New York Police Department working dogs following the September 11, 2001, World Trade Center terrorist attack. J. Am. Vet. Med. Assoc. 2008, 233, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Wismer, T.A.; Murphy, L.A.; Gwaltney-Brant, S.M.; Albretsen, J.C. Management and prevention of toxicoses in search-and-rescue dogs responding to urban disasters. J. Am. Vet. Med. Assoc. 2003, 222, 305–310. [Google Scholar] [CrossRef]
- Yeargin, T.; Buckley, D.; Fraser, A.; Jiang, X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am. J. Infect. Control. 2016, 44, 1365–1373. [Google Scholar] [CrossRef]
- Hardison, R.L.; Nelson, S.W.; Barriga, D.; Ghere, J.M.; Fenton, G.A.; James, R.R.; Stewart, M.J.; Lee, S.D.; Calfee, M.W.; Ryan, S.P.; et al. Efficacy of detergent-based cleaning methods against coronavirus MHV-A59 on porous and non-porous surfaces. J. Occup. Environ. Hyg. 2022, 19, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef] [PubMed]
- Shivkumar, M.; Adkin, P.; Owen, L.; Laird, K. Investigation of the stability and risks of fomite transmission of human coronavirus OC43 on leather. FEMS Microbiol. Lett. 2021, 368, 112. [Google Scholar] [CrossRef] [PubMed]
- Paton, S.; Spencer, A.; Garratt, I.; Thompson, K.A.; Dinesh, I.; Aranega-Bou, P.; Stevenson, D.; Clark, S.; Dunning, J.; Bennett, A.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Virus and Viral RNA in Relation to Surface Type and Contamination Concentration. Appl. Environ. Microbiol. 2021, 87, e0052621. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Shivkumar, M.; Cross, R.B.M.; Laird, K. Porous surfaces: Stability and recovery of coronaviruses. Interface Focus 2022, 12, 20210039. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, H.F.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Preiser, W.; Doerr, H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005, 194, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Owen, L.; Shivkumar, M.; Laird, K. The Stability of Model Human Coronaviruses on Textiles in the Environment and during Health Care Laundering. mSphere 2021, 6, e00316-21. [Google Scholar] [CrossRef]
- Kasloff, S.B.; Leung, A.; Strong, J.E.; Funk, D.; Cutts, T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021, 11, 984. [Google Scholar] [CrossRef]
- Virtanen, J.; Aaltonen, K.; Kivistö, I.; Sironen, T. Survival of SARS-CoV-2 on Clothing Materials. Adv. Virol. 2021, 2021, 6623409. [Google Scholar] [CrossRef]

| Surface | Percent Reduction in Viable Virus | |||
|---|---|---|---|---|
| Water | Baby Wipe | 0.5% Chlorhexidine | 70% IPA | |
| Kong | >99.9 * | >99.9 * | >99.0 * | 99.0 |
| Bumper | 99.8 | 99.6 | >99.9 * | >99.9 * |
| Tennis Ball | 79.2 | 72.8 | >99.0 * | >99.0 * |
| Leather | 0 | 0 | 70.4 | 95.0 |
| Nylon | 99.7 | 99.9 | >99.9 * | >99.9 * |
| Velcro | 59.3 | 52.0 | 95.6 | >99.0 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grady, S.L.; Sebeck, N.M.; Theodore, M.; Meidenbauer, K.L. Routine Decontamination of Surfaces Relevant to Working Dogs: Neutralization of Superficial Coronavirus Contamination. Animals 2022, 12, 1823. https://doi.org/10.3390/ani12141823
Grady SL, Sebeck NM, Theodore M, Meidenbauer KL. Routine Decontamination of Surfaces Relevant to Working Dogs: Neutralization of Superficial Coronavirus Contamination. Animals. 2022; 12(14):1823. https://doi.org/10.3390/ani12141823
Chicago/Turabian StyleGrady, Sarah L., Natalie M. Sebeck, Mellisa Theodore, and Karen L. Meidenbauer. 2022. "Routine Decontamination of Surfaces Relevant to Working Dogs: Neutralization of Superficial Coronavirus Contamination" Animals 12, no. 14: 1823. https://doi.org/10.3390/ani12141823
APA StyleGrady, S. L., Sebeck, N. M., Theodore, M., & Meidenbauer, K. L. (2022). Routine Decontamination of Surfaces Relevant to Working Dogs: Neutralization of Superficial Coronavirus Contamination. Animals, 12(14), 1823. https://doi.org/10.3390/ani12141823






