Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing Treatments
2.2. Behavioral Measurements
2.3. Molecular Measurements
Feces and Blood
2.4. Feathers
2.5. Statistical Analysis
3. Results
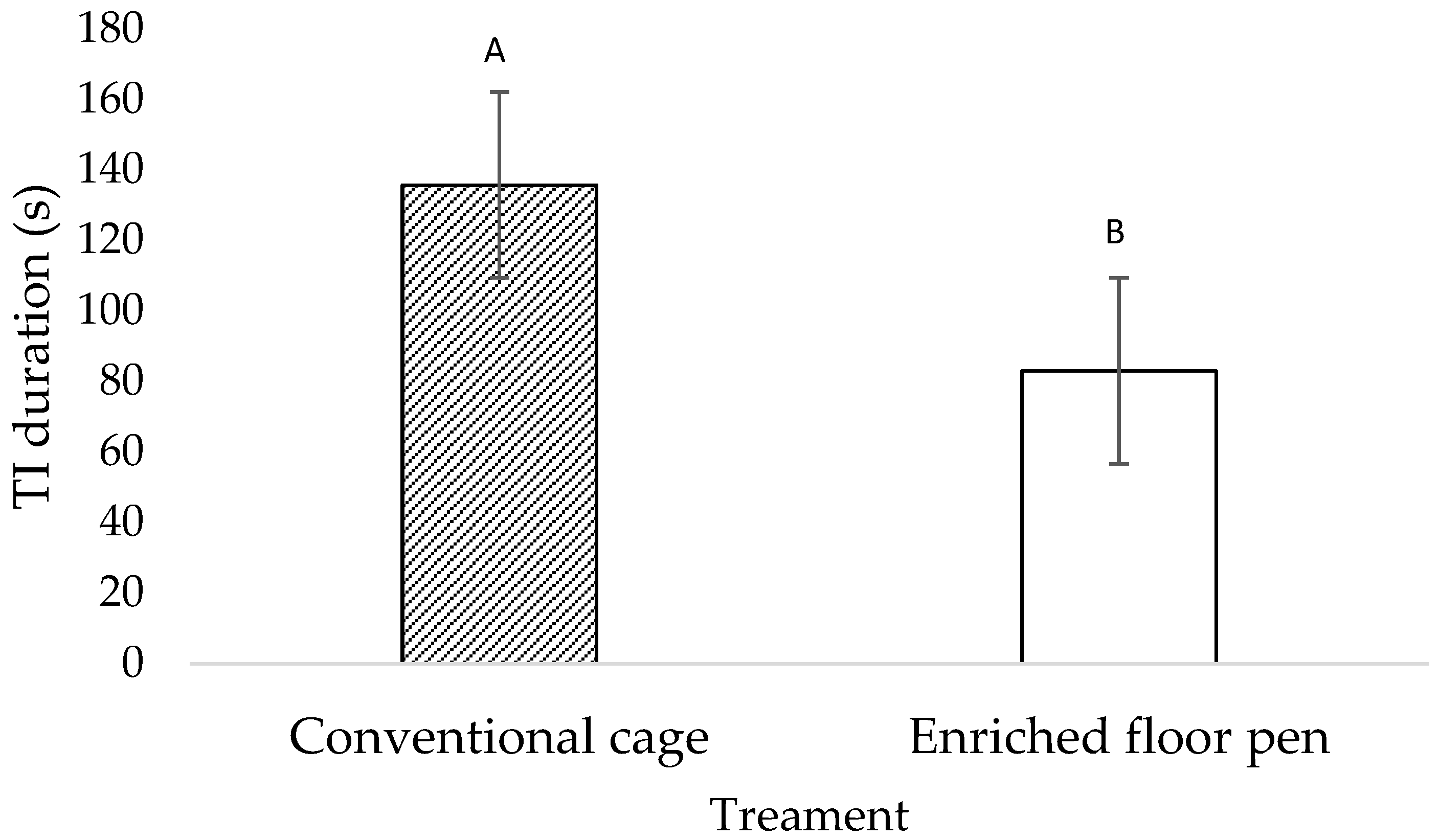
3.1. Behavioral Measures
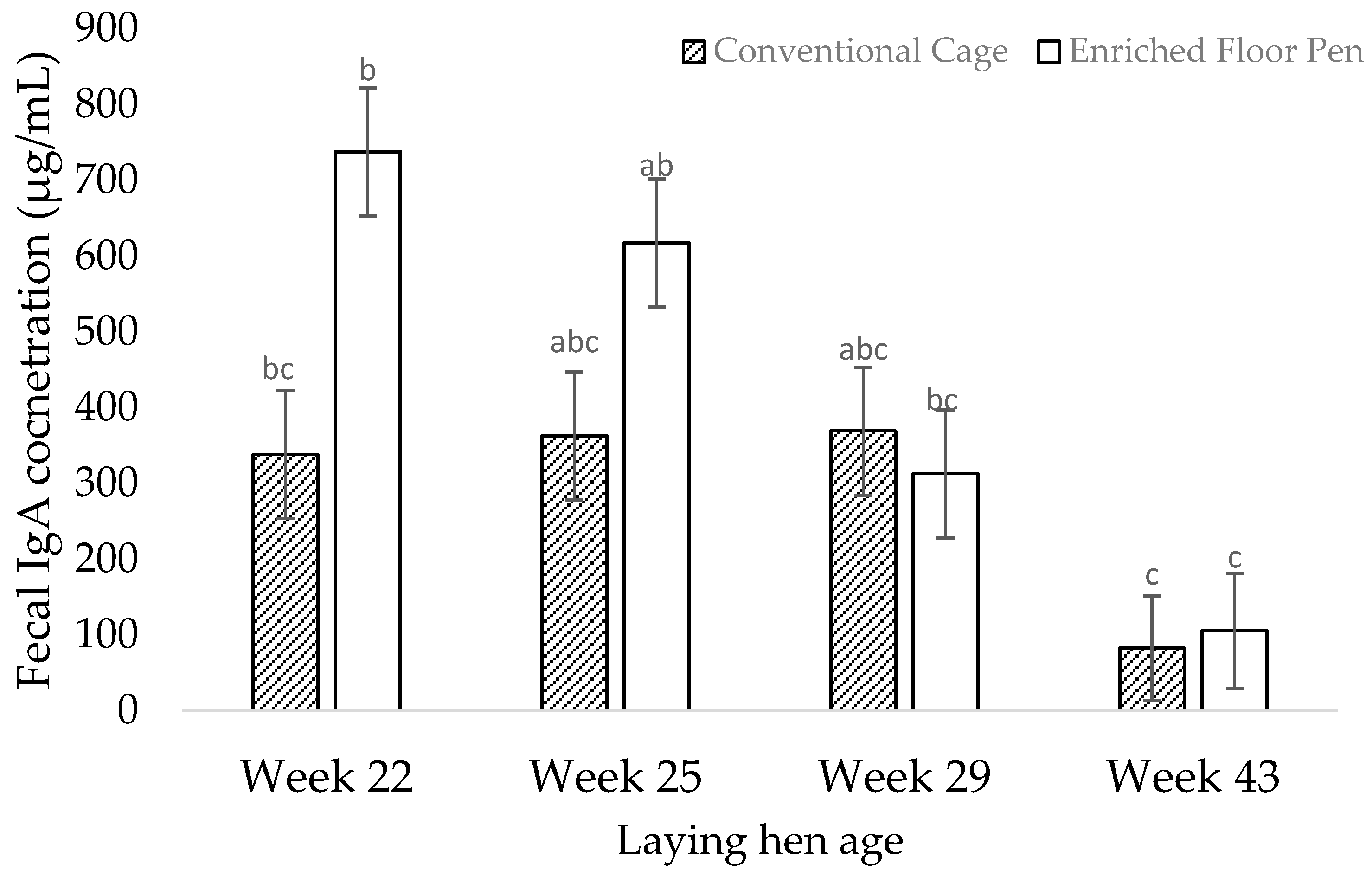
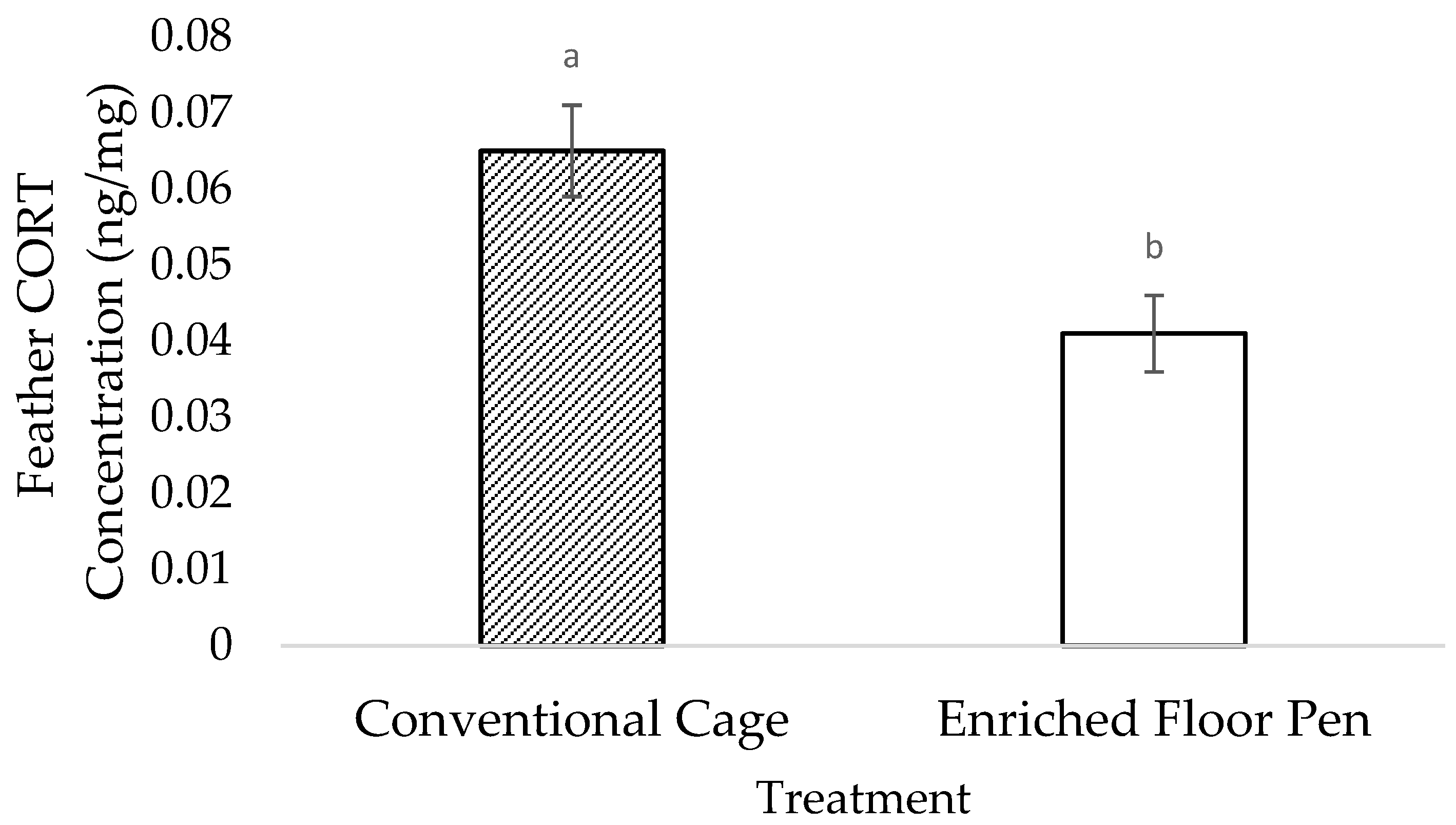
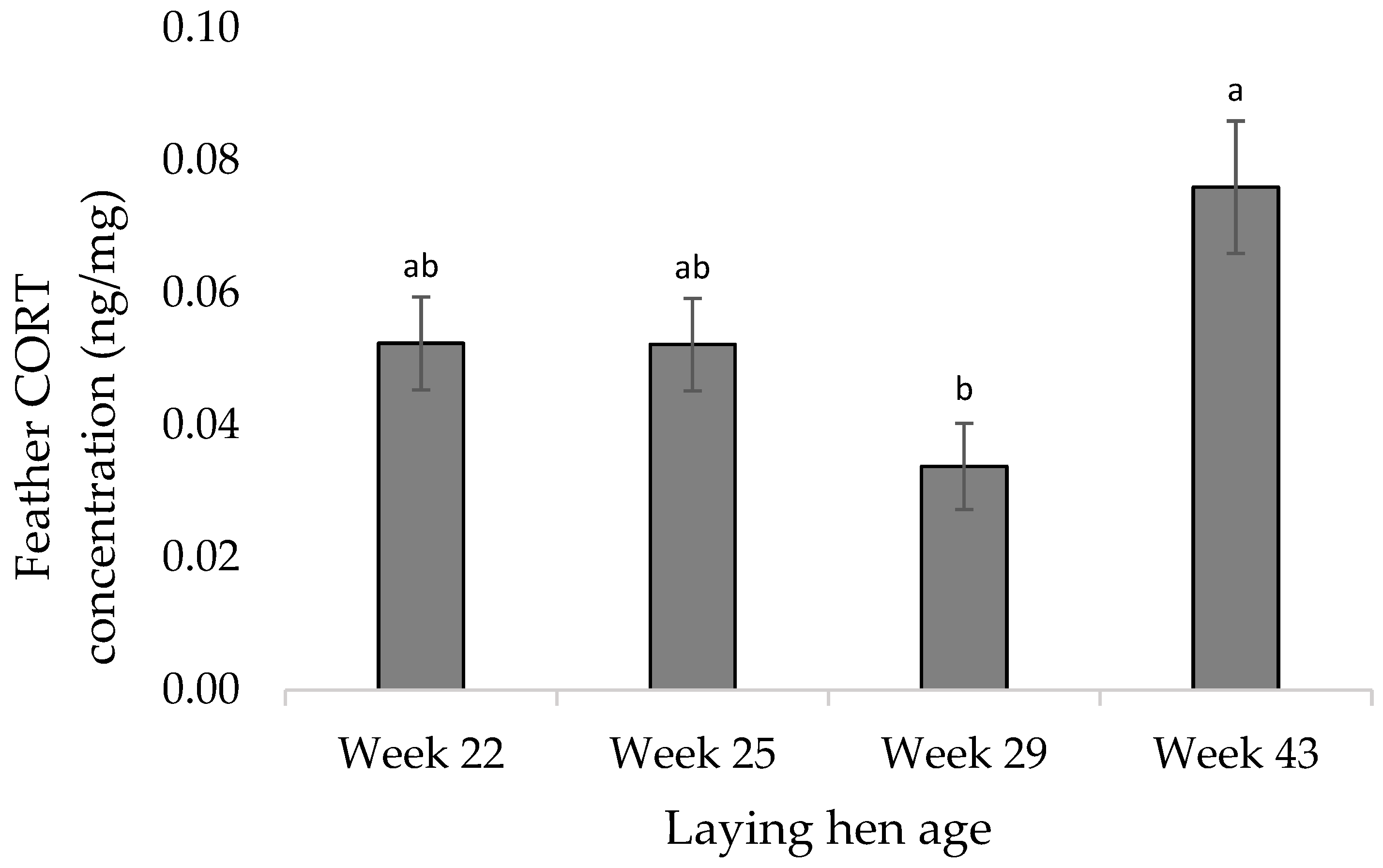
3.2. Molecular Measures
4. Discussion
4.1. Behavioral Measures
4.2. Molecular Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, S1–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartcher, K.M.; Jones, B. The welfare of layer hens in cage and cage-free housing systems. World’s Poult. Sci. J. 2017, 73, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.G.; Campbell, A.M.; Crump, A.; Arnott, G.; Newberry, R.C.; Jacobs, L. Effect of Environmental Complexity and Stocking Density on Fear and Anxiety in Broiler Chickens. Animals 2021, 11, 2383. [Google Scholar] [CrossRef]
- Davis, M.; Walker, D.L.; Miles, L.; Grillon, C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs. Anxiety. Neuropsychopharmacology 2010, 35, 105–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Haas, E.N.; Kops, M.S.; Bolhuis, J.E.; Groothuis, T.G.; Ellen, E.D.; Rodenburg, T.B. The relation between fearfulness in young and stress-response in adult laying hens, on individual and group level. Physiol. Behav. 2012, 107, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.-C.; Canali, E.; Jones, R. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [Green Version]
- Fogelholm, J.; Inkabi, S.; Höglund, A.; Abbey-Lee, R.; Johnsson, M.; Jensen, P.; Henriksen, R.; Wright, D. Genetical Genomics of Tonic Immobility in the Chicken. Genes 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, D.; Li, J.; Bao, J. Effects of Furnished Cage Type on Behavior and Welfare of Laying Hens. Asian-Australas. J. Anim. Sci. 2015, 29, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Tong, Q.; Zheng, W.; Tu, J.; Li, B. Effects of nest boxes in natural mating colony cages on fear, stress, and feather damage for layer breeders 1,2,3. J. Anim. Sci. 2019, 97, 4464–4474. [Google Scholar] [CrossRef]
- Tauson, R.; Wahlström, A.; Abrahamsson, P. Effect of Two Floor Housing Systems and Cages on Health, Production, and Fear Response in Layers. J. Appl. Poult. Res. 1999, 8, 152–159. [Google Scholar] [CrossRef]
- Yilmaz Dikmen, B.; Ipek, A.; Şahan, Ü.; Petek, M.; Sözcü, A. Egg production and welfare of laying hens kept in different housing systems (conventional, enriched cage, and free range). Poult. Sci. 2016, 95, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- Crump, A.; Arnott, G.; Bethell, E.J. Affect-Driven Attention Biases as Animal Welfare Indicators: Review and Methods. Animals 2018, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Košťál, Ľ.; Skalná, Z.; Pichová, K. Use of cognitive bias as a welfare tool in poultry. J. Anim. Sci. 2020, 98, S63. [Google Scholar] [CrossRef]
- Campbell, D.L.; Taylor, P.; Hernandez, C.E.; Stewart, M.; Belson, S.; Lee, C. An attention bias test to assess anxiety states in laying hens. PeerJ 2019, 10, e3707. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.L.; Dickson, E.J.; Lee, C. Application of open field, tonic immobility, and attention bias tests to hens with different ranging patterns. PeerJ 2019, 7, e8122. [Google Scholar] [CrossRef]
- Monk, J.E.; Belson, S.; Lee, C. Pharmacologically-induced stress has minimal impact on judgement and attention biases in sheep. Sci. Rep. 2019, 9, 11446. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.E.; Lee, C.; Belson, S.; Colditz, I.G.; Campbell, D.L. The influence of pharmacologically-induced affective states on attention bias in sheep. PeerJ 2019, 7, e7033. [Google Scholar] [CrossRef]
- Luo, L.; Reimert, I.; De Haas, E.N.; Kemp, B.; Bolhuis, J.E. Effects of early and later life environmental enrichment and personality on attention bias in pigs (Sus scrofa domesticus). Anim. Cogn. 2019, 22, 959–972. [Google Scholar] [CrossRef] [Green Version]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Häffelin, K.; Lindenwald, R.; Kaufmann, F.; Döhring, S.; Spindler, B.; Preisinger, R.; Rautenschlein, S.; Kemper, N.; Andersson, R. Corticosterone in feathers of laying hens: An assay validation for evidence-based assessment of animal welfare. Poult. Sci. 2020, 99, 4685–4694. [Google Scholar] [CrossRef]
- Harris, C.M.; Madliger, C.L.; Love, O.P. An evaluation of feather corticosterone as a biomarker of fitness and an ecologically relevant stressor during breeding in the wild. Oecologia 2017, 183, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Voit, M.; Merle, R.; Baumgartner, K.; Von Fersen, L.; Reese, L.; Ladwig-Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals 2020, 10, 2054. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking stress and immunity: Immunoglobulin A as a non-invasive physiological biomarker in animal welfare studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef]
- Ablimit, A.; Kühnel, H.; Strasser, A.; Upur, H. Abnormal Savda syndrome: Long-term consequences of emotional and physical stress on endocrine and immune activities in an animal model. Chin. J. Integr. Med. 2012, 19, 603–609. [Google Scholar] [CrossRef]
- Guhad, F.; Hau, J. Salivary IgA as a marker of social stress in rats. Neurosci. Lett. 1996, 216, 137–140. [Google Scholar] [CrossRef]
- Jarillo-Luna, A.; Rivera-Aguilar, V.; Garfias, H.R.; Lara-Padilla, E.; Kormanovsky, A.; Campos-Rodríguez, R. Effect of repeated restraint stress on the levels of intestinal IgA in mice. Psychoneuroendocrinology 2007, 32, 681–692. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Falla, J.; Boujedaini, N.; Soulimani, R. The impact of high anxiety level on cellular and humoral immunity in mice. NeuroImmunoModulation 2009, 17, 1–8. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.; Calefi, A.; Cruz, D.; Aloia, T.; Zager, A.; Astolfi-Ferreira, C.; Ferreira, J.P.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tong, Q.; Shi, Z.; Li, H.; Wang, Y.; Li, B.; Yan, G.; Chen, H.; Zheng, W. Effects of chronic heat stress and ammonia concentration on blood parameters of laying hens. Poult. Sci. 2020, 99, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Gourkow, N.; Phillips, C.J. Effect of cognitive enrichment on behavior, mucosal immunity and upper respiratory disease of shelter cats rated as frustrated on arrival. Prev. Vet. Med. 2016, 131, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary exercise increases IgA concentration and polymeric Ig receptor expression in the rat submandibular gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Kimura, F.; Aizawa, K.; Tanabe, K.; Shimizu, K.; Kon, M.; Lee, H.; Akimoto, T.; Akama, T.; Kono, I. A rat model of saliva secretory immunoglobulin: A suppression caused by intense exercise. Scand. J. Med. Sci. Sports 2007, 18, 367–372. [Google Scholar] [CrossRef]
- Souza, C.; Miotto, B.; Bonin, C.; Camargo, M. Lower serum IgA levels in horses kept under intensive sanitary management and physical training. Animal 2010, 4, 2080–2083. [Google Scholar] [CrossRef]
- Robson, P.J.; Alston, T.D.; Myburgh, K.H. Prolonged suppression of the innate immune system in the horse following an 80 km endurance race. Equine Vet. J. 2010, 35, 133–137. [Google Scholar] [CrossRef]
- Peters, I.R.; Calvert, E.L.; Hall, E.; Day, M.J. Measurement of Immunoglobulin Concentrations in the Feces of Healthy Dogs. Clin. Vaccine Immunol. 2004, 11, 841. [Google Scholar] [CrossRef] [Green Version]
- Ricci, F.G.; Terkelli, L.R.; Venancio, E.J.; Justino, L.; Dos Santos, B.Q.; Baptista, A.A.S.; Oba, A.; Souza, B.D.D.O.; Bracarense, A.P.F.R.L.; Hirooka, E.Y.; et al. Tryptophan Attenuates the Effects of OTA on Intestinal Morphology and Local IgA/IgY Production in Broiler Chicks. Toxins 2020, 13, 5. [Google Scholar] [CrossRef]
- Royo, F.; Lyberg, K.; Abelson, K.; Carlsson, H.; Hau, J. Effect of repeated confined single housing of young pigs on faecal excretion of cortisol and IgA. Scand. J. Lab. Anim. Sci. 2005, 31, 33–37. [Google Scholar] [CrossRef]
- Romero, L.M.; Reed, J.M. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Adams, A. Effects of Floor Versus Cage Rearing and Feeder Space on Growth, Long Bone Development, and Duration of Tonic Immobility in Single Comb White Leghorn Pullets. Poult. Sci. 1994, 73, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Faure, J.M. Tonic immobility (“righting time”) in laying hens housed in cages and pens. Appl. Anim. Ethol. 1981, 7, 369–372. [Google Scholar] [CrossRef]
- Monk, J.E.; Doyle, R.E.; Colditz, I.G.; Belson, S.; Cronin, G.M.; Lee, C. Towards a more practical attention bias test to assess affective state in sheep. PLoS ONE 2018, 13, e0190404. [Google Scholar] [CrossRef] [Green Version]
- Steimer, T. The biology of fear- and anxiety-related behaviors. Dialog Clin. Neurosci. 2002, 4, 231–249. [Google Scholar] [CrossRef]
- Albentosa, M.; Kjaer, J.; Nicol, C. Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br. Poult. Sci. 2003, 44, 333–344. [Google Scholar] [CrossRef]
- Fraisse, F.; Cockrem, J.F. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006, 47, 110–119. [Google Scholar] [CrossRef]
- Campo, J.L.; Prieto, M.T.; Dávila, S.G. Effects of Housing System and Cold Stress on Heterophil-to-Lymphocyte Ratio, Fluctuating Asymmetry, and Tonic Immobility Duration of Chickens. Poult. Sci. 2008, 87, 621–626. [Google Scholar] [CrossRef]
- Jones, R.B. Fear and adaptability in poultry: Insights, implications and imperatives. World’s Poult. Sci. J. 1996, 52, 131–174. [Google Scholar] [CrossRef]
- Sefton, A. The Interactions of Cage Size, Cage Level, Social Density, Fearfulness, and Production of Single Comb White Leghorns. Poult. Sci. 1976, 55, 1922–1926. [Google Scholar] [CrossRef]
- Sefton, A.E.; Crober, D.C. Social and physical environmental influences on caged single comb white leghorn layers. Can. J. Anim. Sci. 1976, 56, 733–738. [Google Scholar] [CrossRef]
- Craig, J.; Craig, T.; Dayton, A. Fearful behavior by caged hens of two genetic stocks. Appl. Anim. Ethol. 1983, 10, 263–273. [Google Scholar] [CrossRef]
- Hughes, B.O.; Duncan, I.J.H. The influence of strain and environmental factors upon feather pecking and cannibalism in fowls. Br. Poult. Sci. 1972, 13, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Okpokho, N.A.; Craig, J.V.; Milliken, G.A. Density and group size effects on caged hens of two genetic stocks differing in escape and avoidance behavior. Poult. Sci. 1987, 66, 1905–1910. [Google Scholar] [CrossRef]
- Ouart, M.D.; Adams, A.W. Effects of Cage Design and Bird Density on Layers. Poult. Sci. 1982, 61, 1606–1613. [Google Scholar] [CrossRef]
- Hansen, R.S. Nervousness and Hysteria of Mature Female Chickens. Poult. Sci. 1976, 55, 531–543. [Google Scholar] [CrossRef]
- Brilot, B.O.; Bateson, M. Water bathing alters threat perception in starlings. Biol. Lett. 2012, 8, 379–381. [Google Scholar] [CrossRef] [Green Version]
- Nordquist, R.E.; Heerkens, J.L.; Rodenburg, T.B.; Boks, S.; Ellen, E.D.; van der Staay, F.J. Laying hens selected for low mortality: Behaviour in tests of fearfulness, anxiety and cognition. Appl. Anim. Behav. Sci. 2011, 131, 110–122. [Google Scholar] [CrossRef]
- Engel, O.; Müller, H.W.; Klee, R.; Francke, B.; Mills, D.S. Effectiveness of imepitoin for the control of anxiety and fear associated with noise phobia in dogs. J. Vet. Intern. Med. 2019, 33, 2675–2684. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, M.R.L.V.; Karrow, N.A.; Newman, A.; Widowski, T.M. Effects of Maternal Stress on Measures of Anxiety and Fearfulness in Different Strains of Laying Hens. Front. Vet. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Yudko, E.B.; Rodgers, R.; Blanchard, D. Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behav. Brain Res. 1993, 58, 155–165. [Google Scholar] [CrossRef]
- Bolles, R.C.; Fanselow, M.S. A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci. 1980, 3, 291–301. [Google Scholar] [CrossRef]
- Fanselow, M.S. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learn. Motiv. 1986, 17, 16–39. [Google Scholar] [CrossRef]
- Eriksson, E.; Royo, F.; Lyberg, K.; Carlsson, H.-E.; Hau, J. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp. Physiol. 2004, 89, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, T.B.; Tuyttens, F.A.M.; Sonck, B.; De Reu, K.; Herman, L.; Zoons, J. Welfare, Health, and Hygiene of Laying Hens Housed in Furnished Cages and in Alternative Housing Systems. J. Appl. Anim. Welf. Sci. 2005, 8, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Cox, N.A.; Guard, J.; Fedorka-Cray, P.; Buhr, R.J.; Gast, R.K.; Abdo, Z.; Rigsby, L.L.; Plumblee, J.R.; Karcher, D.M.; et al. Microbiological impact of three commercial laying hen housing systems. Poult. Sci. 2015, 94, 544–551. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef]
- Calefi, A.S.; Honda, B.T.B.; Costola-De-Souza, C.; de Siqueira, A.; Namazu, L.B.; Quinteiro-Filho, W.M.; Fonseca, J.G.D.S.; Aloia, T.P.A.; Piantino-Ferreira, A.J.; Palermo-Neto, J. Effects of long-term heat stress in an experimental model of avian necrotic enteritis. Poult. Sci. 2014, 93, 1344–1353. [Google Scholar] [CrossRef]
- Calefi, A.; Quinteiro-Filho, W.; Ferreira, A.; Palermo-Neto, J. Neuroimmunomodulation and heat stress in poultry. World’s Poult. Sci. J. 2017, 73, 493–504. [Google Scholar] [CrossRef]
- Slawinska, A.; Mendes, S.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems 2019, 178, 10–15. [Google Scholar] [CrossRef]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef]
- Guardia, S.; Konsak, B.; Combes, S.; Levenez, F.; Cauquil, L.; Guillot, J.-F.; Moreau-Vauzelle, C.; Lessire, M.; Juin, H.; Gabriel, I. Effects of stocking density on the growth performance and digestive microbiota of broiler chickens. Poult. Sci. 2011, 90, 1878–1889. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Qin, X.; Sun, S.; Xiao, Z.; Dong, X.; Shahid, M.S.; Yin, D.; Yuan, J. Proteome and microbiota analysis reveals alterations of liver-gut axis under different stocking density of Peking ducks. PLoS ONE 2018, 13, e0198985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, C.; Zhang, T.; Yan, L.; Qiu, L.; Yin, H.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; et al. Dietary 25-hydroxyvitamin D improves intestinal health and microbiota of laying hens under high stocking density. Poult. Sci. 2021, 100, 101132. [Google Scholar] [CrossRef]
- Saki, A.A.; Zamani, P.; Rahmati, M.; Mahmoudi, H. The effect of cage density on laying hen performance, egg quality, and excreta minerals. J. Appl. Poult. Res. 2012, 21, 467–475. [Google Scholar] [CrossRef]
- Kang, H.K.; Park, S.B.; Kim, S.H.; Kim, C.H. Effects of stock density on the laying performance, blood parameter, corticosterone, litter quality, gas emission and bone mineral density of laying hens in floor pens. Poult. Sci. 2016, 95, 2764–2770. [Google Scholar] [CrossRef]
- Li, W.; Wei, F.; Xu, B.; Sun, Q.; Deng, W.; Ma, H.; Bai, J.; Li, S. Effect of stocking density and alpha-lipoic acid on the growth performance, physiological and oxidative stress and immune response of broilers. Asian-Australas. J. Anim. Sci. 2019, 32, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Weimer, S.L.; Robison, C.I.; Tempelman, R.J.; Jones, D.R.; Karcher, D.M. Laying hen production and welfare in enriched colony cages at different stocking densities. Poult. Sci. 2019, 98, 3578–3586. [Google Scholar] [CrossRef]
- Von Eugen, K.; Nordquist, R.E.; Zeinstra, E.; van der Staay, F.J. Stocking Density Affects Stress and Anxious Behavior in the Laying Hen Chick During Rearing. Animals 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
| Scenario | Procedure | Total Test Duration | Data Recorded |
|---|---|---|---|
| Testing begins | Play first alarm call | 300 s | n/a |
| No. birds begin feeding | Allow test to run 300 s | 300 s | All birds receive 300 s maximum latency to begin feeding |
| One bird begins feeding | Play first alarm call and allow test to run for 300 s | 300 s | Latency to begin feeding for bird that began feeding. Other two birds receive maximum latency of 300 s |
| Two birds begin feeding | Play first alarm call and allow test to run for 300 s. Play second alarm call at 300 s and allow test to run until 420 s. | 420 s | Latencies to begin feeding for two birds. Third bird receives maximum latency of 300 s. Latencies to resume feeding for two birds that began feeding if they feed before 420 s. |
| All three birds begin feeding before 270 s | Play first alarm call and allow test to run until the third bird begins feeding. Allow birds to feed for 5 s and play second alarm call. Allow test to run until 300 s | 300 s | Latencies to begin feeding. Latencies to resume feeding for all three birds if they resume feeding prior to 300 s. |
| All three birds begin feeding between 270–300 s | Play first alarm call and allow test to run until the third bird eats. Allow birds to feed for 5 s and play second alarm call. Extend testing duration to 420 s. | 420 s | Latencies to begin feeding. Latencies to resume feeding for all three birds if they resume feeding before 420 s. |
| Measure | Conventional Cage | Enriched Floor Pen |
|---|---|---|
| Latency to begin feeding (s) | 99.17 ± 19.23 a | 145.97 ± 24.52 b |
| Latency to resume feeding (s) | 54.21 ± 13.63 | 54.13 ± 13.87 |
| Birds begin feeding (%) | 91.66 A | 77.77 B |
| Birds resume feeding (%) | 87.87 a | 82.14 b |
| Vigilance behavior score (0–4 score) 1 | 2.35 ± 0.26 | 2.33 ± 0.27 |
| Freeze (% birds) | 55 | 35 |
| Erect (% birds) | 55 | 44 |
| Neck stretch (% birds) | 65 | 55 |
| Look (% birds) | 80 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.M.; Johnson, A.M.; Persia, M.E.; Jacobs, L. Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens. Animals 2022, 12, 1803. https://doi.org/10.3390/ani12141803
Campbell AM, Johnson AM, Persia ME, Jacobs L. Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens. Animals. 2022; 12(14):1803. https://doi.org/10.3390/ani12141803
Chicago/Turabian StyleCampbell, Andrew M., Alexa M. Johnson, Michael E. Persia, and Leonie Jacobs. 2022. "Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens" Animals 12, no. 14: 1803. https://doi.org/10.3390/ani12141803
APA StyleCampbell, A. M., Johnson, A. M., Persia, M. E., & Jacobs, L. (2022). Effects of Housing System on Anxiety, Chronic Stress, Fear, and Immune Function in Bovan Brown Laying Hens. Animals, 12(14), 1803. https://doi.org/10.3390/ani12141803






