Echocardiography and MALDI-TOF Identification of Myosin-Binding Protein C3 A74T Gene Mutations Involved Healthy and Mutated Bengal Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
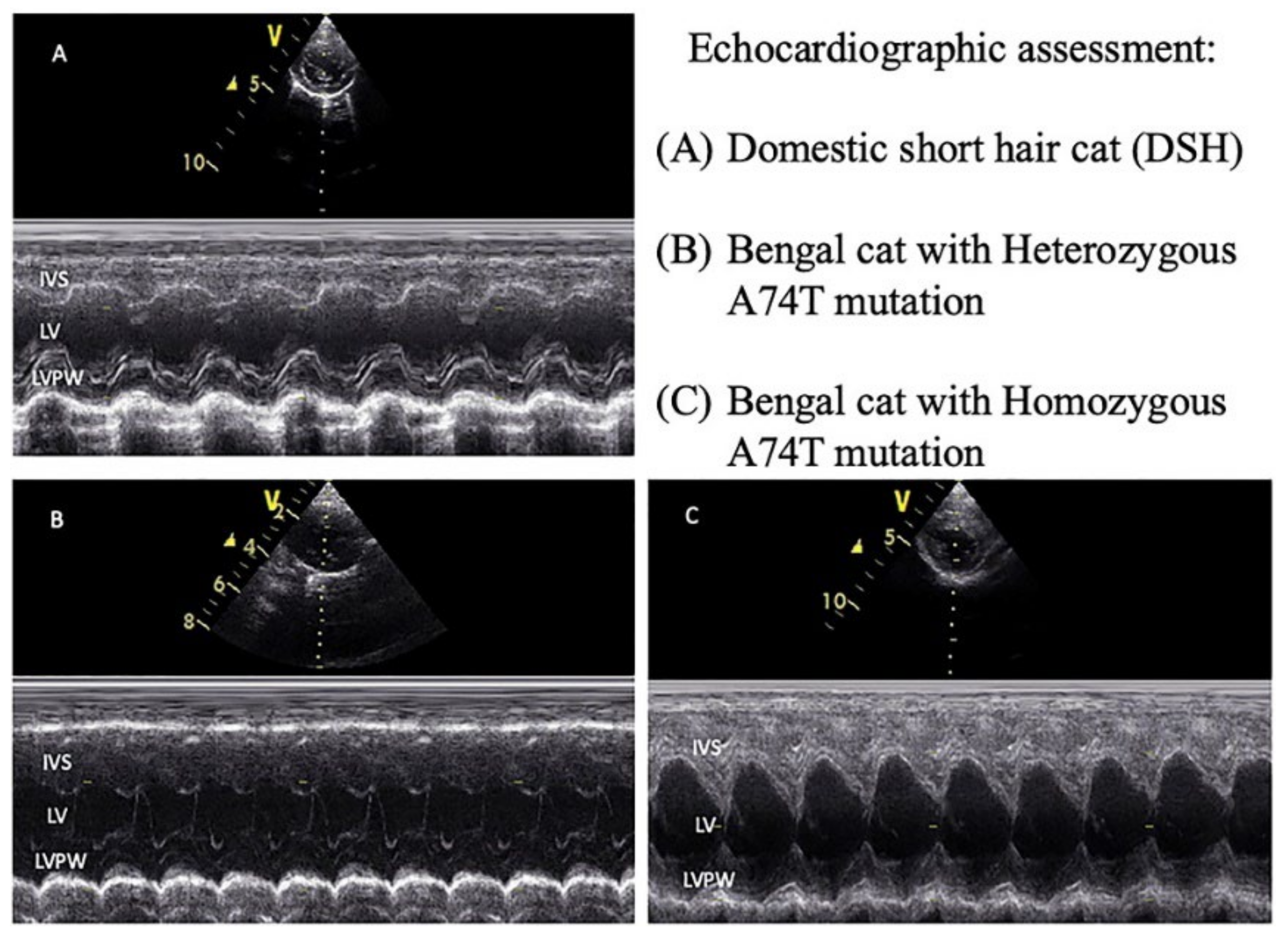
2.2. Echocardiography
2.3. DNA Extraction and Polymerase Chain Reaction (PCR)
2.4. DNA Sequencing
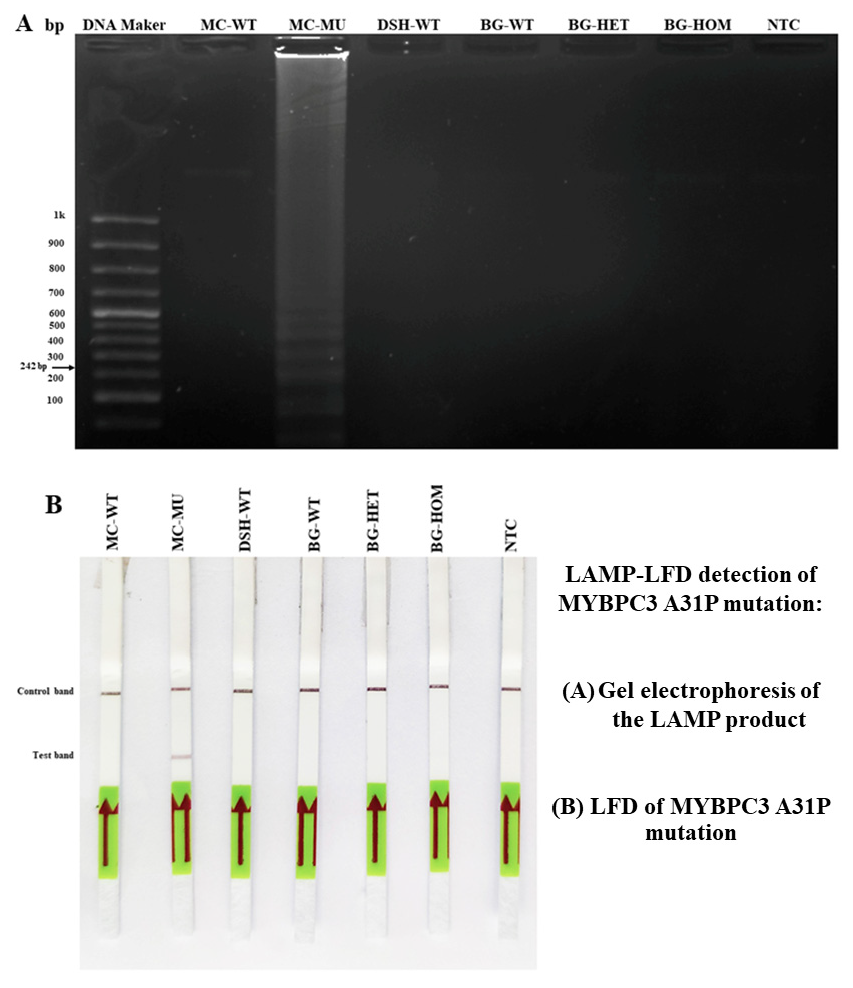
2.5. MYBPC3-LAMP-LFD Detection
2.6. Serum Peptide Analysis Using MALDI-TOF MS
2.7. Identification of Peptide by LC-MS/MS
2.8. Statistical Analysis
3. Results
3.1. The Baseline Characteristics of the Study Animals
3.2. Echocardiographic Parameters of the Study Animals
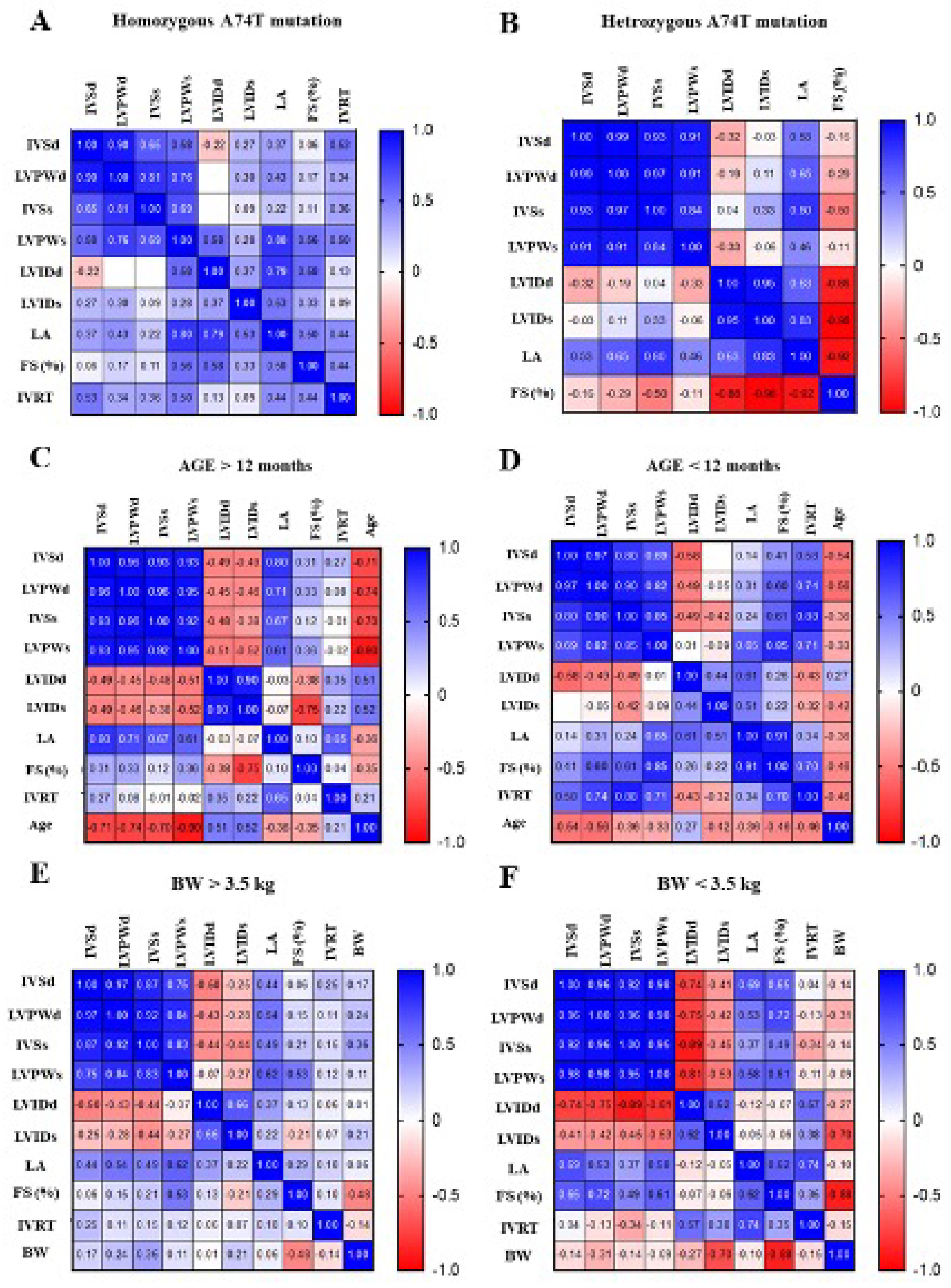
3.3. The Correlation Matrix of Genotype–Phenotype and Echocardiographic Parameters in Animals
3.4. LAMP-LFD Detection for MYBPC3-A31P Mutation in Study Animals
3.5. Verification of Specific SNP in MYBPC3 Gene Mutation by DNA Sequencing
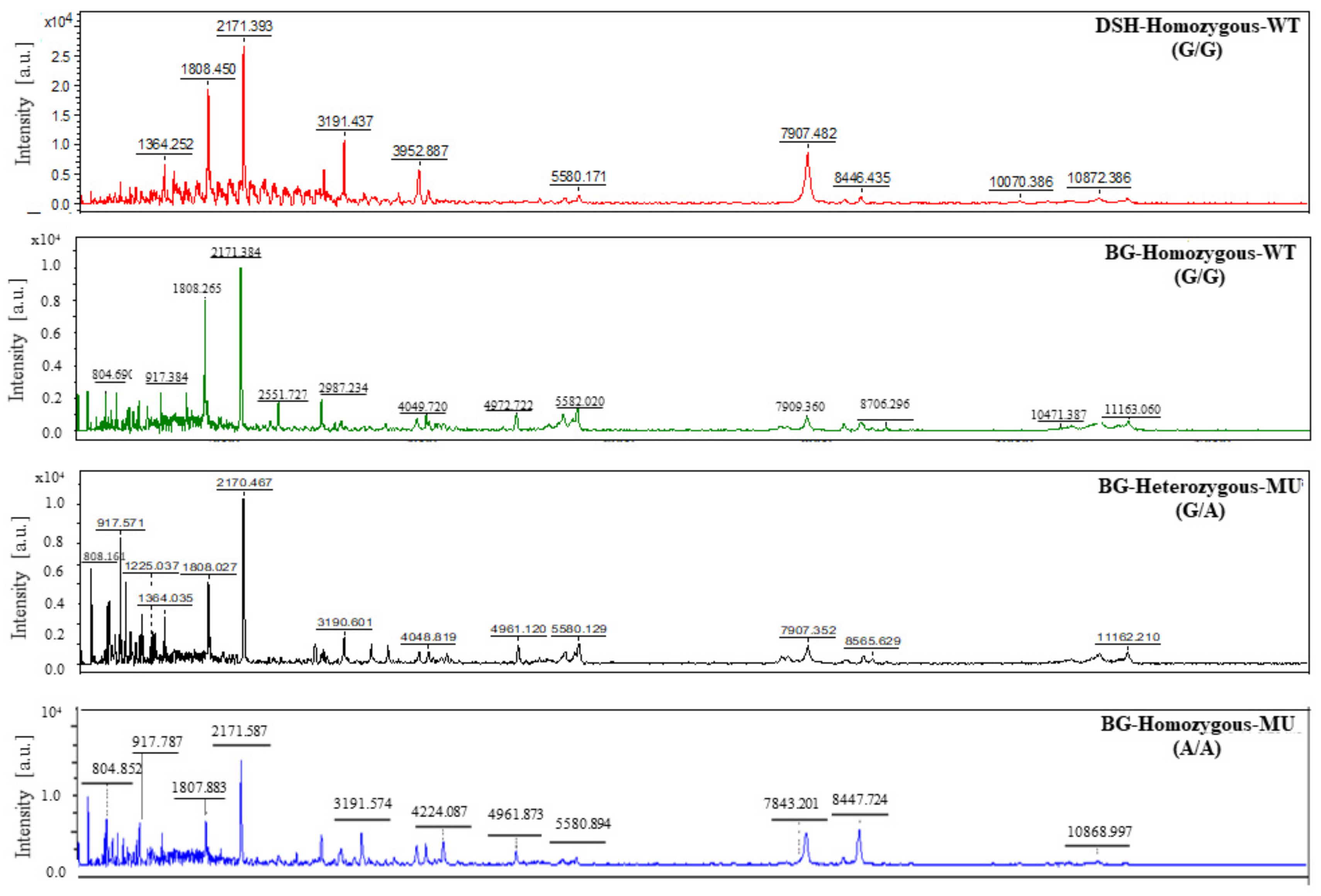
3.6. Results of Peptide Analysis Using MALDI-TOF
3.7. Peptide Identification and Protein Target
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longeri, M.; Ferrari, P.; Knafelz, P.; Mezzelani, A.; Marabotti, A.; Milanesi, L.; Pertica, G.; Polli, M.; Brambilla, P.G.; Kittleson, M.; et al. Myosin-binding protein C DNA variants in domestic cats (A 31 P, A 74 T, R 820 W) and their association with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2013, 27, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, P.R.; Liu, S.K.; Maron, B.J. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy: An animal model of human disease. Circulation 1995, 92, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.R.; Brodbelt, D.C.; Luis, F.V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centers (the CatScan study). J. Vet. Cardiol. 2015, 17, S244–S257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meurs, K.M.; Sanchez, X.; David, R.M.; Bowles, N.E.; Towbin, J.A.; Reiser, P.J.; Kittleson, J.A.; Munro, M.J.; Dryburgh, K.; MacDonald, K.A.; et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum. Mol. Genet. 2005, 14, 3587–3593. [Google Scholar] [CrossRef]
- Meurs, K.M.; Norgard, M.M.; Ederer, M.M.; Hendrix, K.P.; Kittleson, M.D. A substitution mutation in the myosin-binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007, 90, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Meurs, K.M.; Norgard, M.M.; Kuan, M.; Haggstrom, J. Analysis of 8 sarcomeric candidate genes for feline hypertrophic cardiomyopathy mutations in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2009, 2, 840–843. [Google Scholar] [CrossRef]
- Granström, S.; Nyberg Godiksen, M.T.; Christiansen, M.; Pipper, C.B.; Willesen, J.T.; Koch, J. Prevalence of hypertrophic cardiomyopathy in a cohort of British Shorthair cats in Denmark. J. Vet. Intern. Med. 2011, 25, 866–871. [Google Scholar] [CrossRef]
- Trehiou-Sechi, E.; Tissier, R.; Gouni, V.; Misbach, C.; Petit, A.M.P.; Balouka, D.; Carlos Sampedrano, C.; Castaignet, M.; Pouchelon, J.L.; Chetboul, V. Comparative echocardiographic and clinical features of hypertrophic cardiomyopathy in 5 breeds of cats: A retrospective analysis of 344 cases (2001–2011). J. Vet. Intern. Med. 2012, 26, 532–541. [Google Scholar] [CrossRef]
- Marian, A.J. Hypertrophic cardiomyopathy: From genetics to treatment. Eur. J. Clin. Investig. 2010, 40, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Kittleson, M.D.; Meurs, K.M.; Harris, S.P. The genetic basis of hypertrophic cardiomyopathy in cats and humans. J. Vet. Cardiol. 2015, 17, S53–S73. [Google Scholar] [CrossRef] [Green Version]
- Sabater-Molina, M.; Pérez-Sánchez, I.; Hernández Del Rincón, J.P.; Gimeno, J.R. Genetics of hypertrophic cardiomyopathy: A review of current state. Clin. Genet. 2018, 93, 3–14. [Google Scholar] [CrossRef]
- Wess, G.; Schinner, C.; Weber, K.; Küchenhoff, H.; Hartmann, K. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats. J. Vet. Intern. Med. 2010, 24, 527–532. [Google Scholar] [CrossRef]
- Abbott, J.A. Feline hypertrophic cardiomyopathy: An update. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 685–700. [Google Scholar] [CrossRef]
- Fuentes, V.L.; Abbott, J.; Chetboul, V.; Côté, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef] [Green Version]
- Pareek, C.S.; Smoczynski, R.; Tretyn, A. Sequencing technologies and genome sequencing. J. Appl. Genet. 2011, 52, 413–435. [Google Scholar] [CrossRef] [Green Version]
- Dhama, K.; Karthik, K.; Chakraborty, S.; Tiwari, R.; Kapoor, S.; Kumar, A.; Thomas, P. Loop-mediated isothermal amplification of DNA (LAMP): A new diagnostic tool that lights the world of diagnosis of animal and human pathogens: A review. Pak. J. Biol. Sci. 2014, 17, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Saechue, B.; Kamiyama, N.; Wang, Y.; Fukuda, C.; Watanabe, K.; Soga, Y.; Goto, M.; Dewayani, A.; Ariki, S.; Hirose, H.; et al. Development of a portable reverse transcription loop-mediated isothermal amplification system to detect the E1 region of Chikungunya virus cost-effectively. Genes Cells 2020, 25, 615–625. [Google Scholar] [CrossRef]
- Varona, M.; Anderson, J.L. Advances in Mutation Detection Using Loop-Mediated Isothermal Amplification. ACS Omega 2021, 6, 3463–3469. [Google Scholar] [CrossRef]
- Sukumolanan, P.; Phanakrop, N.; Thaisakun, S.; Roytrakul, S.; Petchdee, S. Analysis of the Serum Peptidomics Profile for Cats with Sarcomeric Gene Mutation and Hypertrophic Cardiomyopathy. Front. Vet. Sci. 2021, 8, 771408. [Google Scholar] [CrossRef]
- Brown, S.; Atkins, C.; Bagley, R.; Carr, A.; Cowgill, L.; Davidson, M.; Egner, B.; Elliott, J.; Henik, R.; Labato, M.; et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2007, 21, 542–558. [Google Scholar] [CrossRef]
- Abbott, J.A.; Maclean, H.N. Two-dimensional echocardiographic assessment of the feline left atrium. J. Vet. Intern. Med. 2006, 20, 111–119. [Google Scholar] [CrossRef]
- Demeekul, K.; Sukumolanan, P.; Bootcha, R.; Panprom, C.; Petchdee, S. A Cardiac Protection of Germinated Brown Rice During Cardiopulmonary Bypass Surgery and Simulated Myocardial Ischemia. J. Inflamm. Res. 2021, 14, 3307–3319. [Google Scholar] [CrossRef]
- Godiksen, M.T.; Granstrøm, S.; Koch, J.; Christiansen, M. Hypertrophic cardiomyopathy in young Maine Coon cats caused by the p. A31P cMyBP-C mutation-the clinical significance of having the mutation. Acta Vet. Scand. 2011, 53, 7. [Google Scholar] [CrossRef] [Green Version]
- Sukumolanan, P.; Demeekul, K.; Petchdee, S. Development of a Loop-mediated Isothermal Amplification Assay Coupled with a Lateral Flow Dipstick Test for Detection of Myosin Binding Protein C3 A31P Mutation in Maine Coon Cats. Front. Vet. Sci. 2022, 9, 819694. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry method for protein quantitation. In The Protein Protocols Handbook; Springer: Humana Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar] [CrossRef]
- Ploypetch, S.; Roytrakul, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Krobthong, S.; Suriyaphol, G. Salivary proteomics of canine oral tumors using MALDI-TOF mass spectrometry and LC-tandem mass spectrometry. PLoS ONE 2019, 14, e0219390. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.J.; Spraggins, J.M.; Caprioli, R.M. Protein identification strategies in MALDI imaging mass spectrometry: A brief review. Curr. Opin. Chem. Biol. 2019, 48, 64–72. [Google Scholar] [CrossRef]
- Ploypetch, S.; Roytrakul, S.; Phaonakrop, N.; Kittisenachai, S.; Leetanasaksakul, K.; Pisamai, S.; Kalpravidh, C.; Rungsipipat, A.; Suriyaphol, G. In-gel digestion coupled with mass spectrometry (GeLC-MS/MS)-based salivary proteomic profiling of canine oral tumors. BMC Vet. Res. 2020, 16, 335. [Google Scholar] [CrossRef]
- Yimpring, N.; Roytrakul, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Krobthong, S.; Suriyaphol, G. Proteomic profiles of unilateral cryptorchidism in pigs at different ages using MALDI-TOF mass spectrometry and in-gel digestion coupled with mass spectrometry (GeLC-MS/MS) approach. BMC Vet. Res. 2020, 16, 373. [Google Scholar] [CrossRef]
- Berg, M.; Parbel, A.; Pettersen, H.; Fenyö, D.; Björkesten, L. Detection of artifacts and peptide modifications in liquid chromatography/mass spectrometry data using two-dimensional signal intensity map data visualization. Rapid Commun. Mass Spectrom. 2006, 20, 1558–1562. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophor. Int. J. 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Harris, S.P.; Lyons, R.G.; Bezold, K.L. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ. Res. 2011, 108, 751–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiburek, L.; Vesela, K.; Hansikova, H.; Hulkova, H.; Zeman, J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am. J. Physiol. Cell Physiol. 2009, 296, C1218–C1226. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, P.; Horvath, R.; Macmillan, C.; Peters, J.; Jaksch, M. Reversion of hypertrophic cardiomyopathy in a patient with deficiency of the mitochondrial copper-binding protein Sco2: Is there a potential effect of copper? J. Inherit. Metab. Dis. 2004, 27, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jaksch, M.; Ogilvie, I.; Yao, J.; Kortenhaus, G.; Bresser, H.G.; Gerbitz, K.D.; Shoubridge, E.A. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000, 9, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Mesecke, N.; Terziyska, N.; Kozany, C.; Baumann, F.; Neupert, W.; Hell, K.; Herrmann, J.M. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 2005, 121, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Mesecke, N.; Bihlmaier, K.; Grumbt, B.; Longen, S.; Terziyska, N.; Hell, K.; Herrmann, J.M. The zinc-binding protein Hot13 promotes oxidation of the mitochondrial import receptor Mia40. EMBO Rep. 2008, 9, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Häggström, J.; Andersson, Å.O.; Falk, T.; Nilsfors, L.; OIsson, U.; Kresken, J.G.; Höglund, K.; Rishniw, M.; Tidholm, A.; Ljungvall, I. Effect of Body Weight on Echocardiographic Measurements in 19,866 Pure-Bred Cats with or without Heart Disease. J. Vet. Intern. Med. 2016, 30, 1601–1611. [Google Scholar] [CrossRef]
- Bloemink, M.; Deacon, J.; Langer, S.; Vera, C.; Combs, A.; Leinwand, L.; Geeves, M.A. The hypertrophic cardiomyopathy myosin mutation R453C alters ATP binding and hydrolysis of human cardiac β-myosin. J. Biol. Chem. 2014, 289, 5158–5167. [Google Scholar] [CrossRef] [Green Version]
- Krause, S.; Jacobus, W. Specific enhancement of the cardiac myofibrillar ATPase by bound creatine kinase. J. Biol. Chem. 1992, 267, 2480–2486. [Google Scholar] [CrossRef]




| Parameters | DSH (n = 9) | Bengal | p-Value | ||
|---|---|---|---|---|---|
| A74T-WT (n = 1) | A74T-HET (n = 4) | A74T-HOM (n = 9) | |||
| Age (months) | 20.00 ± 4.60 | 29.00 ± 0.00 | 23.63 ± 9.08 | 14.11 ± 2.43 | 0.47 |
| Body weight (kg) | 4.41 ± 0.49 | 4.40 ± 0.00 | 3.58 ± 0.35 | 4.89 ± 0.58 | 0.56 |
| Male (number (%)) | 3/9 (33.33%) | 0/1 (0%) | 1/4 (25.00%) | 3/9 (33.33%) | - |
| Blood pressure (mmHg) | 111.10 ± 8.24 | 126.67 ± 0.00 | 95.83 ± 6.29 | 108.52 ± 8.20 | 0.60 |
| Parameters | DSH (n = 9) | Bengal | ||
|---|---|---|---|---|
| A74T-WT (n = 1) | A74T-HET (n = 4) | A74T-HOM (n = 9) | ||
| LA (cm) | 0.96 ± 0.04 | 1.08 ± 0.00 | 0.90 ± 0.00 | 1.02 ± 0.04 |
| AO (cm) | 0.78 ± 0.04 | 0.73 ± 0.00 | 0.72 ± 0.02 | 0.70 ± 0.04 |
| LA/AO ratio | 1.30 ± 0.07 | 1.47 ± 0.00 | 1.29 ± 0.01 | 1.44 ± 0.06 |
| IVSd (cm) | 0.48 ± 0.03 | 0.61 ± 0.00 | 0.40 ± 0.06 | 0.57 ± 0.03 |
| LVPWd (cm) | 0.46 ± 0.03 | 0.61 ± 0.00 | 0.40 ± 0.05 | 0.57 ± 0.03 * |
| LVIDd (cm) | 1.21 ± 0.07 | 1.33 ± 0.00 | 1.65 ± 0.02 ## | 1.31 ± 0.06 * |
| IVSs (cm) | 0.59 ± 0.02 | 0.75 ± 0.00 | 0.51 ± 0.06 | 0.70 ± 0.03 ** |
| LVPWs (cm) | 0.58 ± 0.04 | 0.65 ± 0.00 | 0.49 ± 0.08 | 0.69 ± 0.03 * |
| LVIDs (cm) | 0.82 ± 0.07 | 0.78 ± 0.00 | 1.01 ± 0.04 | 0.79 ± 0.03 |
| FS (%) | 40.13 ± 3.88 | 41.30 ± 0.00 | 39.06 ± 1.51 | 41.93 ± 1.67 |
| PV Vmax (m/s) | 0.63 ± 0.02 | 0.76 ± 0.00 | 0.98 ± 0.07 # | 1.12 ± 0.08 †† |
| AV Vmax (m/s) | 0.63 ± 0.02 | 07.00 ± 0.00 | 0.86 ± 0.06 ## | 0.73 ± 0.03 |
| IVRT (ms) | 39.00 ± 0.24 | 50.00 ± 0.00 | 50.00 ± 0.00 # | 51.67 ± 3.12 † |
| Peptide Mass (Da) | Sample Group | Expected Protein |
|---|---|---|
| 3199 | a, c, d |
|
| 916 | b, c, d |
|
| 804/805 | b, d |
|
| 808 | c |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demeekul, K.; Sukumolanan, P.; Panprom, C.; Thaisakun, S.; Roytrakul, S.; Petchdee, S. Echocardiography and MALDI-TOF Identification of Myosin-Binding Protein C3 A74T Gene Mutations Involved Healthy and Mutated Bengal Cats. Animals 2022, 12, 1782. https://doi.org/10.3390/ani12141782
Demeekul K, Sukumolanan P, Panprom C, Thaisakun S, Roytrakul S, Petchdee S. Echocardiography and MALDI-TOF Identification of Myosin-Binding Protein C3 A74T Gene Mutations Involved Healthy and Mutated Bengal Cats. Animals. 2022; 12(14):1782. https://doi.org/10.3390/ani12141782
Chicago/Turabian StyleDemeekul, Kanokwan, Pratch Sukumolanan, Chattida Panprom, Siriwan Thaisakun, Sittiruk Roytrakul, and Soontaree Petchdee. 2022. "Echocardiography and MALDI-TOF Identification of Myosin-Binding Protein C3 A74T Gene Mutations Involved Healthy and Mutated Bengal Cats" Animals 12, no. 14: 1782. https://doi.org/10.3390/ani12141782
APA StyleDemeekul, K., Sukumolanan, P., Panprom, C., Thaisakun, S., Roytrakul, S., & Petchdee, S. (2022). Echocardiography and MALDI-TOF Identification of Myosin-Binding Protein C3 A74T Gene Mutations Involved Healthy and Mutated Bengal Cats. Animals, 12(14), 1782. https://doi.org/10.3390/ani12141782







