Main Factors Influencing the Gut Microbiota of Datong Yaks in Mixed Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Data Processing
2.4. Ecological Assembly Process of the Gut Microbiota
3. Results
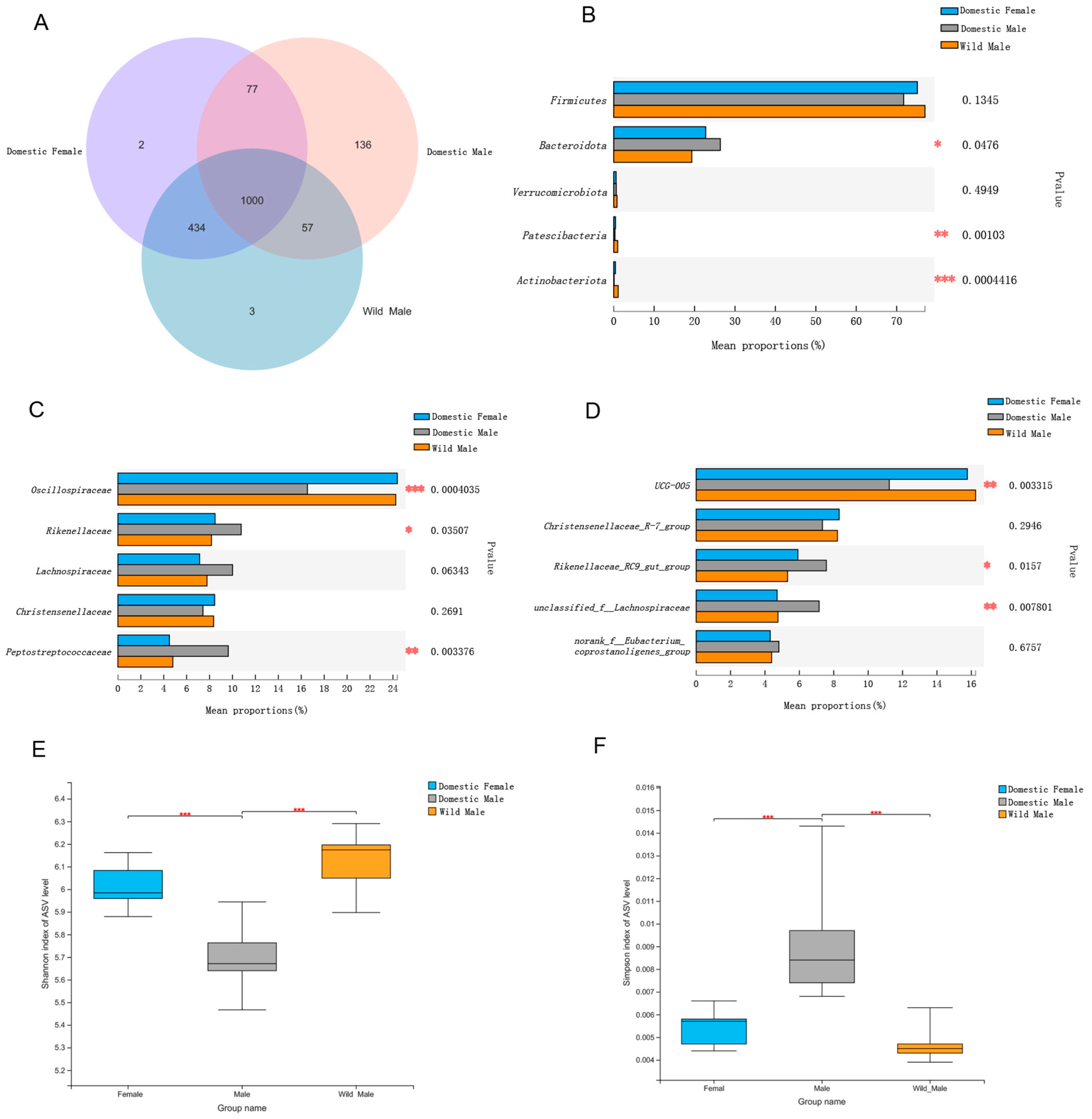
3.1. Comparison of Gut Microbial Diversity among Domestic Females and Males and Wild Males
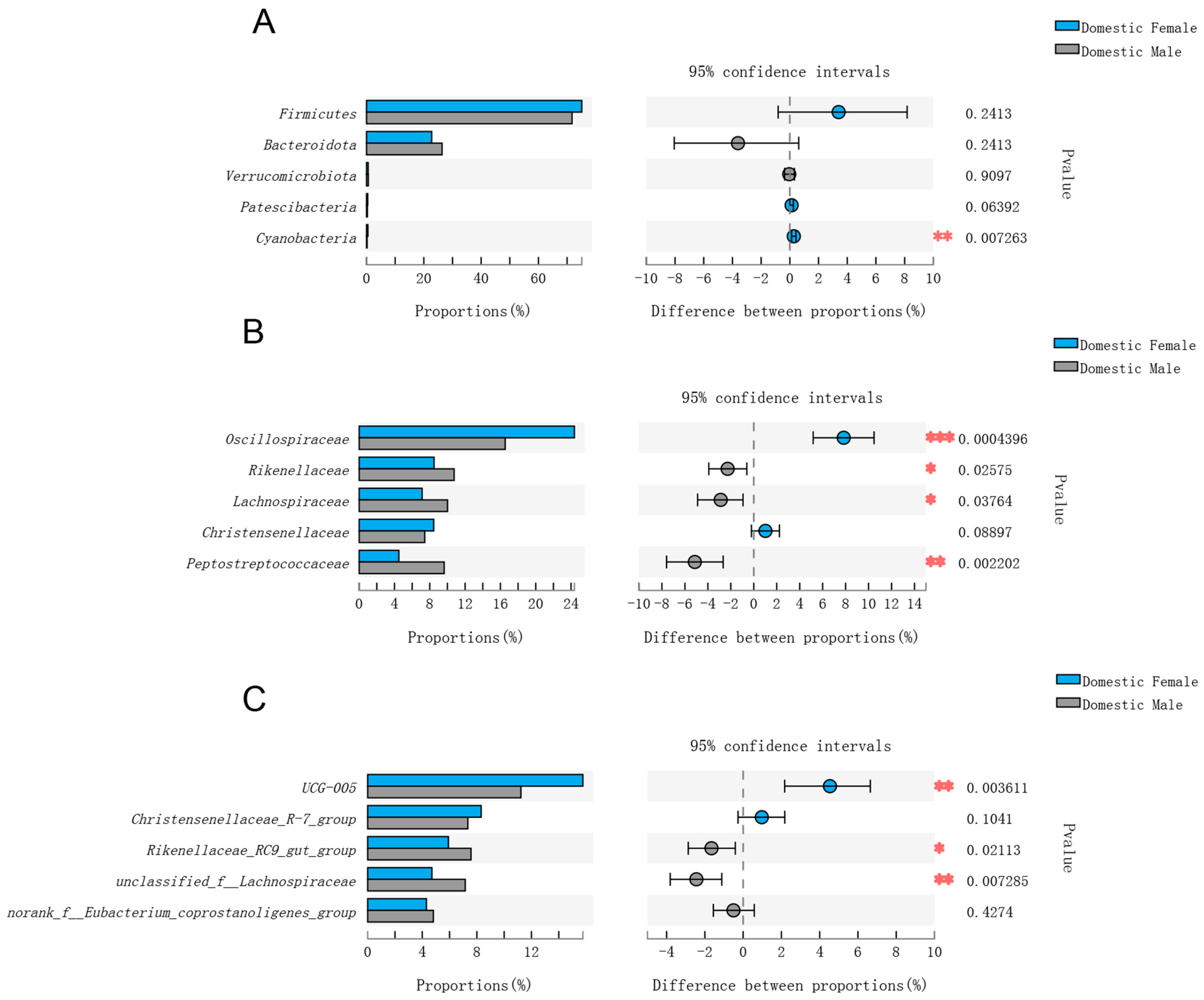
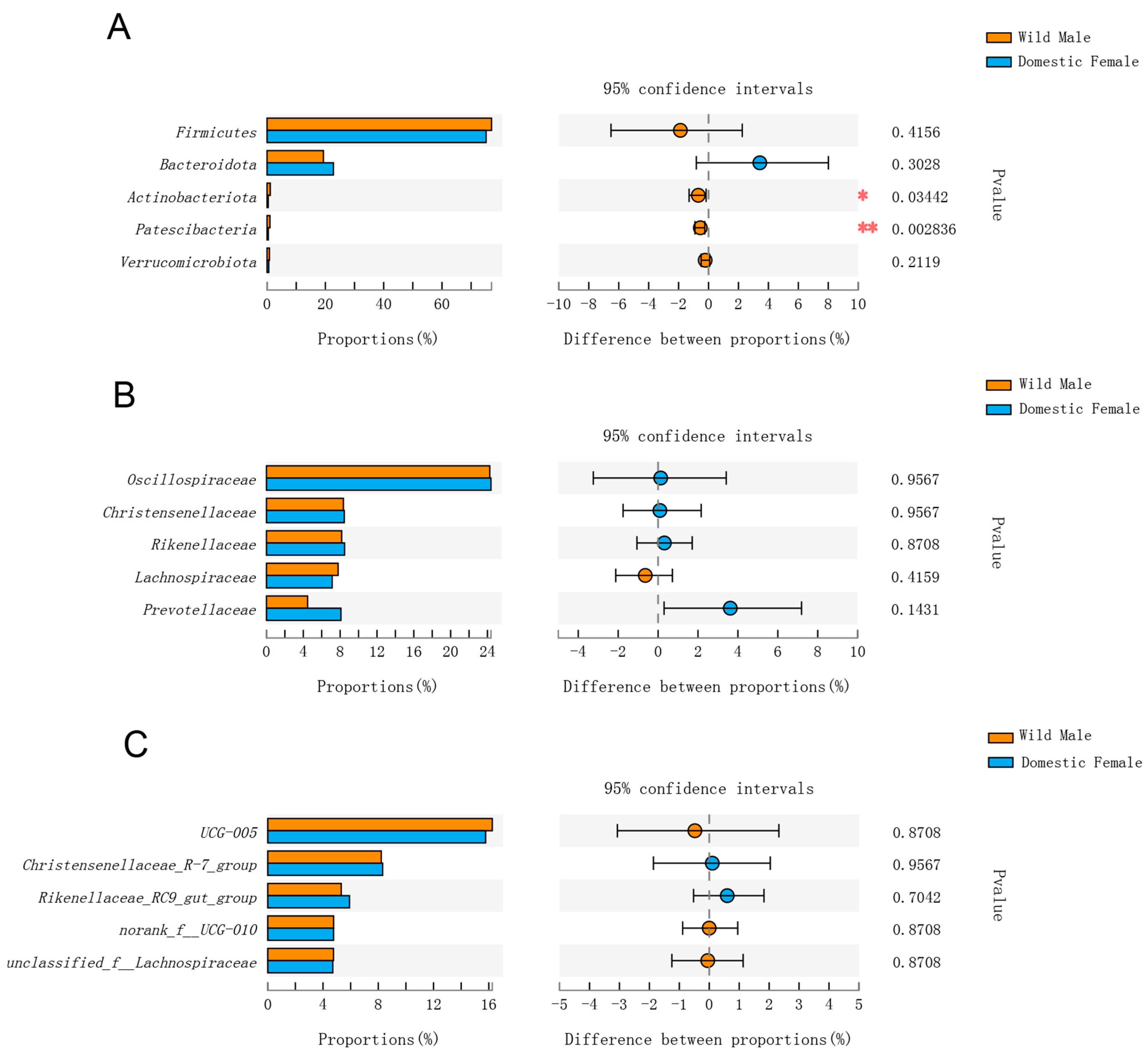
3.2. Comparison of Gut Microbial Composition among Domestic Females and Males and Wild Males
3.3. Comparisons of Gut Microbial Composition in Domestic Females and Males and Wild Males
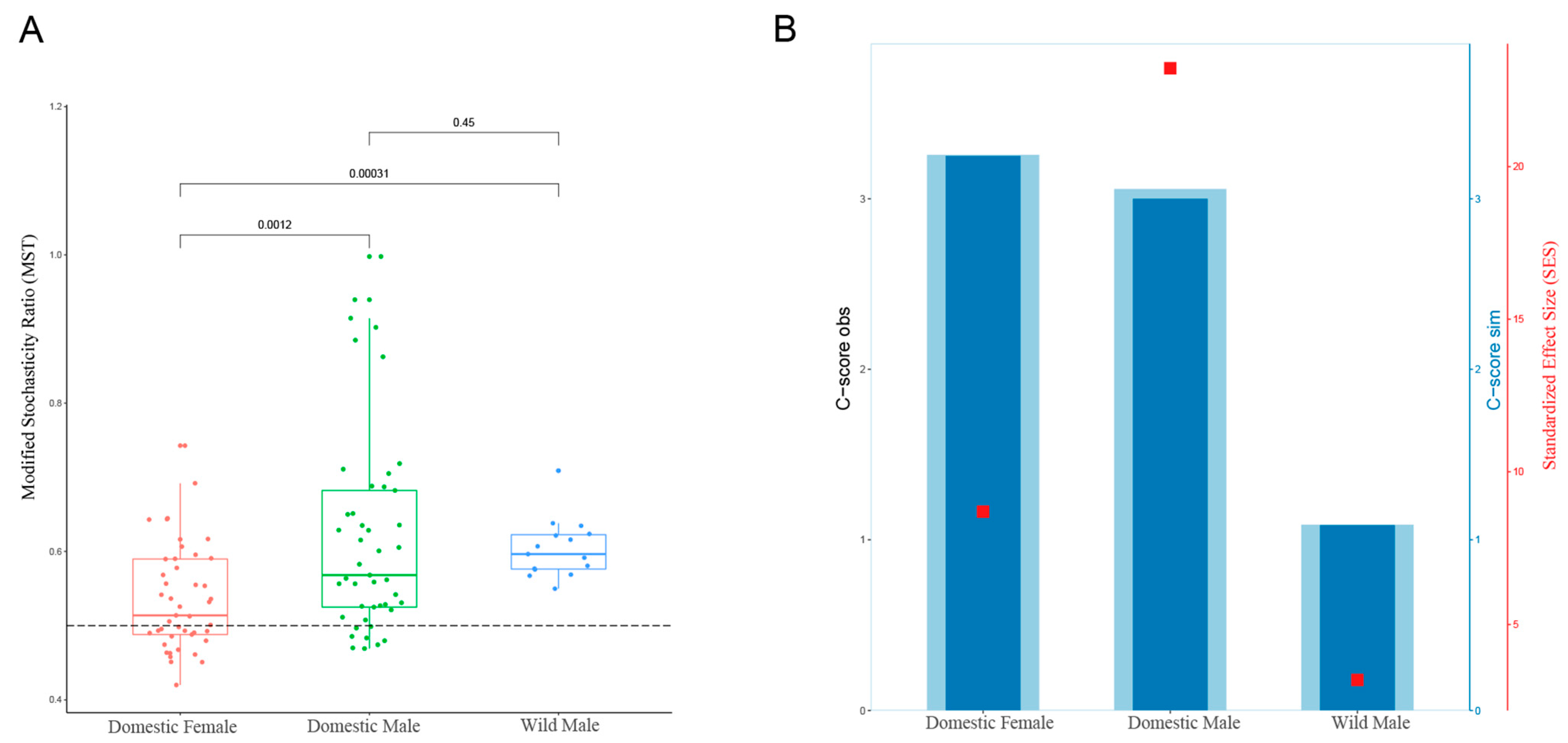
3.4. Ecological Assembly Process of the Gut Microbiota in Domestic Females and Males and in Wild Males
4. Discussion
4.1. Main Factors Influencing the Gut Microbial Diversity in Domestic Males and Females
4.2. Main Factors Influencing Gut Microbial Diversity in Domestic and Wild Males
4.3. Gut Microbial Diversity in Domestic Females and Wild Males during Estrus
4.4. Effects of Different Gut Microbial Compositions on Domestic Females and Males and Wild Males
4.5. Ecological Assembly Processes Reflecting the Living Status of Datong Yaks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Df | Sums of Sqs | MeanSqs | F.Models | R2 | Pr(>F) |
|---|---|---|---|---|---|---|
| ALL Samples | 2 | 1.7853 | 0.8927 | 8.5963 | 0.4278 | 0.001 |
| Residuals | 23 | 2.3884 | 0.1038 | - | 0.5722 | - |
| Total | 25 | 4.1737 | - | - | 1.0000 | - |
| Name | Df | Sums of Sqs | MeanSqs | F.Models | R2 | Pr(>F) |
|---|---|---|---|---|---|---|
| Domestic Males and Females | 1 | 1.4024 | 1.4024 | 13.2632 | 0.4242 | 0.001 |
| Residuals | 18 | 1.9032 | 0.1057 | - | 0.5758 | - |
| Total | 19 | 3.3056 | - | - | 1.0000 | - |
| Name | Df | Sums of Sqs | MeanSqs | F.Models | R2 | Pr(>F) |
|---|---|---|---|---|---|---|
| Domestic Males and Wild Male | 1 | 0.9339 | 0.9339 | 9.0800 | 0.4307 | 0.001 |
| Residuals | 12 | 1.2343 | 0.1029 | - | 0.5693 | - |
| Total | 13 | 2.1682 | - | - | 1.0000 | - |
| Name | Df | Sums of Sqs | MeanSqs | F.Models | R2 | Pr(>F) |
|---|---|---|---|---|---|---|
| Domestic Females and Wild Male | 1 | 0.1145 | 0.1145 | 1.1479 | 0.0758 | 0.18 |
| Residuals | 14 | 1.3969 | 0.0998 | - | 0.9242 | - |
| Total | 15 | 1.5114 | - | - | 1.0000 | - |
References
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The Yak Genome and Adaptation to Life at High Altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, X.; Liang, C.; Guo, X.; Ding, X.; Chu, M.; Wang, H.; Pei, J.; Bao, P.; Yan, P. Bibliometric Analysis of Yak Based on the China National Knowledge Infrastructure Database. Pratacultural Sci. 2019, 36, 2151–2158. [Google Scholar]
- Yuan, K.; Wang, X. The Breeding Progress of Datong Yak in Qinghai Plateau. China Cattle Sci. 2019, 45, 28–32. [Google Scholar]
- Wu, S.; Cui, Z.; Chen, X.; Wang, P.; Yao, J. Changed Caecal Microbiota and Fermentation Contribute to the Beneficial Effects of Early Weaning with Alfalfa Hay, Starter Feed, and Milk Replacer on the Growth and Organ Development of Yak Calves. Animals 2019, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.A.; Ma, S.Q.; Li, Z.R.; Li, X.L.; Madigosky, S.R. Seasonal Changes of Rumen and Intestine Morphology of the Qinghai Yak (Bos grunniens). Vet. World 2018, 11, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhou, Z.; Guan, J.; Xia, B.; Luo, X.; Yang, Y.; Fu, Y.; Sun, Q. Dynamic Changes of Yak (Bos grunniens) Gut Microbiota during Growth Revealed by Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis and Metagenomics. Asian-Australas J. Anim. Sci. 2017, 30, 957–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, W.; Zhou, M.; Ma, T.; Bi, S.; Wang, W.; Zhang, Y.; Huang, X.; Guan, L.L.; Long, R. Survey of Rumen Microbiota of Domestic Grazing Yak during Different Growth Stages Revealed Novel Maturation Patterns of Four Key Microbial Groups and Their Dynamic Interactions. Anim. Microbiome 2020, 2, 23. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wu, X.; Shang, S.; Yan, J.; Chen, Y.; Zhang, H.; Tang, X. Characterization of the Gut Microbiota in the Golden Takin (Budorcas taxicolor bedfordi). AMB Express 2017, 7, 81. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, Y.; Li, D.; Xu, H.; Wu, J.; Wen, A.; Xie, M.; Ni, Q.; Zhang, M.; Peng, G.; et al. Characterization of the Gut Microbiota in Six Geographical Populations of Chinese Rhesus Macaques (Macaca mulatta), Implying an Adaptation to High-Altitude Environment. Microb. Ecol. 2018, 76, 565–577. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Yang, S.; Zhou, J.; Zhang, T.; Qi, L.; Sun, X.; Fan, M.; Xu, S.; Cha, M.; et al. Comparative Analysis of the Gut Microbiota Composition between Captive and Wild Forest Musk Deer. Front. Microbiol. 2017, 8, 1705. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Xu, T.-W.; Wang, X.-G.; Geng, Y.-Y.; Liu, H.-J.; Hu, L.-Y.; Zhao, N.; Kang, S.-P.; Zhang, W.-M.; Xu, S.-X. The Effect of Transitioning between Feeding Methods on the Gut Microbiota Dynamics of Yaks on the Qinghai–Tibet Plateau. Animals 2020, 10, 1641. [Google Scholar] [CrossRef]
- Dande, S.S.; Bhatt, V.D.; Patil, N.V.; Joshi, C.G. The Camel Faecal Metagenome under Different Systems of Management: Phylogenetic and Gene-Centric Approach. Livest. Sci. 2015, 178, 108–118. [Google Scholar] [CrossRef]
- Song, P.; Qin, W.; Huang, Y.; Wang, L.; Cai, Z.; Zhang, T. Grazing Management Influences Gut Microbial Diversity of Livestock in the Same Area. Sustainability 2020, 12, 4160. [Google Scholar] [CrossRef]
- Ren, C.; Sylvia, K. Sexual Dimorphism in the Gut Microbiome. IU J. Undergrad. Res. 2018, 4, 12–16. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.-Y.; Park, M.-S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Schmidt, E.; Mykytczuk, N.; Schulte-Hostedde, A.I. Effects of the Captive and Wild Environment on Diversity of the Gut Microbiome of Deer Mice (Peromyscus maniculatus). ISME J. 2019, 13, 1293–1305. [Google Scholar] [CrossRef]
- Moeller, A.H.; Peeters, M.; Ndjango, J.-B.; Li, Y.; Hahn, B.H.; Ochman, H. Sympatric Chimpanzees and Gorillas Harbor Convergent Gut Microbial Communities. Genome. Res. 2013, 23, 1715–1720. [Google Scholar] [CrossRef]
- Huang, B.-H.; Chang, C.-W.; Huang, C.-W.; Gao, J.; Liao, P.-C. Composition and Functional Specialists of the Gut Microbiota of Frogs Reflect Habitat Differences and Agricultural Activity. Front. Microbiol. 2018, 8, 2670. [Google Scholar] [CrossRef]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-Fat Diet Alters Gut Microbiota Physiology in Mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Trosvik, P.; de Muinck, E.J.; Rueness, E.K.; Fashing, P.J.; Beierschmitt, E.C.; Callingham, K.R.; Kraus, J.B.; Trew, T.H.; Moges, A.; Mekonnen, A.; et al. Multilevel Social Structure and Diet Shape the Gut Microbiota of the Gelada Monkey, the Only Grazing Primate. Microbiome 2018, 6, 84. [Google Scholar] [CrossRef]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, Gut Microbiome, and Cardiovascular Disease Risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Meng, D.; Gong, M.; Li, H.; Wen, W.; Wang, Y.; Zhou, J. Effects of Sex and Diet on Gut Microbiota of Farmland-Dependent Wintering Birds. Front. Microbiol. 2020, 11, 587873. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in Gut Microbiota Associated with Age, Sex, and Stool Consistency in Healthy Japanese Subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wen, Q.; Wang, Y.; Wang, Z.; Tan, Z.; Wu, K. Sex Differences in Intestinal Microbial Composition and Function of Hainan Special Wild Boar. Animals 2020, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ma, T.; Tang, W.; Li, D.; Mishra, S.K.; Xu, Z.; Wang, Q.; Jie, H. Gut Microbiome of Chinese Forest Musk Deer Examined across Gender and Age. BioMed Res. Int. 2019, 2019, e9291216. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Steinel, N.; Weber, J.; Ma, L.; Smith, C.; Correa, D.; Zhu, B.; Bolnick, D.; Wang, G. The Gut Microbiota Response to Helminth Infection Depends on Host Sex and Genotype. ISME J. 2020, 14, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-Specific Association between the Gut Microbiome and High-Fat Diet-Induced Metabolic Disorders in Mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Han, G.; Lee, H.J.; Jeong, S.E.; Jeon, C.O.; Hyun, S. Comparative Analysis of Drosophila Melanogaster Gut Microbiota with Respect to Host Strain, Sex, and Age. Microb. Ecol. 2017, 74, 207–216. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J. Neurogastroenterol. Motil. 2021, 27, 314–325. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, L.; Fu, R.; Cheng, L.; Yan, S.; Mahtab, N.; Song, Y. Effects of Foraging Site Distances on the Intestinal Bacterial Community Compositions of the Sympatric Wintering Hooded Crane (Grus monacha) and Domestic Duck (Anas platyrhynchos domesticus). Avian Res. 2021, 12, 20. [Google Scholar] [CrossRef]
- Sevellec, M.; Derome, N.; Bernatchez, L. Holobionts and Ecological Speciation: The Intestinal Microbiota of Lake Whitefish Species Pairs. Microbiome 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yan, J.; Wang, H.; Jiang, F.; Song, P.; Cai, Z.; Zhang, T. Microbial Biogeography along the Gastrointestinal Tract Segments of Sympatric Subterranean Rodents (Eospalax baileyi and Eospalax cansus). Animals 2021, 11, 3297. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yang, H.; Han, S.; Feng, L.; Wang, T.; Ge, J. Comparison of the Gut Microbiota Composition between Wild and Captive Sika Deer (Cervus nippon hortulorum) from Feces by High-Throughput Sequencing. AMB Express 2017, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.; German, D.P.; Lujan, N.K.; Jackson, C.R. Gut Microbiomes of Sympatric Amazonian Wood-Eating Catfishes (Loricariidae) Reflect Host Identity and Little Role in Wood Digestion. Ecol. Evol. 2020, 10, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic Processes Shape Microeukaryotic Community Assembly in a Subtropical River across Wet and Dry Seasons. Microbiome 2019, 7, 138. [Google Scholar] [CrossRef]
- Mo, Y.; Peng, F.; Gao, X.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.; Xue, Y.; Yang, J. Low Shifts in Salinity Determined Assembly Processes and Network Stability of Microeukaryotic Plankton Communities in a Subtropical Urban Reservoir. Microbiome 2021, 9, 128. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the Roles of Immigration and Chance in Shaping Prokaryote Community Structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Li, B.; Gao, H.; Song, P.; Liang, C.; Jiang, F.; Xu, B.; Liu, D.; Zhang, T. Captivity Shifts Gut Microbiota Communities in White-Lipped Deer (Cervus albirostris). Animals 2022, 12, 431. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.S.R.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Method 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package. 2019. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1778707 (accessed on 11 April 2022).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; RStudio. Create Elegant Data Visualisations Using the Grammar of Graphics. 2019. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 11 April 2022).
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A General Framework for Quantitatively Assessing Ecological Stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A.; et al. Stochasticity, Succession, and Environmental Perturbations in a Fluidic Ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef]
- Stone, L.; Roberts, A. The Checkerboard Score and Species Distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex Differences in Lipid Metabolism Are Affected by Presence of the Gut Microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Yuan, X.; Jia, Q. Dynamics of 4 Kinds of Serum Hormones Concentrations of Qinghai Datong Growing Yak. J. Agric. Univ. Hebei 2002, 25, 61–65. [Google Scholar]
- Luo, X.; Ma, Z.; Xu, J.; Tong, Z.; Chen, S. Investigation of Dynamic Changes of Several Hormones in Reproduction Season in Yak Cows. J. Grassl. Forage Sci. 2010, 1–7. [Google Scholar]
- Wang, Z.; Zhang, H.; Zhang, J.; Xu, S.; Peng, W.; Yang, Q. Effect of LH and FSH on Ovaries Development of Pubertal Female Yak. Chin. Qinghai J. Anim. Vet. Sci. 2017, 47, 24–28. [Google Scholar]
- Wang, Z.; Zhang, H.; Zhang, J.; Xu, S.; Peng, W. Secretion Patterns of Some Reproductive Hormones at Stages of Estrus and Anestrus in the Female Yak around Puberal Time. Anim. Husb. Vet. Med. 2017, 49, 5–8. [Google Scholar]
- Qiao, Z. Behaviors, Sex Hormones and Sex Pheromones during Reproductive Period of Captive Amur Tigers. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2018. [Google Scholar]
- Hicks, A.L.; Lee, K.J.; Couto-Rodriguez, M.; Patel, J.; Sinha, R.; Guo, C.; Olson, S.H.; Seimon, A.; Seimon, T.A.; Ondzie, A.U.; et al. Gut Microbiomes of Wild Great Apes Fluctuate Seasonally in Response to Diet. Nat. Commun. 2018, 9, 1786. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, Q.; Ma, H.; Wang, L.; Yang, Y.; Luo, W.; Qiu, Q. Genome-Wide Variation within and between Wild and Domestic Yak. Mol. Ecol. Resour. 2014, 14, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Genome Assembly of Wild Yak and Construction of Yak Pan-Genome. Master’s Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Yu, S.; Chen, B. Characteristics of Reproduction and Related Hormone Changes around Oestrus in Yak. Acta Zool. Sin. 1997, 43, 178–183. [Google Scholar]
- Ren, T.; Boutin, S.; Humphries, M.M.; Dantzer, B.; Gorrell, J.C.; Coltman, D.W.; McAdam, A.G.; Wu, M. Seasonal, Spatial, and Maternal Effects on Gut Microbiome in Wild Red Squirrels. Microbiome 2017, 5, 163. [Google Scholar] [CrossRef]
- Moeller, A.H.; Foerster, S.; Wilson, M.L.; Pusey, A.E.; Hahn, B.H.; Ochman, H. Social Behavior Shapes the Chimpanzee Pan-Microbiome. Sci. Adv. 2016, 2, e1500997. [Google Scholar] [CrossRef]
- Jiang, F.; Gao, H.; Qin, W.; Song, P.; Wang, H.; Zhang, J.; Liu, D.; Wang, D.; Zhang, T. Marked Seasonal Variation in Structure and Function of Gut Microbiota in Forest and Alpine Musk Deer. Front. Microbiol. 2021, 12, 699797. [Google Scholar] [CrossRef]
- Qin, W.; Song, P.; Lin, G.; Huang, Y.; Wang, L.; Zhou, X.; Li, S.; Zhang, T. Gut Microbiota Plasticity Influences the Adaptability of Wild and Domestic Animals in Co-Inhabited Areas. Front. Microbiol. 2020, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Menke, S.; Meier, M.; Mfune, J.K.E.; Melzheimer, J.; Wachter, B.; Sommer, S. Effects of Host Traits and Land-Use Changes on the Gut Microbiota of the Namibian Black-Backed Jackal (Canis mesomelas). FEMS Microbiol. Ecol. 2017, 93, fix123. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, L.; Fan, C.; Liu, C.; Li, W.; Li, J.; Zhao, X.; Jia, S.; Zhang, Y. Domestication Shapes the Community Structure and Functional Metagenomic Content of the Yak Fecal Microbiota. Front. Microbiol. 2021, 12, 594075. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, W.; Li, S.; Wu, N.; Wen, Z.; Xie, J.; Ma, H.; Zhang, S. Main Factors Influencing the Gut Microbiota of Datong Yaks in Mixed Group. Animals 2022, 12, 1777. https://doi.org/10.3390/ani12141777
Qin W, Li S, Wu N, Wen Z, Xie J, Ma H, Zhang S. Main Factors Influencing the Gut Microbiota of Datong Yaks in Mixed Group. Animals. 2022; 12(14):1777. https://doi.org/10.3390/ani12141777
Chicago/Turabian StyleQin, Wen, Shuang Li, Nan Wu, Zhouxuan Wen, Jiuxiang Xie, Hongyi Ma, and Shoudong Zhang. 2022. "Main Factors Influencing the Gut Microbiota of Datong Yaks in Mixed Group" Animals 12, no. 14: 1777. https://doi.org/10.3390/ani12141777
APA StyleQin, W., Li, S., Wu, N., Wen, Z., Xie, J., Ma, H., & Zhang, S. (2022). Main Factors Influencing the Gut Microbiota of Datong Yaks in Mixed Group. Animals, 12(14), 1777. https://doi.org/10.3390/ani12141777






