Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
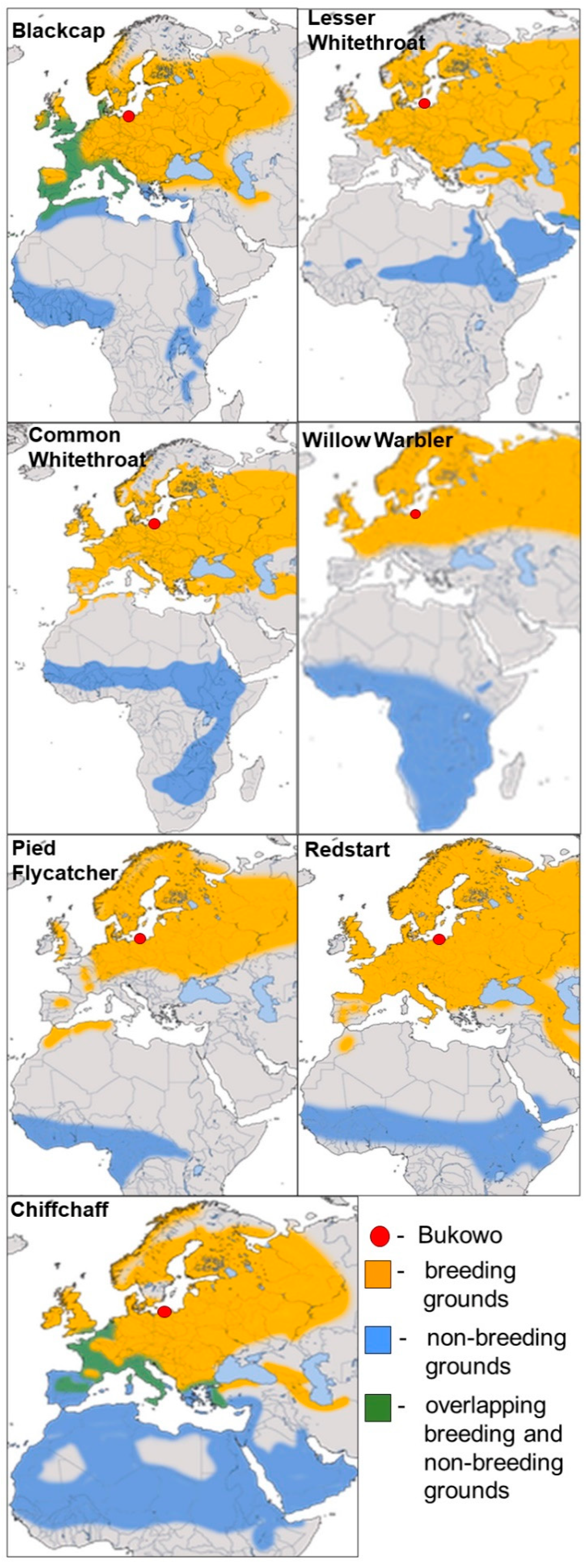
2.1. Species Selected for Analyses
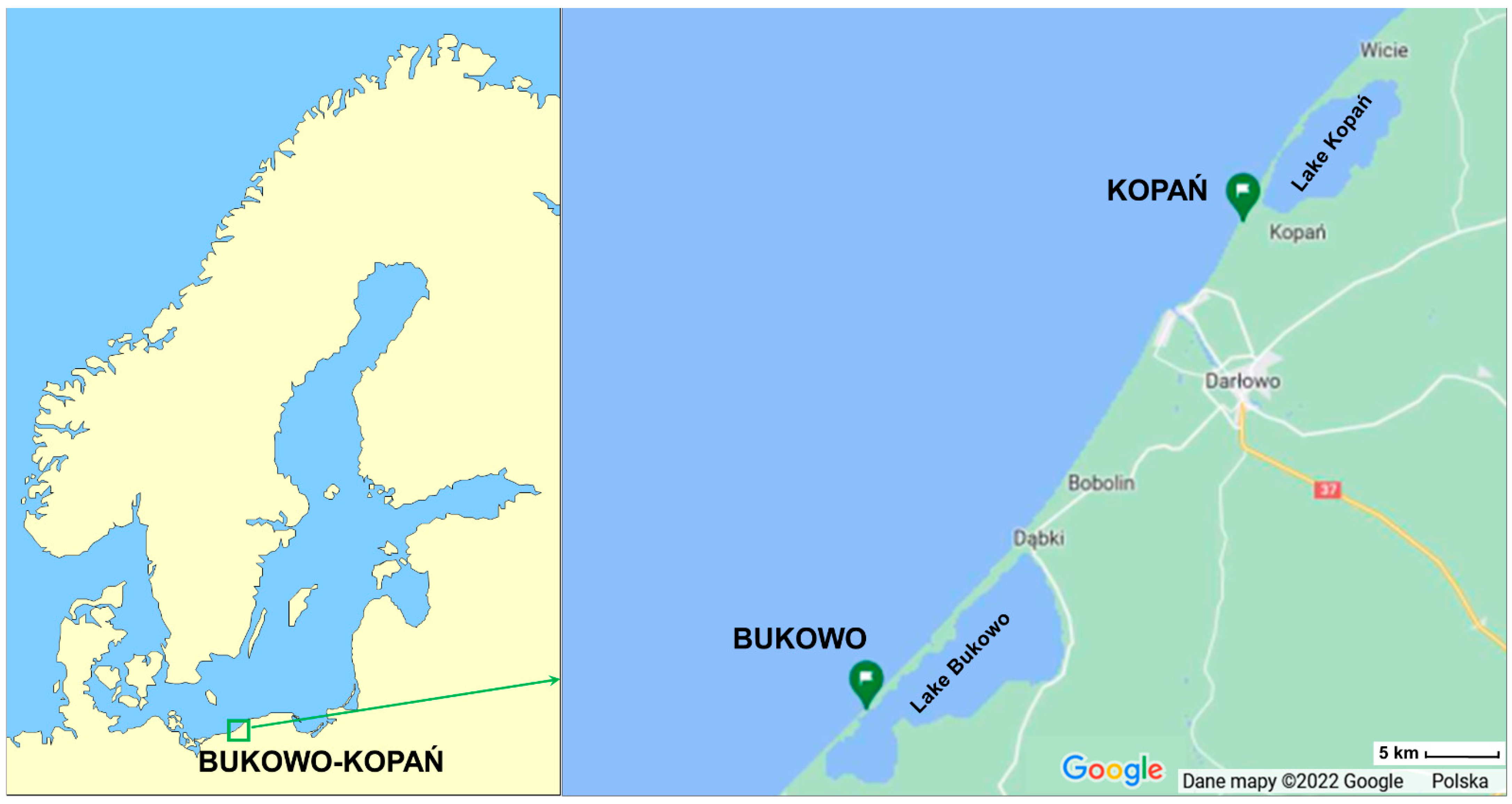
2.2. Study Site and Sampling
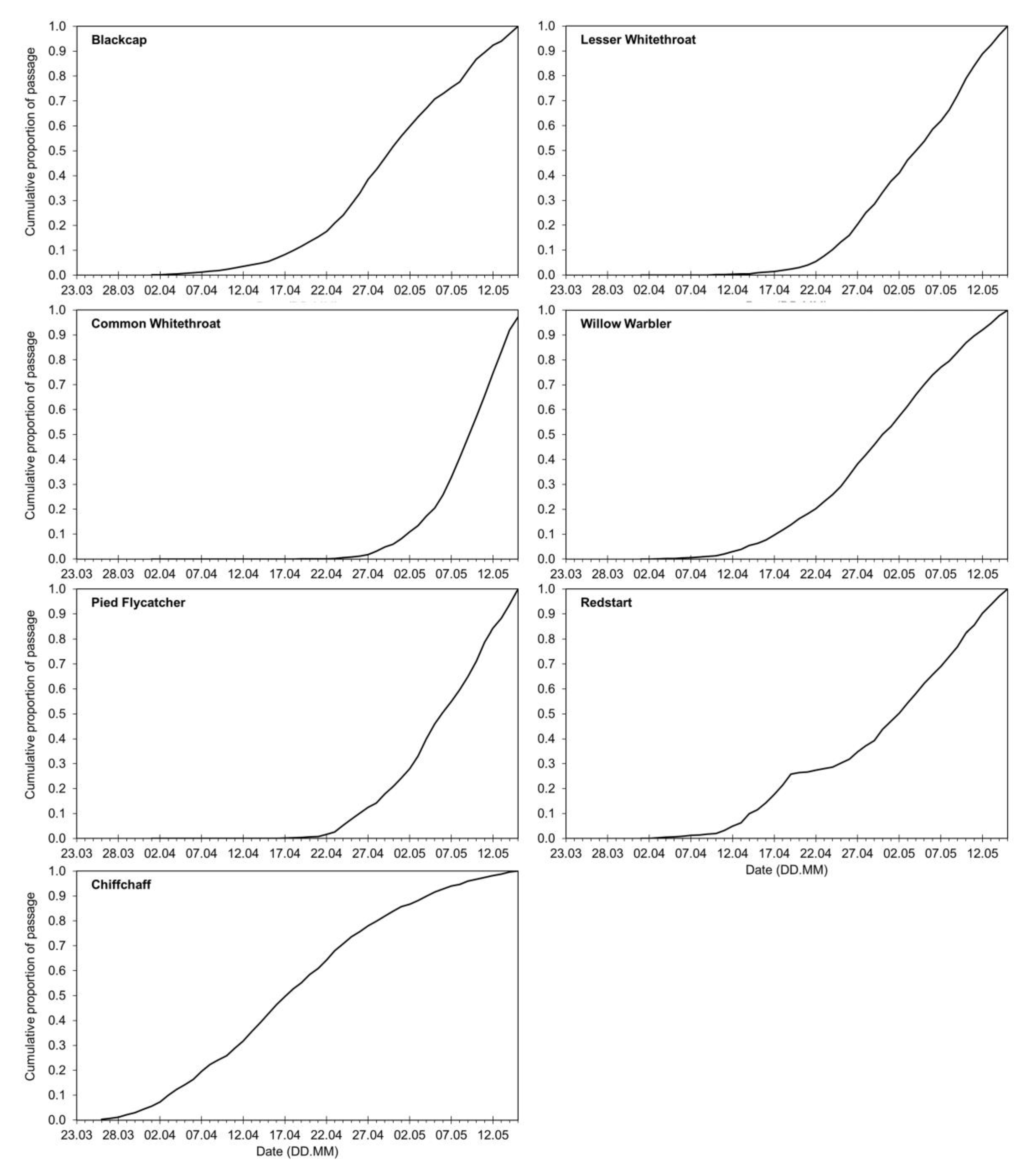
2.3. Calculating the Annual Anomaly of Spring Migration at Bukowo for Each Species
2.4. Ranges of Months When Each Climate Index Might Influence a Migrant
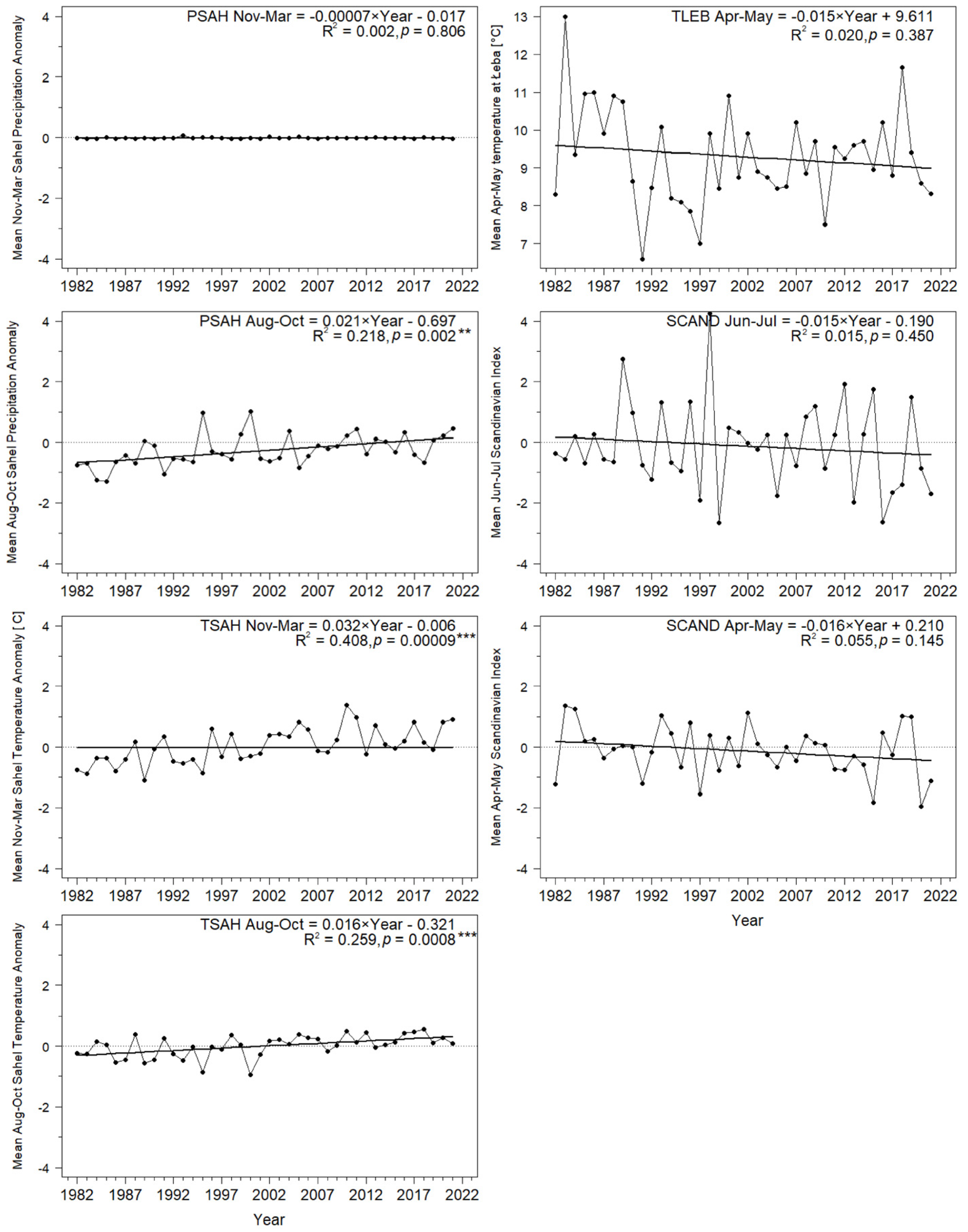
2.5. Climate Indices
2.5.1. North Atlantic Oscillation (NAO)
2.5.2. Indian Ocean Dipole (IOD)
2.5.3. Southern Oscillation (SOI)
2.5.4. Sahel Precipitation Index (PSAH)
2.5.5. Sahel Temperature Anomaly (TSAH)
2.5.6. Local Temperatures at the Baltic Coast (TLEB)
2.5.7. Scandinavian Pattern (SCAND)
2.6. Statistical Analyses
3. Results and Discussion
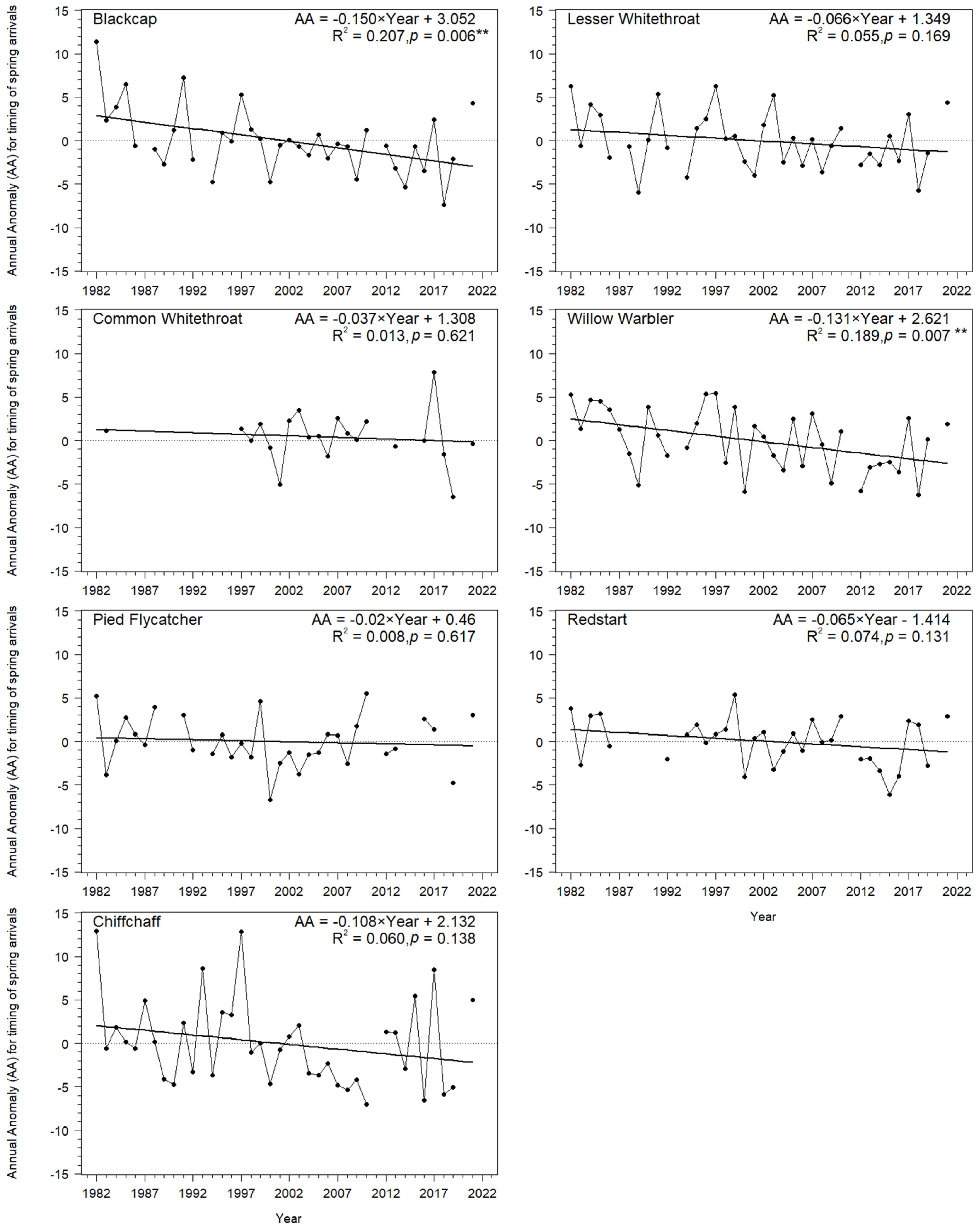
3.1. Long-Term Trends in Timing of Migrants’ Spring Passage at the Baltic Sea Coast
3.2. Relationships between Timing of Migrants’ Spring Arrival in Europe and the Large-Scale Climate Indices
3.2.1. Combined Influence of Climate Indices on Spring Migration of the Seven Migrants at Bukowo
3.2.2. Influence of the North Atlantic Oscillation (NAO)
3.2.3. Influence of the Indian Ocean Dipole (IOD)
3.2.4. Influence of the Southern Oscillation Index (SOI)
3.2.5. Influence of Precipitation (PSAH) and Temperatures (TSAH) in the Sahel on Migrants
3.2.6. Influence of the Local Temperatures (TLEB) during Migration
3.2.7. Influence of the Scandinavian Pattern (SCAND) during Summer and Spring
3.3. Carry-Over Effects of the Condition of Migrants and Timing at Life Stages Prior to Spring Migration Pattern
- Birds’ condition during stay and on departure from the wintering grounds.Climate conditions at the wintering grounds influence the body condition of birds, which then has carry-over effects on the timing of their departure and their condition on arrival at the breeding grounds [49,50,51,52,126,132]. Abundant food enables migrants to replace their flight feathers efficiently and produce good-quality feathers that will withstand the rigours of migration [48,52,133]. Early moult of flight feathers leaves the migrants sufficient time for efficient pre-migratory fuelling [48,52,133]. During wet winters in the tropics, migrants were able to fuel faster and accumulate larger fuel loads and arrive at the breeding grounds the following spring in better condition than in dry years [24,49,51,134];
- Timing of departure from the wintering grounds.After wet winters, the migrants are able to depart from the wintering grounds earlier, and thus arrive earlier at the breeding grounds, than after dry winters [22,23,49,51,134]. In both moist and dry wintering habitats, above average rainfall before spring migration advances the migrants’ departure [24,49,50,134,135,136];
- Duration and frequency of stopovers.Large fuel reserves on departure from the wintering grounds enable birds to migrate faster, with fewer and shorter stopovers [14,25,126]. Favourable conditions during the stopovers on southward migration in northern and eastern Africa, through which insectivorous Lesser Whitethroats and Bonelli’s Warblers Phylloscopus orientalis migrate, had a carry-over effect the following spring, more than six months later; early arrival was observed at a stopover site in Eilat, Israel [137]. Unfavourable conditions on route delay passage, as shown by longer stopovers and later spring arrival in the breeding grounds of some migrants after the 2011 drought in the Horn of Africa than in the other years [19]. By adjusting the rate of migration to the environmental conditions encountered at stopover sites, migrants appear to be able to fine-tune their arrival time to the conditions at the breeding grounds [34,116];
- Survival of migration and overwintering.Below average rainfall in the wintering grounds decreases the survival rates of migrants [25,135,138,139], which would in turn affect the demography of the breeding population [14,116]. After wet years on the wintering grounds with a high survival rate of migrants, individuals of poor quality are able to successfully complete migration, including immatures less experienced than adults, which potentially results in a delay in the overall mean arrival date at northern sites as compared with dry years [22,116]. The decreasing trends of many trans-Saharan migrants have been attributed to droughts in the Sahel [25,140,141].
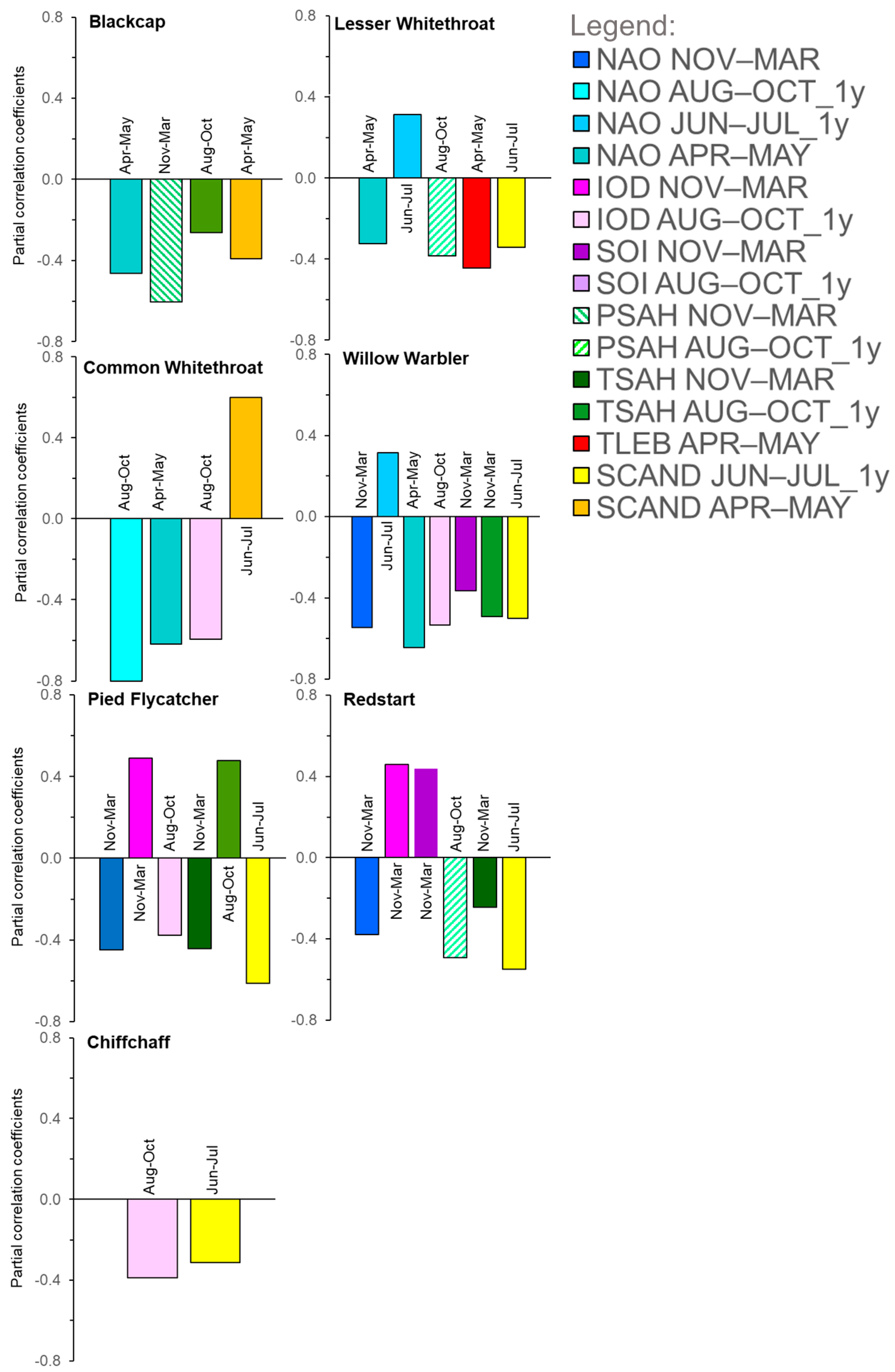
3.4. Combined Effects of Climate Indices on the Patterns of Spring Arrivals of Long-Distance Migrants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Location and Methods of Work at the Bird Ringing Station Bukowo, Baltic Sea Coast, Poland

Appendix B
| Year | Location | N Blackcap | N Lesser Whitethroat | N Common Whitethroat | N Willow Warbler | N Pied Flycatcher | N Common Redstart | N Chiffchaff | N Nets |
|---|---|---|---|---|---|---|---|---|---|
| 1982 | Kopań | 88 | 123 | – | 1016 | 39 | 15 | 90 | 57 |
| 1983 | Kopań | 80 | 186 | 29 | 498 | 36 | 23 | 100 | 57 |
| 1984 | Kopań | 22 | 48 | – | 180 | 16 | – | 52 | 42 |
| 1985 | Kopań | 13 | 42 | – | 189 | 42 | 37 | 39 | 51 |
| 1986 | Kopań | 24 | 12 | – | 62 | 39 | 13 | 29 | 50 |
| 1987 | Kopań | – | – | – | 76 | 14 | – | 42 | 45 |
| 1988 | Kopań | 35 | 25 | – | 64 | 16 | – | 28 | 45 |
| 1989 | Kopań | 11 | 40 | – | 84 | – | – | 48 | 45 |
| 1990 | Kopań | – | 20 | – | 34 | – | – | 31 | 45 |
| 1991 | Kopań | 28 | 50 | – | 52 | 15 | – | 77 | 45 |
| 1992 | Kopań | 16 | 31 | – | 31 | 11 | 12 | 18 | 37 |
| 1993 | Kopań | – | – | – | – | – | – | 73 | – |
| 1994 | Kopań | 60 | 85 | – | 71 | 17 | 25 | 58 | 47 |
| 1995 | Kopań | 35 | 99 | – | 79 | 12 | 31 | 59 | 47 |
| 1996 | Kopań | 64 | 88 | – | 108 | 62 | 16 | 51 | 47 |
| 1997 | Kopań | 110 | 80 | 12 | 161 | 93 | 70 | 63 | 47 |
| 1998 | Kopań | 117 | 112 | – | 80 | 30 | 32 | 92 | 47 |
| 1999 | Kopań | 133 | 80 | 30 | 133 | 78 | 136 | 70 | 47 |
| 2000 | Kopań | 120 | 89 | 34 | 72 | 46 | 63 | 85 | 47 |
| 2001 | Kopań | 180 | 147 | 12 | 95 | 20 | 32 | 91 | 57 |
| 2002 | Kopań | 136 | 99 | 13 | 92 | 20 | 37 | 61 | 47 |
| 2003 | Kopań | 175 | 67 | 12 | 58 | 22 | 20 | 91 | 46 |
| 2004 | Kopań | 163 | 108 | 17 | 99 | 95 | 99 | 92 | 47 |
| 2005 | Kopań | 280 | 228 | 23 | 177 | 79 | 65 | 132 | 47 |
| 2006 | Kopań | 297 | 126 | 19 | 107 | 57 | 54 | 108 | 47 |
| 2007 | Kopań | 212 | 183 | 33 | 147 | 18 | 43 | 87 | 54 |
| 2008 | Kopań | 150 | 182 | 55 | 115 | 27 | 51 | 129 | 50 |
| 2009 | Kopań | 257 | 155 | 30 | 107 | 15 | 53 | 131 | 46 |
| 2010 | Kopań | 288 | 163 | 15 | 85 | 51 | 38 | 148 | 35 |
| 2011 | – | – | – | – | – | – | – | – | – |
| 2012 | Bukowo | 334 | 63 | – | 138 | – | 30 | 89 | 56 |
| 2013 | Bukowo | 391 | 115 | 25 | 85 | 12 | 30 | 88 | 51 |
| 2014 | Bukowo | 224 | 103 | – | 71 | – | 18 | 120 | 48 |
| 2015 | Bukowo | 198 | 62 | – | 42 | – | 11 | 75 | 51 |
| 2016 | Bukowo | 374 | 54 | 12 | 72 | 11 | 28 | 87 | 52 |
| 2017 | Bukowo | 186 | 71 | – | 78 | 21 | 33 | 47 | 52 |
| 2018 | Bukowo | 279 | 89 | 14 | 59 | – | 28 | 110 | 52 |
| 2019 | Bukowo | 295 | 88 | 47 | 73 | 38 | 40 | 138 | 52 |
| 2020 | – | – | – | – | – | – | – | – | – |
| 2021 | Bukowo | 326 | 91 | 33 | 68 | 19 | 39 | 89 | 52 |
| N years | 35 | 36 | 19 | 37 | 32 | 30 | 38 |
| No | Parameter | ß Slope | SE | R2 | t38 | p | ß Slope × 40 Years |
|---|---|---|---|---|---|---|---|
| 1 | NAO Nov–Mar | 0.0026 | 0.0085 | 0.002 | 0.37 | 0.7608 | 0.1040 |
| 2 | NAO Aug–Oct_1y | −0.0077 | 0.0084 | 0.02 | −0.92 | 0.3660 | −0.3080 |
| 3 | NAO Jun–Jul_1y | −0.0223 | 0.0112 | 0.09 | −1.91 | 0.0636 | −0.8920 |
| 4 | NAO Apr–May | −0.0111 | 0.0112 | 0.03 | −0.99 | 0.3293 | −0.4440 |
| 5 | IOD Nov–Mar | 0.0087 | 0.0024 | 0.25 | 3.57 | 0.00098 | 0.3480 |
| 6 | IOD Aug–Oct_1y | 0.0206 | 0.0102 | 0.10 | 2.00 | 0.0522 | 0.8240 |
| 7 | SOI Nov–Mar | 0.0254 | 0.0141 | 0.08 | 1.80 | 0.0792 | 1.0160 |
| 8 | SOI Aug–Oct_1y | 0.0113 | 0.0116 | 0.02 | 0.98 | 0.3360 | 0.4520 |
| 9 | PSAH Aug–Oct_1y | 0.0211 | 0.0066 | 0.22 | 3.26 | 0.0023 | 0.8440 |
| 10 | PSAH Nov–Mar | 0.00007 | 0.0003 | 0.002 | −0.25 | 0.8061 | 0.0028 |
| 11 | TSAH Nov–Mar | 0.0319 | 0.0062 | 0.41 | 5.13 | 0.000009 | 1.2760 |
| 12 | TSAH Aug–Oct_1y | 0.0159 | 0.0044 | 0.26 | 3.65 | 0.0008 | 0.6360 |
| 13 | TLEB Apr–May | −0.0153 | 0.0175 | 0.02 | −0.88 | 0.3873 | −0.6120 |
| 14 | SCAND Jun–Jul_1y | −0.0151 | 0.0198 | 0.02 | −0.76 | 0.4500 | −0.6040 |
| 15 | SCAN Apr–May | −0.0165 | 0.0111 | 0.055 | −1.49 | 0.1451 | −0.6600 |
| Species | ß Slope | SE | R2 | t | p | 40 Years × ß (Days) |
|---|---|---|---|---|---|---|
| Blackcap Sylvia atricapilla | −0.1499 | 0.0503 | 0.2074 | −2.982 | 0.0053 | −6.0 |
| Lesser Whitethroat Curruca curruca | −0.0663 | 0.0471 | 0.0550 | −1.406 | 0.1690 | −2.7 |
| Common Whitethroat Curruca communis | −0.0367 | 0.0731 | 0.0131 | −0.503 | 0.6211 | −1.5 |
| Willow Warbler Phylloscopus trochilus | −0.1312 | 0.0459 | 0.1892 | −2.858 | 0.0071 | −5.2 |
| Pied Flycatcher Ficedula hypoleuca | −0.0238 | 0.0471 | 0.0085 | −0.506 | 0.6169 | −1.0 |
| Common Redstart Phoenicurus phoenicurus | −0.0647 | 0.0417 | 0.0742 | −1.551 | 0.1310 | −2.6 |
| Chiffchaff Phylloscopus collybita | −0.1079 | 0.0711 | 0.0601 | −1.517 | 0.1381 | −4.3 |
| NAO Aug–Oct_1y | NAO Jun–Jul_1y | NAO Apr–May | IOD Nov–Mar | IOD Aug–Oct_1y | SOI Nov–Mar | SOI Aug–Oct_1y | PSAH Nov–Mar | PSAH Aug–Oct_1y | TLEB Apr–May | SCAND Jun–Jul_1y | SCAND Apr–May | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAO Nov–Mar | −0.07 | 0.10 | 0.06 | 0.02 | 0.15 | −0.16 | −0.30 | 0.08 | 0.16 | 0.24 | 0.03 | −0.06 |
| NAO Aug–Oct_1y | 0.18 | −0.20 | −0.17 | −0.09 | −0.12 | 0.02 | −0.12 | −0.20 | −0.10 | 0.23 | 0.33 | |
| NAO Jun–Jul_1y | 0.04 | −0.09 | −0.09 | −0.12 | −0.05 | 0.05 | −0.11 | 0.11 | 0.19 | 0.14 | ||
| NAO Apr–May | −0.30 | −0.17 | 0.02 | 0.19 | 0.13 | −0.10 | 0.28 | −0.05 | 0.09 | |||
| IOD Nov–Mar | 0.63 | −0.04 | −0.23 | −0.18 | 0.43 | −0.09 | 0.24 | −0.07 | ||||
| IOD Aug–Oct_1y | −0.27 | −0.45 | −0.22 | 0.28 | 0.23 | 0.08 | 0.12 | |||||
| SOI Nov–Mar | 0.86 | −0.09 | 0.28 | −0.17 | 0.02 | −0.24 | ||||||
| SOI Aug–Oct_1y | −0.11 | 0.13 | −0.12 | 0.07 | −0.18 | |||||||
| PSAH Nov–Mar | −0.09 | −0.07 | −0.06 | 0.16 | ||||||||
| PSAH Aug–Oct_1y | −0.09 | −0.07 | −0.23 | |||||||||
| TLEB Apr–May | 0.17 | 0.53 | ||||||||||
| SCAND Jun–Jul_1y | 0.23 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,20 = 2.56, AdjR2 = 40.9% | |||||||
| NAO_NOV_MAR | −0.16 | 0.20 | −0.81 | 0.4268 | 2.37 | 0.03 | −0.05 |
| NAO_AUG_OCT_1y | 0.06 | 0.17 | 0.36 | 0.7222 | 1.80 | 0.01 | 0.22 |
| NAO_JUN_JUL_1y | 0.04 | 0.14 | 0.31 | 0.7622 | 1.22 | 0.00 | 0.12 |
| NAO_APR_MAY | −0.42 | 0.16 | −2.70 | 0.0139 | 1.47 | 0.27 | −0.53 |
| IOD_NOV_MAR | 0.31 | 0.24 | 1.30 | 0.2096 | 3.49 | 0.08 | 0.34 |
| IOD_AUG_OCT_1y | −0.26 | 0.20 | −1.25 | 0.2266 | 2.55 | 0.07 | −0.22 |
| SOI_NOV_MAR | −0.26 | 0.31 | −0.84 | 0.4110 | 5.70 | 0.03 | −0.13 |
| SOI_AUG_OCT_1y | 0.16 | 0.34 | 0.47 | 0.6406 | 7.10 | 0.01 | 0.16 |
| PSAH_NOV_MAR | −0.02 | 0.15 | −0.11 | 0.9135 | 1.33 | 0.00 | 0.05 |
| PSAH_AUG_OCT_1y | −0.54 | 0.25 | −2.18 | 0.0415 | 3.79 | 0.19 | −0.25 |
| TSAH_NOV_MAR | −0.15 | 0.27 | −0.56 | 0.5809 | 4.59 | 0.02 | 0.03 |
| TSAH_AUG_OCT_1y | −0.25 | 0.29 | −0.89 | 0.3860 | 5.01 | 0.04 | 0.03 |
| TLEB_APR_MAY | −0.02 | 0.20 | −0.10 | 0.9237 | 2.41 | 0.00 | 0.09 |
| SCAND_JUN_JUL_1y | −0.26 | 0.17 | −1.54 | 0.1390 | 1.71 | 0.11 | −0.38 |
| SCAND_APR_MAY | −0.33 | 0.19 | −1.72 | 0.1017 | 2.28 | 0.13 | −0.48 |
| YearN | 0.00 | 0.01 | −0.53 | 0.6028 | 1.23 | 0.01 | −0.46 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,3 = 1.20, AdjR2 = 0.14% | |||||||
| NAO_NOV_MAR | 0.99 | 1.10 | 0.90 | 0.4324 | 26.70 | 0.21 | 0.55 |
| NAO_AUG_OCT_1y | −1.43 | 0.48 | −2.97 | 0.0593 | 7.06 | 0.75 | −0.60 |
| NAO_JUN_JUL_1y | −0.34 | 0.45 | −0.75 | 0.5074 | 5.19 | 0.16 | −0.30 |
| NAO_APR_MAY | −0.51 | 0.74 | −0.69 | 0.5406 | 12.89 | 0.14 | −0.13 |
| SOI_NOV_MAR | −1.38 | 1.11 | −1.24 | 0.3024 | 36.00 | 0.34 | −0.30 |
| SOI_AUG_OCT_1y | 1.18 | 1.19 | 0.99 | 0.3934 | 35.80 | 0.25 | 0.47 |
| IOD_NOV_MAR | 0.53 | 0.79 | 0.66 | 0.5536 | 12.95 | 0.13 | 0.49 |
| IOD_AUG_OCT_1y | −0.77 | 0.86 | −0.90 | 0.4354 | 14.33 | 0.21 | −0.11 |
| PSAH_NOV_MAR | −0.25 | 0.77 | −0.32 | 0.7707 | 16.73 | 0.03 | −0.29 |
| PSAH_AUG_OCT_1y | −0.46 | 1.15 | −0.40 | 0.7142 | 28.32 | 0.05 | −0.34 |
| TSAH_AUG_OCT_1y | −1.04 | 1.39 | −0.75 | 0.5098 | 38.27 | 0.16 | −0.35 |
| TSAH_NOV_MAR | 0.49 | 0.95 | 0.51 | 0.6437 | 20.95 | 0.08 | 0.45 |
| SCAND_JUN_JUL_1y | 0.17 | 0.94 | 0.18 | 0.8677 | 16.42 | 0.01 | −0.19 |
| SCAND_APR_MAY | 1.09 | 1.35 | 0.81 | 0.4778 | 42.65 | 0.18 | 0.48 |
| TLEB_APR_MAY | −0.79 | 1.80 | −0.44 | 0.6922 | 84.41 | 0.06 | −0.35 |
| YearN | 0.01 | 0.02 | 0.40 | 0.7176 | 9.19 | 0.05 | −0.34 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,3 = 2.56, AdjR2 = 28.5% | |||||||
| NAO_NOV_MAR | −0.45 | 0.23 | −1.97 | 0.0662 | 2.36 | 0.20 | −0.37 |
| NAO_AUG_OCT_1y | 0.05 | 0.21 | 0.25 | 0.8078 | 1.90 | 0.00 | 0.20 |
| NAO_JUN_JUL_1y | −0.21 | 0.17 | −1.18 | 0.2540 | 1.37 | 0.08 | −0.35 |
| NAO_APR_MAY | −0.03 | 0.18 | −0.19 | 0.8542 | 1.47 | 0.00 | −0.06 |
| SOI_NOV_MAR | 0.24 | 0.38 | 0.63 | 0.5382 | 6.61 | 0.02 | 0.22 |
| SOI_AUG_OCT_1y | −0.22 | 0.45 | −0.49 | 0.6310 | 8.97 | 0.01 | 0.03 |
| IOD_NOV_MAR | 0.49 | 0.31 | 1.57 | 0.1364 | 4.29 | 0.13 | 0.50 |
| IOD_AUG_OCT_1y | −0.30 | 0.24 | −1.24 | 0.2334 | 2.61 | 0.09 | −0.18 |
| PSAH_NOV_MAR | −0.04 | 0.20 | −0.23 | 0.8247 | 1.73 | 0.00 | 0.01 |
| PSAH_AUG_OCT_1y | −0.08 | 0.30 | −0.27 | 0.7920 | 4.04 | 0.00 | 0.25 |
| TSAH_NOV_MAR | −0.46 | 0.33 | −1.37 | 0.1908 | 4.98 | 0.10 | −0.19 |
| TSAH_AUG_OCT_1y | 0.39 | 0.33 | 1.16 | 0.2622 | 4.92 | 0.08 | 0.52 |
| TLEB_APR_MAY | 0.10 | 0.24 | 0.43 | 0.6704 | 2.54 | 0.01 | 0.18 |
| SCAND_JUN_JUL_1y | −0.56 | 0.25 | −2.26 | 0.0384 | 2.74 | 0.24 | −0.62 |
| SCAND_APR_MAY | −0.22 | 0.24 | −0.90 | 0.3796 | 2.54 | 0.05 | −0.26 |
| YearN | 0.00 | 0.01 | −0.59 | 0.5654 | 1.25 | 0.02 | −0.60 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,22 = 1.54, AdjR2 = 18.5% | |||||||
| NAO_NOV_MAR | 0.13 | 0.23 | 0.58 | 0.5710 | 1.24 | 0.00 | 0.04 |
| NAO_AUG_OCT_1y | −0.21 | 0.19 | −1.09 | 0.2883 | 2.44 | 0.00 | −0.05 |
| NAO_JUN_JUL_1y | 0.04 | 0.16 | 0.26 | 0.7938 | 1.47 | 0.12 | −0.35 |
| NAO_APR_MAY | −0.31 | 0.18 | −1.73 | 0.0969 | 2.28 | 0.08 | −0.27 |
| IOD_NOV_MAR | 0.34 | 0.27 | 1.27 | 0.2188 | 2.36 | 0.01 | 0.11 |
| IOD_AUG_OCT_1y | −0.33 | 0.23 | −1.43 | 0.1676 | 3.39 | 0.07 | 0.26 |
| SOI_NOV_MAR | −0.30 | 0.34 | −0.88 | 0.3880 | 5.35 | 0.03 | −0.19 |
| SOI_AUG_OCT_1y | 0.32 | 0.38 | 0.85 | 0.4048 | 1.74 | 0.05 | −0.23 |
| PSAH_NOV_MAR | 0.09 | 0.18 | 0.50 | 0.6245 | 2.49 | 0.08 | −0.29 |
| PSAH_AUG_OCT_1y | −0.56 | 0.28 | −1.96 | 0.0624 | 6.77 | 0.03 | 0.17 |
| TSAH_AUG_OCT_1y | −0.46 | 0.33 | −1.38 | 0.1817 | 1.26 | 0.00 | 0.05 |
| TSAH_NOV_MAR | 0.06 | 0.32 | 0.20 | 0.8455 | 1.68 | 0.04 | −0.20 |
| TLEB_APR_MAY | −0.05 | 0.23 | −0.22 | 0.8310 | 1.55 | 0.01 | 0.09 |
| SCAND_JUN_JUL_1y | −0.18 | 0.19 | −0.96 | 0.3485 | 3.74 | 0.15 | −0.36 |
| SCAND_APR_MAY | −0.31 | 0.22 | −1.38 | 0.1804 | 5.09 | 0.08 | −0.27 |
| YearN | 0.00 | 0.01 | 0.05 | 0.9595 | 4.71 | 0.00 | 0.03 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,20 = 1.65, AdjR2 = 22.9% | |||||||
| NAO_NOV_MAR | −0.09 | 0.23 | −0.38 | 0.7046 | 2.37 | 0.01 | 0.00 |
| NAO_AUG_OCT_1y | −0.05 | 0.20 | −0.26 | 0.7982 | 1.80 | 0.00 | 0.02 |
| NAO_JUN_JUL_1y | 0.24 | 0.16 | 1.46 | 0.1591 | 1.22 | 0.10 | 0.34 |
| NAO_APR_MAY | −0.23 | 0.18 | −1.32 | 0.2017 | 1.47 | 0.08 | −0.27 |
| IOD_NOV_MAR | 0.25 | 0.27 | 0.92 | 0.3688 | 3.48 | 0.04 | 0.23 |
| IOD_AUG_OCT_1y | −0.28 | 0.23 | −1.18 | 0.2505 | 2.55 | 0.07 | −0.22 |
| SOI_NOV_MAR | −0.20 | 0.35 | −0.56 | 0.5794 | 5.71 | 0.02 | −0.08 |
| SOI_AUG_OCT_1y | 0.00 | 0.39 | 0.01 | 0.9921 | 7.10 | 0.00 | 0.03 |
| PSAH_NOV_MAR | −0.10 | 0.17 | −0.56 | 0.5785 | 1.33 | 0.02 | −0.09 |
| PSAH_AUG_OCT_1y | −0.41 | 0.29 | −1.44 | 0.1643 | 3.78 | 0.09 | −0.17 |
| TSAH_AUG_OCT_1y | −0.30 | 0.33 | −0.91 | 0.3727 | 5.00 | 0.04 | −0.06 |
| TSAH_NOV_MAR | 0.24 | 0.31 | 0.75 | 0.4597 | 4.58 | 0.03 | 0.25 |
| TLEB_APR_MAY | −0.19 | 0.23 | −0.85 | 0.4068 | 2.41 | 0.03 | −0.13 |
| SCAND_JUN_JUL_1y | −0.32 | 0.19 | −1.66 | 0.1123 | 1.71 | 0.12 | −0.37 |
| SCAND_APR_MAY | −0.14 | 0.22 | −0.65 | 0.5246 | 2.27 | 0.02 | −0.21 |
| YearN | 0.00 | 0.01 | −0.33 | 0.7463 | 1.23 | 0.01 | −0.29 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,21 = 4.81, AdjR2 = 62.8% | |||||||
| NAO_NOV_MAR | −0.21 | 0.15 | −1.38 | 0.1819 | 2.36 | 0.08 | −0.28 |
| NAO_AUG_OCT_1y | −0.16 | 0.13 | −1.19 | 0.2485 | 1.80 | 0.06 | −0.24 |
| NAO_JUN_JUL_1y | 0.18 | 0.11 | 1.59 | 0.1278 | 1.25 | 0.11 | 0.33 |
| NAO_APR_MAY | −0.48 | 0.12 | −3.87 | 0.0009 | 1.55 | 0.42 | −0.64 |
| IOD_NOV_MAR | 0.06 | 0.18 | 0.34 | 0.7354 | 3.36 | 0.01 | 0.07 |
| IOD_AUG_OCT_1y | −0.30 | 0.16 | −1.86 | 0.0762 | 2.56 | 0.14 | −0.37 |
| SOI_NOV_MAR | −0.24 | 0.23 | −1.02 | 0.3185 | 5.48 | 0.05 | −0.21 |
| SOI_AUG_OCT_1y | 0.09 | 0.26 | 0.33 | 0.7463 | 6.81 | 0.01 | 0.07 |
| PSAH_NOV_MAR | −0.22 | 0.12 | −1.87 | 0.0752 | 1.32 | 0.14 | −0.37 |
| PSAH_AUG_OCT_1y | −0.37 | 0.19 | −1.92 | 0.0682 | 3.73 | 0.15 | −0.35 |
| TSAH_AUG_OCT_1y | −0.35 | 0.22 | −1.56 | 0.1332 | 4.94 | 0.10 | −0.29 |
| TSAH_NOV_MAR | −0.14 | 0.22 | −0.67 | 0.5094 | 4.61 | 0.02 | −0.14 |
| TLEB_APR_MAY | −0.30 | 0.16 | −1.94 | 0.0659 | 2.42 | 0.15 | −0.38 |
| SCAND_JUN_JUL_1y | −0.40 | 0.13 | −3.12 | 0.0052 | 1.67 | 0.32 | −0.56 |
| SCAND_APR_MAY | 0.20 | 0.15 | 1.32 | 0.2005 | 2.27 | 0.08 | 0.27 |
| YearN | 0.00 | 0.00 | 0.00 | 0.9962 | 1.24 | 0.00 | −0.01 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Full Model: F16,20 = 1.08, AdjR2 = 3.3% | |||||||
| NAO_NOV_MAR | −0.28 | 0.26 | −1.06 | 0.3031 | 2.53 | 0.05 | −0.42 |
| NAO_AUG_OCT_1y | 0.02 | 0.21 | 0.12 | 0.9092 | 1.71 | 0.00 | −0.14 |
| NAO_JUN_JUL_1y | 0.09 | 0.18 | 0.50 | 0.6237 | 1.24 | 0.01 | 0.08 |
| NAO_APR_MAY | −0.13 | 0.20 | −0.66 | 0.5178 | 1.55 | 0.02 | −0.23 |
| SOI_NOV_MAR | −0.08 | 0.40 | −0.20 | 0.8462 | 5.94 | 0.00 | −0.13 |
| SOI_AUG_OCT_1y | 0.06 | 0.44 | 0.14 | 0.8913 | 7.21 | 0.00 | −0.04 |
| IOD_NOV_MAR | 0.18 | 0.30 | 0.59 | 0.5605 | 3.34 | 0.02 | 0.12 |
| IOD_AUG_OCT_1y | −0.27 | 0.26 | −1.04 | 0.3112 | 2.53 | 0.05 | −0.36 |
| PSAH_NOV_MAR | 0.03 | 0.19 | 0.14 | 0.8877 | 1.37 | 0.00 | −0.04 |
| PSAH_AUG_OCT_1y | 0.27 | 0.32 | 0.86 | 0.3994 | 3.71 | 0.04 | −0.09 |
| TSAH_ NOV_MAR | 0.00 | 0.36 | 0.00 | 0.9987 | 4.88 | 0.00 | −0.22 |
| TSAH_ AUG_OCT_1y | 0.10 | 0.36 | 0.27 | 0.7914 | 4.96 | 0.00 | −0.21 |
| SCAND_JUN_JUL_1y | −0.49 | 0.21 | −2.31 | 0.0316 | 1.66 | 0.21 | −0.51 |
| SCAND_APR_MAY | 0.08 | 0.25 | 0.33 | 0.7430 | 2.32 | 0.01 | 0.23 |
| TLEB_APR_MAY | 0.05 | 0.26 | 0.18 | 0.8615 | 2.51 | 0.00 | −0.08 |
| YearN | 0.00 | 0.01 | 0.63 | 0.5353 | 1.23 | 0.02 | 0.56 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| Best Model: F4,32 = 9.82, AdjR2 = 49.5% | |||||||
| NAO_APR_MAY | −0.46 | 0.12 | −3.81 | 0.001 | 1.05 | 0.31 | −0.56 |
| PSAH_AUG_OCT_1y | −0.60 | 0.13 | −4.57 | 0.000 | 1.24 | 0.40 | −0.34 |
| TSAH_AUG_OCT_1y | −0.26 | 0.13 | −2.02 | 0.052 | 1.21 | 0.11 | −0.50 |
| SCAND_APR_MAY | −0.39 | 0.12 | −3.26 | 0.003 | 1.03 | 0.25 | −0.63 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F5,31 = 5.02, AdjR2 = 36.8% | |||||||
| NAO_APR_MAY | −0.46 | 0.12 | −3.81 | 0.001 | 1.05 | 0.31 | −0.56 |
| PSAH_AUG_OCT_1y | −0.60 | 0.13 | −4.57 | 0.000 | 1.24 | 0.40 | −0.34 |
| TSAH_AUG_OCT_1y | −0.26 | 0.13 | −2.02 | 0.052 | 1.21 | 0.11 | −0.50 |
| SCAND_APR_MAY | −0.39 | 0.12 | −3.26 | 0.003 | 1.03 | 0.25 | −0.63 |
| NAO_APR_MAY | −0.46 | 0.12 | −3.81 | 0.001 | 1.05 | 0.31 | −0.56 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F4,15 = 6.93, AdjR2 = 55.5% | |||||||
| NAO_APR_MAY | −0.52 | 0.19 | −2.76 | 0.0147 | 1.59 | 0.34 | −0.62 |
| NAO_AUG_OCT_1y | −0.89 | 0.18 | −5.05 | 0.0001 | 1.82 | 0.63 | −0.81 |
| IOD_AUG_OCT_1y | −0.57 | 0.20 | −2.80 | 0.0135 | 1.56 | 0.34 | −0.59 |
| SCAND_APR_MAY | 0.57 | 0.21 | 2.65 | 0.0182 | 2.05 | 0.32 | 0.60 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F6,26 = 4.113, AdjR2 = 36.8% | |||||||
| NAO_NOV_MAR | −0.31 | 0.15 | −2.09 | 0.0465 | 1.10 | 0.14 | −0.38 |
| IOD_NOV_MAR | 0.58 | 0.22 | 2.64 | 0.0139 | 2.44 | 0.21 | 0.46 |
| SOI_NOV_MAR | 0.37 | 0.15 | 2.49 | 0.0194 | 1.14 | 0.19 | 0.44 |
| PSAH_AUG_OCT_1y | −0.64 | 0.22 | −2.89 | 0.0077 | 2.47 | 0.24 | −0.49 |
| TSAH_AUG_OCT_1y | −0.23 | 0.18 | −1.29 | 0.2080 | 1.60 | 0.06 | −0.25 |
| SCAND_JUN_JUL_1y | −0.59 | 0.18 | −3.37 | 0.0024 | 1.55 | 0.30 | −0.55 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F2,36 = 8.14, AdjR2 = 27.3% | |||||||
| IOD_AUG_OCT_1y | −0.39 | 0.14 | −2.72 | 0.010 | 1.07 | 0.17 | −0.41 |
| SCAND_APR_MAY | −0.31 | 0.14 | −2.20 | 0.035 | 1.07 | 0.12 | −0.34 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F7,30 = 9.79, AdjR2 = 62.4% | |||||||
| NAO_NOV_MAR | −0.43 | 0.12 | −3.55 | 0.001 | 1.45 | 0.30 | −0.54 |
| NAO_JUN_JUL_1y | 0.20 | 0.11 | 1.82 | 0.078 | 1.16 | 0.10 | 0.32 |
| NAO_APR_MAY | −0.49 | 0.11 | −4.63 | 0.000 | 1.11 | 0.42 | −0.65 |
| IOD_AUG_OCT_1y | −0.39 | 0.11 | −3.45 | 0.002 | 1.27 | 0.28 | −0.53 |
| SOI_NOV_MAR | −0.23 | 0.11 | −2.15 | 0.040 | 1.16 | 0.13 | −0.37 |
| TSAH_NOV_MAR | −0.40 | 0.13 | −3.09 | 0.004 | 1.63 | 0.24 | −0.49 |
| SCAND_JUN_JUL_1y | −0.34 | 0.11 | −3.16 | 0.004 | 1.11 | 0.25 | −0.50 |
| Explanatory Variable | Estimate | SE | t | p | VIF | R2 | pR |
|---|---|---|---|---|---|---|---|
| AA Best Model: F6,26 = 5.62, AdjR2 = 46.6% | |||||||
| NAO_NOV_MAR | −0.44 | 0.17 | −2.56 | 0.017 | 1.74 | 0.20 | −0.45 |
| IOD_NOV_MAR | 0.53 | 0.18 | 2.86 | 0.008 | 2.04 | 0.24 | 0.49 |
| IOD_AUG_OCT_1y | −0.35 | 0.17 | −2.07 | 0.048 | 1.71 | 0.14 | −0.38 |
| TSAH_ NOV_MAR | −0.54 | 0.21 | −2.52 | 0.018 | 2.76 | 0.20 | −0.44 |
| TSAH_ AUG_OCT_1y | 0.48 | 0.17 | 2.78 | 0.010 | 1.80 | 0.23 | 0.48 |
| SCAND_JUN_JUL_1y | −0.61 | 0.15 | −3.96 | 0.001 | 1.43 | 0.38 | −0.61 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 5 | 84.36 | 0.00 | 0.22 |
| AA~NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 85.11 | 0.75 | 0.15 |
| AA~NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 85.52 | 1.16 | 0.12 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 85.52 | 1.16 | 0.12 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~NAO_APR_MAY+NAO_JUN_JUL_1y+ PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y | 6 | 94.14 | 0.00 | 0.21 |
| AA~NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y | 5 | 94.99 | 0.85 | 0.13 |
| AA~NAO_JUN_JUL_1y+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y | 5 | 95.26 | 1.13 | 0.12 |
| AA~NAO_APR_MAY+PSAH_AUG_OCT_1y | 4 | 95.34 | 1.21 | 0.11 |
| AA~NAO_APR_MAY+NAO_JUN_JUL_1y+ PSAH_AUG_OCT_1y | 5 | 95.74 | 1.61 | 0.09 |
| AA~PSAH_AUG_OCT_1y | 3 | 95.79 | 1.65 | 0.09 |
| AA~NAO_JUN_JUL_1y+ PSAH_AUG_OCT_1y | 4 | 95.92 | 1.78 | 0.09 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+SOI_NOV_MAR | 5 | 96.03 | 1.90 | 0.08 |
| AA~NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y | 7 | 96.08 | 1.94 | 0.08 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_AUG_OCT_1y+SCAND_APR_MAY | 5 | 47.68 | 0.00 | 0.38 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 48.67 | 0.99 | 0.23 |
| AA~IOD_AUG_OCT_1y+NAO_AUG_OCT_1y+SOI_AUG_OCT_1y | 4 | 48.76 | 1.08 | 0.22 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_AUG_OCT_1y+SCAND_APR_MAY+YearN | 6 | 49.47 | 1.79 | 0.16 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TSAH_NOV_MAR | 7 | 84.84 | 0.00 | 0.30 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_AUG_OCT_1y+TSAH_NOV_MAR | 7 | 85.57 | 0.74 | 0.21 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR | 6 | 85.79 | 0.95 | 0.19 |
| AA~IOD_NOV_MAR+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR+ TSAH_NOV_MAR | 8 | 86.11 | 1.28 | 0.16 |
| AA~IOD_NOV_MAR+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_AUG_OCT_1y+ TSAH_NOV_MAR | 8 | 86.16 | 1.33 | 0.15 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TSAH_NOV_MAR | 7 | 84.84 | 0.00 | 0.30 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_AUG_OCT_1y+TSAH_NOV_MAR | 7 | 85.57 | 0.74 | 0.21 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR | 6 | 85.79 | 0.95 | 0.19 |
| AA~IOD_NOV_MAR+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR+ TSAH_NOV_MAR | 8 | 86.11 | 1.28 | 0.16 |
| AA~IOD_NOV_MAR+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_AUG_OCT_1y+ TSAH_NOV_MAR | 8 | 86.16 | 1.33 | 0.15 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~IOD_AUG_OCT_1y+SCAND_APR_MAY | 3 | 99.41 | 0.00 | 0.12 |
| AA~IOD_AUG_OCT_1y+PSAH_AUG_OCT_1y+SCAND_APR_MAY | 4 | 99.86 | 0.45 | 0.09 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+SCAND_APR_MAY | 4 | 100.46 | 1.04 | 0.07 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 100.47 | 1.05 | 0.07 |
| AA~IOD_AUG_OCT_1y+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 5 | 100.59 | 1.17 | 0.06 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY | 5 | 100.62 | 1.21 | 0.06 |
| AA~IOD_AUG_OCT_1y+NAO_JUN_JUL_1y+SCAND_APR_MAY | 4 | 100.63 | 1.21 | 0.06 |
| AA~IOD_AUG_OCT_1y+SCAND_APR_MAY+SOI_AUG_OCT_1y | 4 | 100.75 | 1.33 | 0.06 |
| AA~IOD_AUG_OCT_1y+SCAND_APR_MAY+SOI_NOV_MAR | 4 | 100.87 | 1.46 | 0.06 |
| AA~PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 4 | 100.92 | 1.51 | 0.05 |
| AA~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_AUG_OCT_1y+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 7 | 101.01 | 1.60 | 0.05 |
| AA~IOD_AUG_OCT_1y+SCAND_APR_MAY+TSAH_NOV_MAR | 4 | 101.07 | 1.65 | 0.05 |
| AA~IOD_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_APR_MAY | 4 | 101.14 | 1.73 | 0.05 |
| AA~IOD_AUG_OCT_1y+IOD_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 6 | 101.17 | 1.75 | 0.05 |
| AA~NAO_APR_MAY+PSAH_AUG_OCT_1y+SCAND_APR_MAY+TSAH_AUG_OCT_1y | 5 | 101.18 | 1.77 | 0.05 |
| AA~IOD_AUG_OCT_1y+NAO_JUN_JUL_1y+PSAH_AUG_OCT_1y+SCAND_APR_MAY | 5 | 101.31 | 1.89 | 0.04 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TSAH_NOV_MAR | 8 | 81.14 | 0.00 | 0.10 |
| A~NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+TLEB_APR_MAY+ TSAH_AUG_OCT_1y | 9 | 81.65 | 0.51 | 0.08 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ TSAH_AUG_OCT_1y | 9 | 81.71 | 0.56 | 0.08 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+ NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TSAH_NOV_MAR | 7 | 81.75 | 0.61 | 0.08 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+TSAH_AUG_OCT_1y | 8 | 82.09 | 0.95 | 0.06 |
| A~NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+TLEB_APR_MAY+TSAH_AUG_OCT_1y | 8 | 82.26 | 1.11 | 0.06 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ TSAH_AUG_OCT_1y | 8 | 82.52 | 1.38 | 0.05 |
| A~NAO_APR_MAY+ PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+TLEB_APR_MAY+TSAH_AUG_OCT_1y | 7 | 82.55 | 1.40 | 0.05 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+TSAH_AUG_OCT_1y | 7 | 82.67 | 1.53 | 0.05 |
| A~NAO_APR_MAY+NAO_JUN_JUL_1y+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+TLEB_APR_MAY+ TSAH_AUG_OCT_1y | 8 | 82.69 | 1.55 | 0.05 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_AUG_OCT_1y+ TSAH_NOV_MAR | 8 | 82.73 | 1.58 | 0.05 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TLEB_APR_MAY+ TSAH_NOV_MAR | 9 | 82.82 | 1.67 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TLEB_APR_MAY+ TSAH_NOV_MAR | 9 | 82.82 | 1.67 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+TSAH_NOV_MAR | 8 | 82.91 | 1.76 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ TLEB_APR_MAY+ TSAH_AUG_OCT_1y | 10 | 82.91 | 1.76 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ SOI_NOV_MAR+ TSAH_AUG_OCT_1y | 10 | 82.93 | 1.78 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+ PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ SOI_NOV_MAR+TSAH_NOV_MAR | 9 | 82.94 | 1.80 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+PSAH_NOV_MAR+SCAND_JUN_JUL_1y+ SOI_AUG_OCT_1y+TSAH_AUG_OCT_1y | 10 | 83.01 | 1.86 | 0.04 |
| A~IOD_AUG_OCT_1y+NAO_APR_MAY+NAO_JUN_JUL_1y+NAO_NOV_MAR+PSAH_AUG_OCT_1y+SCAND_JUN_JUL_1y+SOI_NOV_MAR+ TSAH_NOV_MAR | 9 | 83.13 | 1.98 | 0.04 |
| Model Formula | k | AICc | ΔAICc | Wi |
|---|---|---|---|---|
| AA~IOD_AUG_OCT_1y+IOD_NOV_MAR+ NAO_NOV_MAR+SCAND_JUN_JUL_1y+TSAH_AUG_OCT_1y+ TSAH_NOV_MAR | 7 | 81.77 | 0.00 | 0.22 |
| AA~IOD_AUG_OCT_1y+IOD_NOV_MAR+NAO_JUN_JUL_1y+NAO_NOV_MAR+SCAND_JUN_JUL_1y+TSAH_AUG_OCT_1y+ TSAH_NOV_MAR | 8 | 82.81 | 1.04 | 0.13 |
| AA~IOD_NOV_MAR+ NAO_NOV_MAR+ SCAND_APR_MAY+SCAND_JUN_JUL_1y+TSAH_AUG_OCT_1y+ TSAH_NOV_MAR | 7 | 83.29 | 1.52 | 0.10 |
| AA~NAO_NOV_MAR+ SCAND_APR_MAY+SCAND_JUN_JUL_1y+ TSAH_AUG_OCT_1y+TSAH_NOV_MAR | 6 | 83.30 | 1.53 | 0.10 |
| AA~IOD_AUG_OCT_1y+IOD_NOV_MAR+PSAH_AUG_OCT_1y+ SCAND_JUN_JUL_1y | 5 | 83.32 | 1.56 | 0.10 |
| AA~IOD_NOV_MAR+ NAO_NOV_MAR+SCAND_JUN_JUL_1y+ TSAH_AUG_OCT_1y+TSAH_NOV_MAR | 6 | 83.37 | 1.60 | 0.10 |
| AA~SCAND_APR_MAY+SCAND_JUN_JUL_1y+ TSAH_AUG_OCT_1y | 4 | 83.55 | 1.78 | 0.09 |
| AA~IOD_NOV_MAR+NAO_NOV_MAR+SCAND_JUN_JUL_1y+SOI_NOV_MAR+TSAH_AUG_OCT_1y+ TSAH_NOV_MAR | 7 | 83.67 | 1.90 | 0.08 |
| AA~IOD_AUG_OCT_1y+IOD_NOV_MAR+NAO_JUN_JUL_1y+ PSAH_AUG_OCT_1y+ SCAND_JUN_JUL_1y | 6 | 83.75 | 1.98 | 0.08 |




References
- Sokolov, L.V.; Markovets, M.Y.; Morozov, Y.G. Long-Term Dynamics of the Mean Date of Autumn Migration in Passerines on the Courish Spit of the Baltic Sea. Avian Ecol. Behav. 1999, 2, 1–18. [Google Scholar]
- Cotton, P.A. Avian Migration Phenology and Global Climate Change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehikoinen, A.; Lindén, A.; Karlsson, M.; Andersson, A.; Crewe, T.L.; Dunn, E.H.; Gregory, G.; Karlsson, L.; Kristiansen, V.; Mackenzie, S.; et al. Phenology of the Avian Spring Migratory Passage in Europe and North America: Asymmetric Advancement in Time and Increase in Duration. Ecol. Indic. 2019, 101, 985–991. [Google Scholar] [CrossRef]
- Redlisiak, M.; Remisiewicz, M.; Mazur, A. Sex-Specific Differences in Spring Migration Timing of Song Thrush Turdus philomelos at the Baltic Coast in Relation to Temperatures on the Wintering Grounds. Eur. Zool. J. 2021, 88, 191–203. [Google Scholar] [CrossRef]
- Wood, E.M.; Kellermann, J.L. (Eds.) Phenological Synchrony and Bird Migration. Changing Climate and Seasonal Resources in North America; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Hüppop, O.; Hüppop, K. North Atlantic Oscillation and Timing of Spring Migration in Birds. Proc. R. Soc. B Biol. Sci. 2003, 270, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tryjanowski, P.; Kuźniak, S.; Sparks, T.H. What Affects the Magnitude of Change in First Arrival Dates of Migrant Birds? J. Ornithol. 2005, 146, 200–205. [Google Scholar] [CrossRef]
- Tøttrup, A.P.; Thorup, K.; Rahbek, C. Patterns of Change in Timing of Spring Migration in North European Songbird Populations. J. Avian Biol. 2006, 37, 84–92. [Google Scholar] [CrossRef]
- Sparks, T.; Tryjanowski, P. Patterns of Spring Arrival Dates Differ in Two Hirundines. Clim. Res. 2007, 35, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird Migration Times, Climate Change, and Changing Population Sizes. Glob. Chang. Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Miles, W.T.S.; Bolton, M.; Davis, P.; Dennis, R.; Broad, R.; Robertson, I.; Riddiford, N.J.; Harvey, P.V.; Riddington, R.; Shaw, D.N.; et al. Quantifying Full Phenological Event Distributions Reveals Simultaneous Advances, Temporal Stability and Delays in Spring and Autumn Migration Timing in Long-Distance Migratory Birds. Glob. Chang. Biol. 2017, 23, 1400–1414. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Butchart, S.H.M.; Phillimore, A.B. Temporal Shifts and Temperature Sensitivity of Avian Spring Migratory Phenology: A Phylogenetic Meta-Analysis. J. Anim. Ecol. 2017, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Zaifman, J.; Shan, D.; Ay, A.; Jimenez, A.G. Shifts in Bird Migration Timing in North American Long-Distance and Short-Distance Migrants Are Associated with Climate Change. Int. J. Zool. 2017. [Google Scholar] [CrossRef] [Green Version]
- Gordo, O. Why Are Bird Migration Dates Shifting? A Review of Weather and Climate Effects on Avian Migratory Phenology. Clim. Res. 2007, 35, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Haest, B.; Hüppop, O.; Bairlein, F. Challenging a 15-Year-Old Claim: The North Atlantic Oscillation Index as a Predictor of Spring Migration Phenology of Birds. Glob. Chang. Biol. 2018, 24, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Haest, B.; Hüppop, O.; Bairlein, F. Weather at the Winter and Stopover Areas Determines Spring Migration Onset, Progress, and Advancements in Afro-Palearctic Migrant Birds. Proc. Natl. Acad. Sci. USA 2020, 117, 17056–17062. [Google Scholar] [CrossRef] [PubMed]
- Lerche-Jørgensen, M.; Willemoes, M.; Tøttrup, A.P.; Snell, K.R.S.; Thorup, K. No Apparent Gain from Continuing Migration for More than 3000 Kilometres: Willow Warblers Breeding in Denmark Winter across the Entire Northern Savannah as Revealed by Geolocators. Mov. Ecol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Thorup, K.; Tøttrup, A.P.; Willemoes, M.; Klaassen, R.H.G.; Strandberg, R.; Vega, M.L.; Dasari, H.P.; Araújo, M.B.; Wikelski, M.; Rahbek, C. Resource Tracking within and across Continents in Long-Distance Bird Migrants. Sci. Adv. 2017, 3, e1601360. [Google Scholar] [CrossRef] [Green Version]
- Tøttrup, A.P.; Klaassen, R.H.G.; Kristensen, M.W.; Strandberg, R.; Vardanis, Y.; Lindström, Å.; Rahbek, C.; Alerstam, T.; Thorup, K. Drought in Africa Caused Delayed Arrival of European Songbirds. Science 2012, 338, 1307. [Google Scholar] [CrossRef] [Green Version]
- Hušek, J.; Adamĺk, P.; Cepák, J.; Tryjanowski, P. The Influence of Climate and Population Size on the Distribution of Breeding Dates in the Red-Backed Shrike (Lanius collurio). Ann. Zool. Fennici 2009, 46, 439–450. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Stenseth, N.C.; Matysioková, B. The Indian Ocean Dipole as an Indicator of Climatic Conditions Affecting European Birds. Clim. Res. 2013, 57, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Remisiewicz, M.; Underhill, L.G. Climatic Variation in Africa and Europe Has Combined Effects on Timing of Spring Migration in a Long-Distance Migrant Willow Warbler Phylloscopus trochilus. PeerJ 2020, 8, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remisiewicz, M.; Underhill, L.G. Climate in Africa Sequentially Shapes Spring Passage of Willow Warbler Phylloscopus trochilus across the Baltic Coast. PeerJ 2022, 10, e8770. [Google Scholar] [CrossRef] [PubMed]
- Tomotani, B.M.; Gienapp, P.; de la Hera, I.; Terpstra, M.; Pulido, F.; Visser, M.E. Integrating Causal and Evolutionary Analysis of Life-History Evolution: Arrival Date in a Long-Distant Migrant. Front. Ecol. Evol. 2021, 9, 630823. [Google Scholar] [CrossRef]
- Zwarts, L.; Bijlsma, R.G.; van der Kamp, J.; Wymenga, E. Living on the Edge: Wetlands and Birds in a Changing Sahel; KNNV Publishing: Zeist, The Netherlands, 2009. [Google Scholar]
- Tobolka, M.; Dylewski, L.; Wozna, J.T.; Zolnierowicz, K.M. How Weather Conditions in Non-Breeding and Breeding Grounds Affect the Phenology and Breeding Abilities of White Storks. Sci. Total Environ. 2018, 636, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Saino, N.; Ambrosini, R. Climatic Connectivity between Africa and Europe May Serve as a Basis for Phenotypic Adjustment of Migration Schedules of Trans-Saharan Migratory Birds. Glob. Chang. Biol. 2008, 14, 250–263. [Google Scholar] [CrossRef]
- Fransson, T.; Hall-Karlsson, S. Svensk Ringmärkningsatlas; Naturhistoriska Riksmuseet & Sveriges Ornitologiska Forening: Stockholm, Sweden, 2008; Volume 3. [Google Scholar]
- Bairlein, F.; Dierschke, J.; Dierschke, V.; Salewski, V.; Geiter, O.; Hüppop, K.; Köppen, U.; Fiedler, W. Atlas Des Vogelzugs: Ringfunde Deutscher Brut-Und Gastvögel; AULA-Verlag: Wiebelsheim, Germany, 2014. [Google Scholar]
- Maciąg, T.; Remisiewicz, M.; Nowakowski, J.K.; Redlisiak, M.; Rosińska, K.; Stępniewski, K.; Stępniewska, K.; Szulc, J. Website of the Bird Migration Research Station. Available online: https://en-sbwp.ug.edu.pl/badania/monitoringwyniki/maps-of-ringing-recoveries/ (accessed on 2 June 2022).
- Briedis, M.; Bauer, S.; Adamík, P.; Alves, J.A.; Costa, J.S.; Emmenegger, T.; Gustafsson, L.; Koleček, J.; Krist, M.; Liechti, F.; et al. Broad-Scale Patterns of the Afro-Palaearctic Landbird Migration. Glob. Ecol. Biogeogr. 2020, 29, 722–735. [Google Scholar] [CrossRef]
- Valkama, J.; Saurola, P.; Lehikoinen, A.; Lehikoinen, E.; Piha, M.; Sola, P.; Velmala, W. Suomen Rengastysatlas II. [The Finnish Bird Ringing Atlas, Vol. II]; Finnish Museum of Natural History and Ministry of Environment: Helsinki, Finland, 2014. [Google Scholar]
- Gordo, O.; Sanz, J.J. The Relative Importance of Conditions in Wintering and Passage Areas on Spring Arrival Dates: The Case of Long-Distance Iberian Migrants. J. Ornithol. 2008, 149, 199–210. [Google Scholar] [CrossRef]
- Tøttrup, A.P.; Rainio, K.; Coppack, T.; Lehikoinen, E.; Rahbek, C.; Thorup, K. Local Temperature Fine-Tunes the Timing of Spring Migration in Birds. Integr. Comp. Biol. 2010, 50, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Forchhammer, M.C.; Post, E.; Stenseth, N.C. North Atlantic Oscillation Timing of Long- and Short-Distance Migration. J. Anim. Ecol. 2002, 71, 1002–1014. [Google Scholar] [CrossRef]
- Ahola, M.; Laaksonen, T.; Sippola, K.; Eeva, T.; Rainio, K.; Lehikoinen, E. Variation in Climate Warming along the Migration Route Uncouples Arrival and Breeding Dates. Glob. Chang. Biol. 2004, 10, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- Gordo, O.; Barriocanal, C.; Robson, D. Ecological Impacts of the North Atlantic Oscillation (NAO) in Mediterranean Ecosystems. In Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2011; pp. 153–170. [Google Scholar]
- Karell, P.; Ahola, K.; Karstinen, T.; Valkama, J.; Brommer, J.E. Climate Change Drives Microevolution in a Wild Bird. Nat. Commun. 2011, 2, 207–208. [Google Scholar] [CrossRef] [Green Version]
- Spina, F.; Baillie, S.R.; Bairlein, F.; Fiedler, W.; Thorup, K. The Eurasian African Bird Migration Atlas. Available online: https://migrationatlas.org (accessed on 24 May 2022).
- Saino, N.; Rubolini, D.; Jonzén, N.; Ergon, T.; Montemaggiori, A.; Stenseth, N.C.; Spina, F. Temperature and Rainfall Anomalies in Africa Predict Timing of Spring Migration in Trans-Saharan Migratory Birds. Clim. Res. 2007, 35, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Møller, A.P.; Balbontín, J.; Cuervo, J.J.; Hermosell, I.G.; De Lope, F. Individual Differences in Protandry, Sexual Selection, and Fitness. Behav. Ecol. 2009, 20, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Saino, N.; Ambrosini, R.; Rubolini, D.; von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O.; Lehikoinen, A.; Lehikoinen, E.; . Rainio, K.; et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B Biol. Sci. 2011, 278, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenseth, N.C.; Ottersen, G.; Hurrell, J.W.; Mysterud, A.; Lima, M.; Chan, K.S.; Yoccoz, N.G.; Ådlandsvik, B. Studying Climate Effects on Ecology through the Use of Climate Indices: The North Atlantic Oscillation, El Niño Southern Oscillation and Beyond. Proc. R. Soc. B Biol. Sci. 2003, 270, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Bussière, E.M.S.; Underhill, L.G.; Altwegg, R. Patterns of Bird Migration Phenology in South Africa Suggest Northern Hemisphere Climate as the Most Consistent Driver of Change. Glob. Chang. Biol. 2015, 21, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Ockendon, N.; Leech, D.; Pearce-Higgins, J.W. Climatic Effects on Breeding Grounds Are More Important Drivers of Breeding Phenology in Migrant Birds than Carryover Effects from Wintering Grounds. Biol. Lett. 2013, 9, 20130669. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.E.; Barnard, P.; Poloczanska, E.S.; Hobday, A.J.; Keatley, M.R.; Allsopp, N.; Underhill, L.G. Southern Hemisphere Biodiversity and Global Change: Data Gaps and Strategies. Austral Ecol. 2017, 42, 20–30. [Google Scholar] [CrossRef]
- Redlisiak, M.; Remisiewicz, M.; Nowakowski, J.K. Long-Term Changes in Migration Timing of Song Thrush Turdus philomelos at the Southern Baltic Coast in Response to Temperatures on Route and at Breeding Grounds. Int. J. Biometeorol. 2018, 62, 1595–1605. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Bernitz, Z.; Bernitz, H.; Burman, M.S.; Raijmakers, J.M.H.; Raijmakers, J.H.F.A.; Underhill, L.G.; Rostkowska, A.; Barshep, Y.; Soloviev, S.A.; et al. Contrasting Strategies for Wing-Moult and Pre-Migratory Fuelling in Western and Eastern Populations of Common Whitethroat Sylvia communis. IBIS 2019, 161, 824–838. [Google Scholar] [CrossRef]
- González-Prieto, A.M.; Hobson, K.A. Environmental Conditions on Wintering Grounds and during Migration Influence Spring Nutritional Condition and Arrival Phenology of Neotropical Migrants at a Northern Stopover Site. J. Ornithol. 2013, 154, 1067–1078. [Google Scholar] [CrossRef]
- Ouwehand, J.; Both, C. African Departure Rather than Migration Speed Determines Variation in Spring Arrival in Pied Flycatchers. J. Anim. Ecol. 2017, 86, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Katti, M.; Price, T. Annual Variation in Fat Storage by a Migrant Warbler Overwintering in the Indian Tropics. J. Anim. Ecol. 1999, 68, 815–823. [Google Scholar] [CrossRef]
- Salewski, V.; Altwegg, R.; Erni, B.; Falk, K.H.; Bairlein, F.; Leisler, B. Die Mauser Dreier Paläarktischer Zugvögel Im Westafrikanischen Überwinterungsgebiet. J. Ornithol. 2004, 145, 109–116. [Google Scholar] [CrossRef]
- Marchant, R.; Mumbi, C.; Behera, S.; Yamagata, T. The Indian Ocean Dipole—The Unsung Driver of Climatic Variability in East Africa. Afr. J. Ecol. 2007, 45, 4–16. [Google Scholar] [CrossRef]
- Bueh, C.; Nakamura, H. Scandinavian Pattern and Its Climatic Impact. Q. J. R. Meteorol. Soc. 2007, 133, 2117–2131. [Google Scholar] [CrossRef]
- Trepte, A. Avi-Fauna—Vögel in Deutschland. Available online: https://www.avi-fauna.info/ (accessed on 2 June 2022).
- Cramp, S.; Brooks, D.J. The Birds of the Western Palearctic. Handbook of the Birds of Europe, the Middle East and North Africa. Vol. VI: Warblers; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Tomiałojć, L.; Stawarczyk, T. Awifauna Polski: Rozmieszczenie, Liczebność i Zmiany; PTPP “pro Natura”: Wrocław, Poland, 2003. [Google Scholar]
- Bensch, S.; Bengtsson, G.; Åkesson, S. Patterns of Stable Isotope Signatures in Willow Warbler Phylloscopus trochilus Feathers Collected in Africa. J. Avian Biol. 2006, 37, 323–330. [Google Scholar] [CrossRef]
- Lerche-Jørgensen, M.; Korner-Nievergelt, F.; Tøttrup, A.P.; Willemoes, M.; Thorup, K. Early Returning Long-Distance Migrant Males Do Pay a Survival Cost. Ecol. Evol. 2018, 8, 11434–11449. [Google Scholar] [CrossRef]
- Zhao, T.; Ilieva, M.; Larson, K.; Lundberg, M.; Neto, J.M.; Sokolovskis, K.; Åkesson, S.; Bensch, S. Autumn Migration Direction of Juvenile Willow Warblers (Phylloscopus t. Trochilus and P. t. Acredula) and Their Hybrids Assessed by QPCR SNP Genotyping. Mov. Ecol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Busse, P.; Meissner, W. Bird Ringing Station Manual; De Gruyter Open Ltd.: Warsaw, Poland; Berlin, Germany, 2015. [Google Scholar]
- Nowakowski, J.K.; Stępniewski, K.; Stępniewska, K.; Muś, K.; Szefler, A. Strona www Programu Badawczego “Akcja Bałtycka” [Website of the “Operation Baltic” Research Programme]. Available online: http://akbalt.ug.edu.pl/ (accessed on 30 June 2022).
- Svensson, L. (Ed.) Identification Guide to European Passerines; British Trust for Ornithology: Thetford, Norfolk, UK, 1992. [Google Scholar]
- Demongin, L. Identification Guide to Birds in the Hand; Laurent Demongin: Beauregard-Vendon, France, 2016. [Google Scholar]
- European Climate Assessment & Dataset Royal Netherlands Meteorological Institute (KNMI) European Climate Assessment & Dataset. Available online: https://www.ecad.eu/dailydata/predefinedseries.php (accessed on 2 June 2022).
- Hurrell, J.W. Decadal Trends in the North Atlantic Oscillation: Regional Temperatures and Precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Saji, N.H.; Vinayachandran, P.N. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Scaife, A.A.; Arribas, A.; Blockley, E.; Brookshaw, A.; Clark, R.T.; Dunstone, N.; Eade, R.; Fereday, D.; Folland, C.K.; Gordon, M.; et al. Skillful long-range prediction of European and North American winters. Geophys. Res. Lett. 2014, 5, 6413–6419. [Google Scholar] [CrossRef] [Green Version]
- Comas-Bru, L.; Hernández, A. Reconciling North Atlantic Climate Modes: Revised Monthly Indices for the East Atlantic and the Scandinavian Patterns beyond the 20th Century. Earth Syst. Sci. Data 2018, 10, 2329–2344. [Google Scholar] [CrossRef] [Green Version]
- National Oceanic and Atmospheric Administration US Department of Commerce National Weather Service. Climate Prediction Center. Climate & Weather Linkage. Available online: https://www.cpc.ncep.noaa.gov/ (accessed on 31 May 2022).
- Jones, P.D.; Jonsson, T.; Wheeler, D. Extension to the North Atlantic Oscillation Using Early Instrumental Pressure Observations from Gibraltar and South-West Iceland. Int. J. Climatol. 1997, 17, 1433–1450. [Google Scholar] [CrossRef]
- Osborn, T.J. An Historical and Climatological Note on Snowfalls Associated with Cold Pools in Southern Britain. Weather 2011, 66, 19–21. [Google Scholar] [CrossRef]
- Folland, C.K.; Knight, J.; Linderholm, H.W.; Fereday, D.; Ineson, S.; Hurrel, J.W. The Summer North Atlantic Oscillation: Past, Present, and Future. J. Clim. 2009, 22, 1082–1103. [Google Scholar] [CrossRef]
- Wang, L.; Ting, M. Stratosphere-Troposphere Coupling Leading to Extended Seasonal Predictability of Summer North Atlantic Oscillation and Boreal Climate. Geophys. Res. Lett. 2022, 49, e2021GL096362. [Google Scholar] [CrossRef]
- Osborn, T.J. Recent Variations in the Winter North Atlantic Oscillation. Weather 2006, 61, 353–355. [Google Scholar] [CrossRef]
- Cai, W.; Yang, K.; Wu, L.; Huang, G.; Santoso, A.; Ng, B.; Wang, G.; Yamagata, T. Opposite Response of Strong and Moderate Positive Indian Ocean Dipole to Global Warming. Nat. Clim. Chang. 2021, 11, 27–32. [Google Scholar] [CrossRef]
- Stige, L.C.; Stave, J.; Chan, K.S.; Ciannelli, L.; Pettorelli, N.; Glantz, M.; Herren, H.R.; Stenseth, N.C. The Effect of Climate Variation on Agro-Pastoral Production in Africa. Proc. Natl. Acad. Sci. USA 2006, 103, 3049–3053. [Google Scholar] [CrossRef] [Green Version]
- Hirons, L.; Turner, A. The Impact of Indian Ocean Mean-State Biases in Climate Models on the Representation of the East African Short Rains. J. Clim. 2018, 31, 6611–6631. [Google Scholar] [CrossRef]
- Ashok, K.; Guan, Z.; Yamagata, T. A Look at the Relationship between the ENSO and the Indian Ocean Dipole. J. Meteorol. Soc. Jpn. 2003, 81, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Behera, S.K.; Luo, J.J.; Masson, S.; Rao, S.A.; Sakuma, H.; Yamagata, T. A CGCM Study on the Interaction between IOD and ENSO. J. Clim. 2006, 19, 1688–1705. [Google Scholar] [CrossRef]
- Hong, L.-C.; Lin, H.; Jin, F.-F. Geophysical Research Letters. Geophys. Res. Lett. 2014, 41, 2142–2149. [Google Scholar] [CrossRef]
- Koutavas, A.; Lynch-Stieglitz, J.; Marchitto, T.M.; Sachs, J.P. El Niño-like Pattern in Ice Age Tropical Pacific Sea Surface Temperature. Science 2002, 297, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Moy, C.; Seltzer, G.; Rodbell, D. Variability of El Nino/Southern Oscillation Activity at Millennial Timescales during the Holocene epoch. Nature 2002, 420, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Tudhope, S.; Collins, M. The Past and Future of El Niño. Nature 2003, 424, 261–262. [Google Scholar] [CrossRef]
- Richard, Y.; Fauchereau, N.; Poccard, I.; Rouault, M.; Trzaska, S. 20th Century Droughts in Southern Africa: Spatial and Temporal Variability, Teleconnections with Oceanic and Atmospheric Conditions. Int. J. Climatol. 2001, 21, 873–885. [Google Scholar] [CrossRef]
- McPhaden, M.J.; Santoso, A.; Cai, W. (Eds.) El Niño Southern Oscillation in a Changing Climate; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 253. [Google Scholar]
- Benkenstein, A. Climate Change Adaptation Readiness: Lessons from the 2015/16 El Niño for Climate Readiness in Southern Africa. SAIIA Occas. Pap. 2017, 250, 1–18. [Google Scholar]
- Zhang, R.; Delworth, T.L. Impact of Atlantic Multidecadal Oscillations on India/Sahel Rainfall and Atlantic Hurricanes. Geophys. Res. Lett. 2006, 33, L17712. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, T.M.; Overpeck, J.T.; Anchukaitis, K.J.; Beck, J.W.; Cole, J.E.; Dettman, D.L.; Peck, J.A.; Scholz, C.A.; King, J.W. Atlantic Forcing of Persistent Drought in West Africa. Science 2009, 324, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munemoto, M.; Tachibana, Y. The Recent Trend of Increasing Precipitation in Sahel and the Associated Inter-Hemispheric Dipole of Global SST. Int. J. Climatol. 2012, 32, 1346–1353. [Google Scholar] [CrossRef]
- Joint Institute for the Study of the Atmosphere and the Ocean. Sahel Precipitation Index 1901–2017. Available online: http://research.jisao.washington.edu/data/sahel//sahelprecip19012017 (accessed on 31 May 2022).
- Finch, T.; Pearce-Higgins, J.W.; Leech, D.I.; Evans, K.L. Carry-over Effects from Passage Regions Are More Important than Breeding Climate in Determining the Breeding Phenology and Performance of Three Avian Migrants of Conservation Concern. Biodivers. Conserv. 2014, 23, 2427–2444. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gillies, R.R. Observed Change in Sahel Rainfall, Circulations, African Easterly Waves, and Atlantic Hurricanes since 1979. Int. J. Geophys. 2011, 2011, 259529. [Google Scholar] [CrossRef] [Green Version]
- Klein Tank, A.M.G.; Wijngaard, J.B.; Können, G.P.; Böhm, R.; Demarée, G.; Gocheva, A.; Mileta, M.; Pashiardis, S.; Hejkrlik, L.; Kern-Hansen, C.; et al. Daily Dataset of 20th-Century Surface Air Temperature and Precipitation Series for the European Climate Assessment. Int. J. Climatol. 2002, 22, 1441–1453. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference, version 1.43.6; R Package: Madison, WI, USA, 2019; pp. 1–75. [Google Scholar]
- Kim, S. Ppcor: An R Package for a Fast Calculation to Semi-Partial Correlation Coefficients. Commun. Stat. Appl. Methods 2015, 22, 665–674. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org (accessed on 5 February 2021).
- Vähätalo, A.V.; Rainio, K.; Lehikoinen, A.; Lehikoinen, E. Spring Arrival of Birds Depends on the North Atlantic Oscillation. J. Avian Biol. 2004, 35, 210–216. [Google Scholar] [CrossRef]
- Hedlund, J.S.U.; Jakobsson, S.; Kullberg, C.; Fransson, T. Long-Term Phenological Shifts and Intra-Specific Differences in Migratory Change in the Willow Warbler Phylloscopus trochilus. J. Avian Biol. 2015, 46, 97–106. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Sparks, T.H.; Tryjanowski, P. The Detection of Climate Impacts: Some Methodological Considerations. Int. J. Climatol. 2005, 25, 271–277. [Google Scholar] [CrossRef]
- Rainio, K.; Laaksonen, T.; Ahola, M.; Vähätalo, A.V.; Lehikoinen, E. Climatic Responses in Spring Migration of Boreal and Arctic Birds in Relation to Wintering Area and Taxonomy. J. Avian Biol. 2006, 37, 507–515. [Google Scholar] [CrossRef]
- Jonzén, N.; Lindén, A.; Ergon, T.; Knudsen, E.; Vik, J.O.; Rubolini, D.; Piacentini, D.; Brinch, C.; Spina, F.; Karlsson, L.; et al. Rapid Advance of Spring Arrival Dates in Long-Distance Migratory Birds. Science 2006, 312, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Lehikoinen, E.; Sparks, T.H.; Zalakevicius, M. Arrival and Departure Dates. Adv. Ecol. Res. 2004, 35, 1–31. [Google Scholar]
- Stokke, B.G.; Møller, A.P.; Sæther, B.E.; Rheinwald, G.; Gutscher, H. Weather in the Breeding Area and during Migration Affects the Demography of a Small Long-Distance Passerine Migrant. Auk 2005, 122, 637–647. [Google Scholar] [CrossRef]
- Haest, B.; Hüppop, O.; Bairlein, F. The Influence of Weather on Avian Spring Migration Phenology: What, Where and When? Glob. Chang. Biol. 2018, 24, 5769–5788. [Google Scholar] [CrossRef]
- Zduniak, P.; Yosef, R.; Sparks, T.H.; Smit, H.; Tryjanowski, P. Rapid Advances in the Timing of the Spring Passage Migration through Israel of the Steppe Eagle Aquila Nipalensis. Clim. Res. 2010, 42, 217–222. [Google Scholar] [CrossRef]
- Nussbaumer, R.; Gravey, M.; Nussbaumer, A.; Jackson, C. Investigating the Influence of the Extreme Indian Ocean Dipole on the 2020 Influx of Red-Necked Phalaropes Phalaropus Lobatus in Kenya. Ostrich 2021, 92, 307–315. [Google Scholar] [CrossRef]
- Stone, M. A Plague of Locusts Has Descended on East Africa. Climate Change May Be to Blame. Available online: https://www.nationalgeographic.co.uk/environment-and-conservation/2020/02/a-plague-of-locusts-has-descended-on-east-africa-climate-change-may-be-to-blame (accessed on 31 May 2022).
- Cai, W.; Borlace, S.; Lengaigne, M.; Van Rensch, P.; Collins, M.; Vecchi, G.; Timmermann, A.; Santoso, A.; Mcphaden, M.J.; Wu, L.; et al. Increasing Frequency of Extreme El Niño Events Due to Greenhouse Warming. Nat. Clim. Chang. 2014, 4, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, N.M.A.; Jönsson, P.E.; Wennerberg, L. Geographical Variation in the Timing of Breeding and Moult in Dunlin Calidris Alpina on the Palearctic Tundra. Polar Biol. 2001, 24, 369–377. [Google Scholar] [CrossRef]
- MacMynowski, D.P.; Root, T.L. Climate and the Complexity of Migratory Phenology: Sexes, Migratory Distance, and Arrival Distributions. Int. J. Biometeorol. 2007, 51, 361–373. [Google Scholar] [CrossRef]
- Jenouvrier, S.; Weimerskirch, H.; Barbraud, C.; Park, Y.H.; Cazelles, B. Evidence of a Shift in the Cyclicity of Antarctic Seabird Dynamics Linked to Climate. Proc. R. Soc. B Biol. Sci. 2005, 272, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbraud, C.; Weimerskirch, H. Antarctic Birds Breed Later in Response to Climate Change. Proc. Natl. Acad. Sci. USA 2006, 103, 6248–6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, D.; Barriocanal, C. Ecological Conditions in Wintering and Passage Areas as Determinants of Timing of Spring Migration in Trans-Saharan Migratory Birds. J. Anim. Ecol. 2011, 80, 320–331. [Google Scholar] [CrossRef]
- Franke, A.; Therrien, J.F.; Descamps, S.; Bêty, J. Climatic Conditions during Outward Migration Affect Apparent Survival of an Arctic Top Predator, the Peregrine Falcon Falco Peregrinus. J. Avian Biol. 2011, 42, 544–551. [Google Scholar] [CrossRef]
- Halupka, L.; Wierucka, K.; Sztwiertnia, H.; Klimczuk, E. Conditions at Autumn Stopover Sites Affect Survival of a Migratory Passerine. J. Ornithol. 2017, 158, 979–988. [Google Scholar] [CrossRef] [Green Version]
- Tøttrup, A.P.; Thorup, K.; Rainio, K.; Yosef, R.; Lehikoinen, E.; Rahbek, C. Avian Migrants Adjust Migration in Response to Environmental Conditions En Route. Biol. Lett. 2008, 4, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Balbontín, J.; Møller, A.P.; Hermosell, I.G.; Marzal, A.; Reviriego, M.; De Lope, F. Individual Responses in Spring Arrival Date to Ecological Conditions during Winter and Migration in a Migratory Bird. J. Anim. Ecol. 2009, 78, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Palm, V.; Leito, A.; Truu, J.; Tomingas, O. The Spring Timing of Arrival of Migratory Birds: Dependence on Climate Variables and Migration Route. Ornis Fenn. 2009, 86, 97–108. [Google Scholar]
- Koenig, W.D. Spatial Autocorrelation of Ecological Phenomena. Trends Ecol. Evol. 1999, 14, 22–26. [Google Scholar] [CrossRef]
- Halkka, A.; Lehikoinen, A.; Velmala, W. Do Long-Distance Migrants Use Temperature Variations along the Migration Route in Europe to Adjust the Timing of Their Spring Arrival? Boreal Environ. Res. 2011, 16, 35–48. [Google Scholar]
- Briedis, M.; Hahn, S.; Adamík, P. Cold Spell En Route Delays Spring Arrival and Decreases Apparent Survival in a Long-Distance Migratory Songbird. BMC Ecol. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heino, M.; Puma, M.J.; Ward, P.J.; Gerten, D.; Heck, V.; Siebert, S.; Kummu, M. Two-Thirds of Global Cropland Area Impacted by Climate Oscillations. Nat. Commun. 2018, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- McKellar, A.E.; Marra, P.P.; Hannon, S.J.; Studds, C.E.; Ratcliffe, L.M. Winter Rainfall Predicts Phenology in Widely Separated Populations of a Migrant Songbird. Oecologia 2013, 172, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Tomotani, B.M.; van der Jeugd, H.; Gienapp, P.; de la Hera, I.; Pilzecker, J.; Teichmann, C.; Visser, M.E. Climate Change Leads to Differential Shifts in the Timing of Annual Cycle Stages in a Migratory Bird. Glob. Chang. Biol. 2018, 24, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Elkins, N. Weather and Bird Behaviour, 2nd ed.; T & A D Poyser: London, UK, 2018. [Google Scholar]
- Newton, I. Bird Migration; Collins: London, UK, 2010. [Google Scholar]
- Allan, D.G.; Harrison, J.A.; Herremans, M.; Navarro, R.; Underhill, L.G. Southern African Geography: Its Relevance to Birds. In The Atlas of Southern African Birds. Vol. 1: Non-Passerines; Harrison, J.A., Allan, D.G., Underhill, L.G., Herremans, M., Tree, A.J., Parker, V., Brown, C.J., Eds.; BirdLife South Africa: Johannesburg, South Africa, 1997; pp. lxv–ci. [Google Scholar]
- Lingbeek, B.J.; Higgins, C.L.; Muir, J.P.; Kattes, D.H.; Schwertner, T.W. Arthropod Diversity and Assemblage Structure Response to Deforestation and Desertification in the Sahel of Western Senegal. Glob. Ecol. Conserv. 2017, 11, 165–176. [Google Scholar] [CrossRef]
- Stanley, C.Q.; Dudash, M.R.; Ryder, T.B.; Gregory Shriver, W.; Marra, P.P. Variable Tropical Moisture and Food Availability Underlie Mixed Winter Space-Use Strategies in a Migratory Songbird. Proc. R. Soc. B Biol. Sci. 2021, 288, 1–9. [Google Scholar] [CrossRef]
- Jenni, L.; Winkler, R. The Biology of Moult in Birds; Helm. Bloomsbury Publishing Plc.: London, UK, 2020. [Google Scholar]
- González, A.M.; Bayly, N.J.; Wilson, S.; Hobson, K.A. Shade Coffee or Native Forest? Indicators of Winter Habitat Quality for a Long-Distance Migratory Bird in the Colombian Andes. Ecol. Indic. 2021, 131, 108115. [Google Scholar] [CrossRef]
- Peach, W.; Baillie, S.; Underhill, L.G. Survival of British Sedge Warblers Acrocephalus schoenobaenus in Relation to West African Rainfal. IBIS 1991, 133, 300–305. [Google Scholar] [CrossRef]
- Studds, C.E.; Marra, P.P. Rainfall-Induced Changes in Food Availability Modify the Spring Departure Programme of a Migratory Bird. Proc. R. Soc. B Biol. Sci. 2011, 278, 3437–3443. [Google Scholar] [CrossRef]
- Aloni, I.; Markman, S.; Ziv, Y. Autumn Temperatures at African Wintering Grounds Affect Body Condition of Two Passerine Species during Spring Migration. PLoS ONE 2019, 14, 9–10. [Google Scholar] [CrossRef]
- Szép, T. Relationship between West African Rainfall and the Survival of Central European Sand Martins Riparia riparia. IBIS 1995, 137, 162–168. [Google Scholar] [CrossRef]
- Cowley, E.; Siriwardena, G.M. Long-Term Variation in Survival Rates of Sand Martins Riparia riparia: Dependence on Breeding and Wintering Ground Weather, Age and Sex, and Their Population Consequences. Bird Study 2005, 52, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Beresford, A.E.; Sanderson, F.J.; Donald, P.F.; Burfield, I.J.; Butler, A.; Vickery, J.A.; Buchanan, G.M. Phenology and Climate Change in Africa and the Decline of Afro-Palearctic Migratory Bird Populations. Remote Sens. Ecol. Conserv. 2019, 5, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Vickery, J.A.; Ewing, S.R.; Smith, K.W.; Pain, D.J.; Bairlein, F.; Škorpilová, J.; Gregory, R.D. The Decline of Afro-Palaearctic Migrants and an Assessment of Potential Causes. IBIS 2014, 156, 1–22. [Google Scholar] [CrossRef]
- Pulido, F.; Berthold, P. Current Selection for Lower Migratory Activity Will Drive the Evolution of Residency in a Migratory Bird Population. Proc. Natl. Acad. Sci. USA 2010, 107, 7341–7346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmore, K.E.; Van Doren, B.M.; Conway, G.J.; Curk, T.; Garrido-Garduño, T.; Germain, R.R.; Hasselmann, T.; Hiemer, D.; van der Jeugd, H.P.; Justen, H.; et al. Individual Variability and Versatility in an Eco-evolutionary Model of Avian Migration. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201339. [Google Scholar] [CrossRef]
- World Meteorological Organisation. 2020 Was One of Three Warmest Years on Record. Available online: https://public.wmo.int/en/media/press-release/2020-was-one-of-three-warmest-years-record (accessed on 1 June 2022).
- Nowakowski, J.K. Ringing Data from the Bird Migration Research Station, University of Gdańsk. Available online: https://www.gbif.org/dataset/8186b0c0−925e−11da−8900-b8a03c50a862 (accessed on 1 June 2022).



| Symbol 1 | Climate Index | Months 2 | Baseline Period 3 | Source of Data |
|---|---|---|---|---|
| NAO NOV–MAR | North Atlantic Oscillation Index | Nov–Mar | 1982–2021 | https://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/norm.nao.monthly.b5001.current.ascii.table (accessed on 30 June 2022) |
| NAO AUG–OCT_1y | Aug–Oct_1y | |||
| NAO JUN–JUL_1y | Jun–Jul_1y | |||
| NAO APR–MAY | Apr–May | |||
| IOD NOV–MAR | Indian Ocean Dipole | Nov–Mar | 1981–2010 | https://psl.noaa.gov/gcos_wgsp/Timeseries/DMI/ (accessed on 30 June 2022) |
| IOD AUG–OCT_1y | Aug–Oct_1y | |||
| SOI NOV–MAR | Southern Oscillation Index | Nov–Mar | 1981–2010 | https://psl.noaa.gov/gcos_wgsp/Timeseries/SOI/ (accessed on 30 June 2022) |
| SOI AUG–OCT_1y | Aug–Oct_1y | |||
| PSAH NOV–MAR | Sahel Precipitation Index within 10°–20° N, 20° W–10° E | Nov–Mar | 1982–2021 | https://climexp.knmi.nl/select.cgi?gpcc (accessed on 30 June 2022) |
| PSAH AUG–OCT_1y | Aug–Oct_1y | |||
| TSAH NOV–MAR | Sahel temperature anomaly within 10°–20° N, 20° W–10° E | Nov–Mar | 1982–2021 | http://climexp.knmi.nl/select.cgi?era5_t2m_daily (accessed on 30 June 2022) |
| TSAH AUG–OCT_1y | Aug–Oct_1y | |||
| TLEB APR–MAY | local temperatures in Łeba | Apr–May | 1982–2021 | http://www.ecad.eu (accessed on 30 June 2022) |
| SCAND JUN–JUL_1y | Scandinavian Pattern Index | Jun–Jul_1y | 1981–2010 | ftp://ftp.cpc.ncep.noaa.gov/wd52dg/data/indices/scand_index.tim (accessed on 30 June 2022) |
| SCAND APR–MAY | Apr–May | |||
| Year | 1982 = Year 1 | 1982–2021 | Our database |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remisiewicz, M.; Underhill, L.G. Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa. Animals 2022, 12, 1732. https://doi.org/10.3390/ani12131732
Remisiewicz M, Underhill LG. Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa. Animals. 2022; 12(13):1732. https://doi.org/10.3390/ani12131732
Chicago/Turabian StyleRemisiewicz, Magdalena, and Les G. Underhill. 2022. "Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa" Animals 12, no. 13: 1732. https://doi.org/10.3390/ani12131732
APA StyleRemisiewicz, M., & Underhill, L. G. (2022). Large-Scale Climatic Patterns Have Stronger Carry-Over Effects than Local Temperatures on Spring Phenology of Long-Distance Passerine Migrants between Europe and Africa. Animals, 12(13), 1732. https://doi.org/10.3390/ani12131732






