Comparative Study of the Effects of Two Dietary Sources of Vitamin D on the Bone Metabolism, Welfare and Birth Progress of Sows Fed Protein- and Phosphorus-Reduced Diets
Abstract
:Simple Summary
Abstract
1. Introduction
- (A)
- A positive effect on vitamin D status.
- (B)
- Improved Ca and phosphate (Pi) homeostasis.
- (C)
- A positive influence on bone metabolism.
- (D)
- A lower prevalence of gait changes.
- (E)
- A positive influence on birth progress.
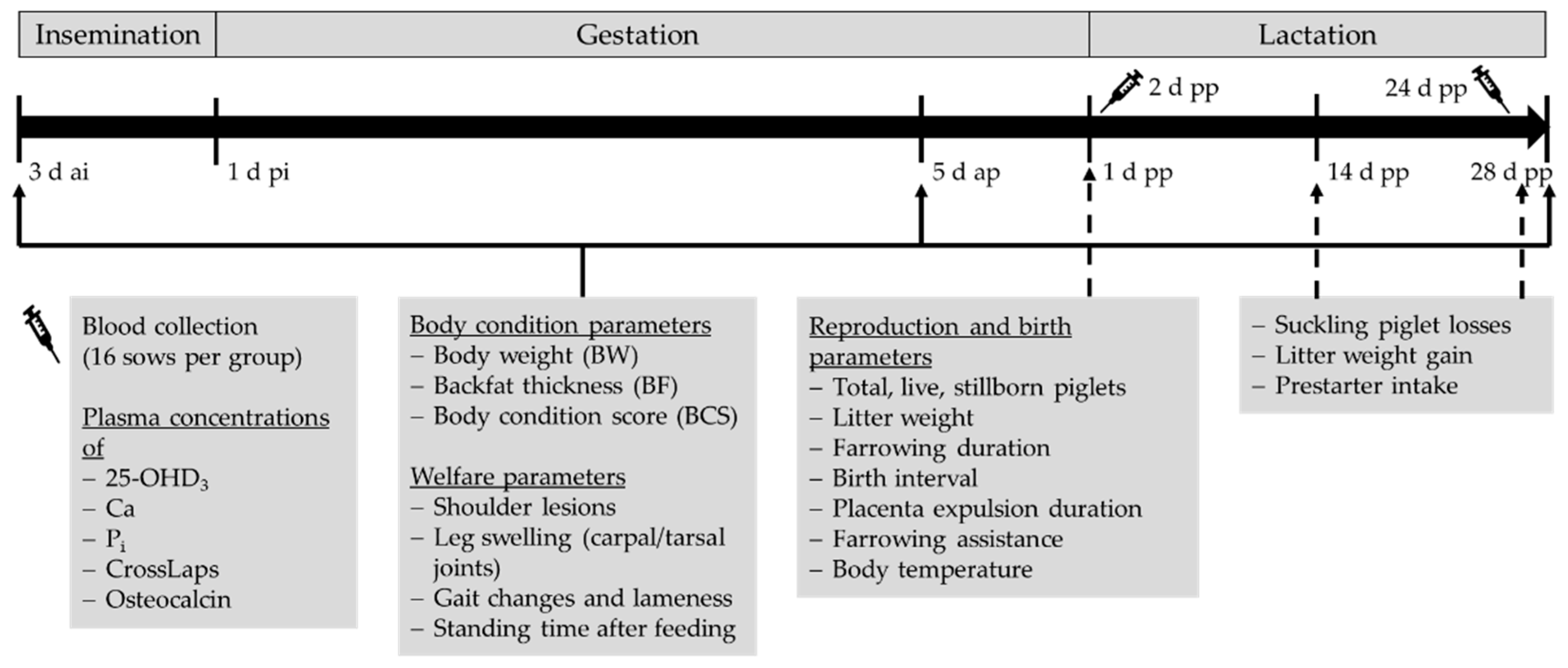
2. Materials and Methods
2.1. Animals and Diets
2.2. Measurements
2.2.1. Sampling and Laboratory Testing
2.2.2. Feed Intake and Body Condition Development
2.2.3. Welfare Parameters
2.2.4. Reproduction and Birth Parameters
2.3. Statistical Analysis
3. Results
3.1. Plasma Concentrations of 25-OHD3, Ca, Pi, CrossLaps and Osteocalcin
3.2. Feed Intake and Body Condition Development
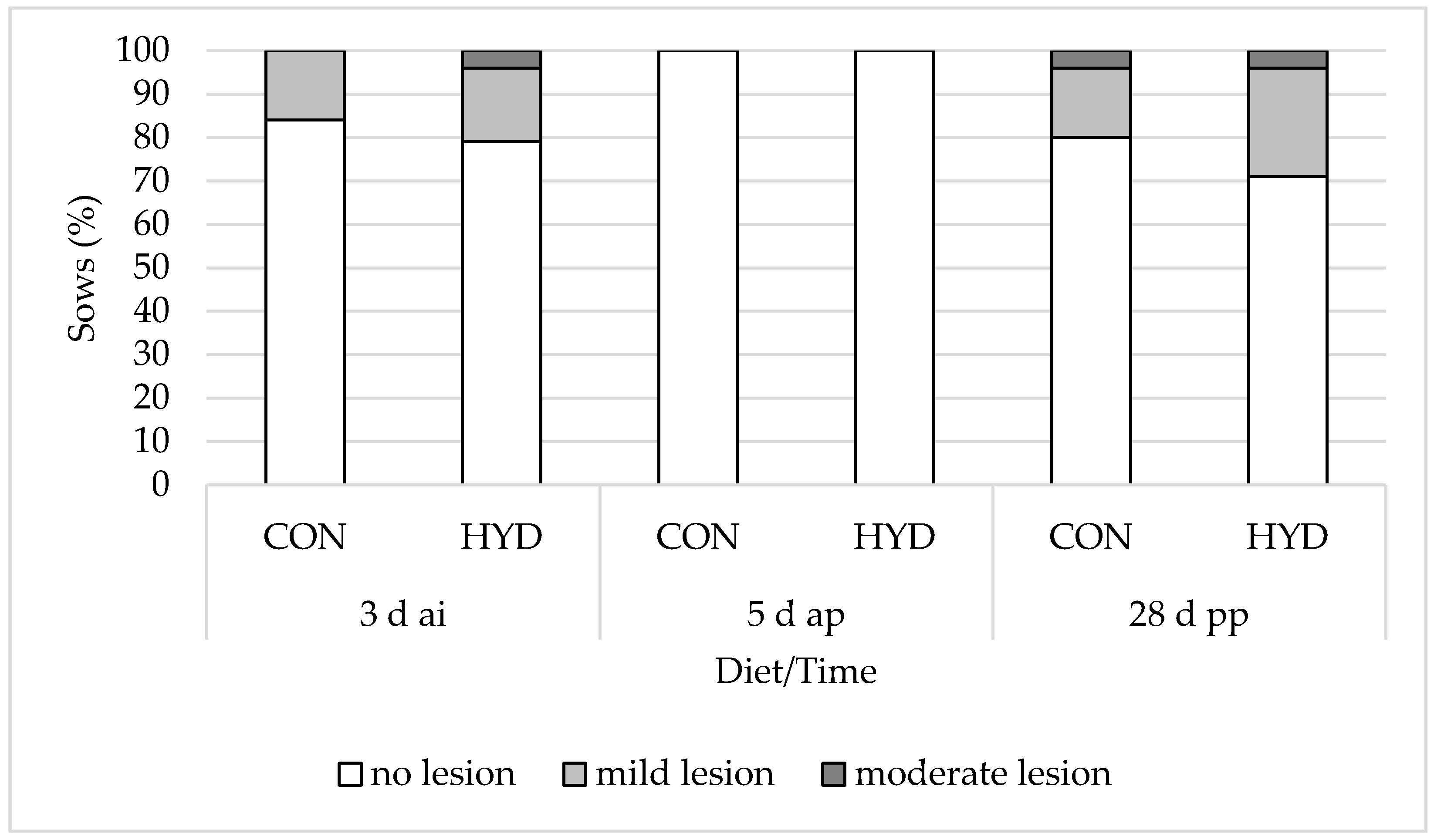
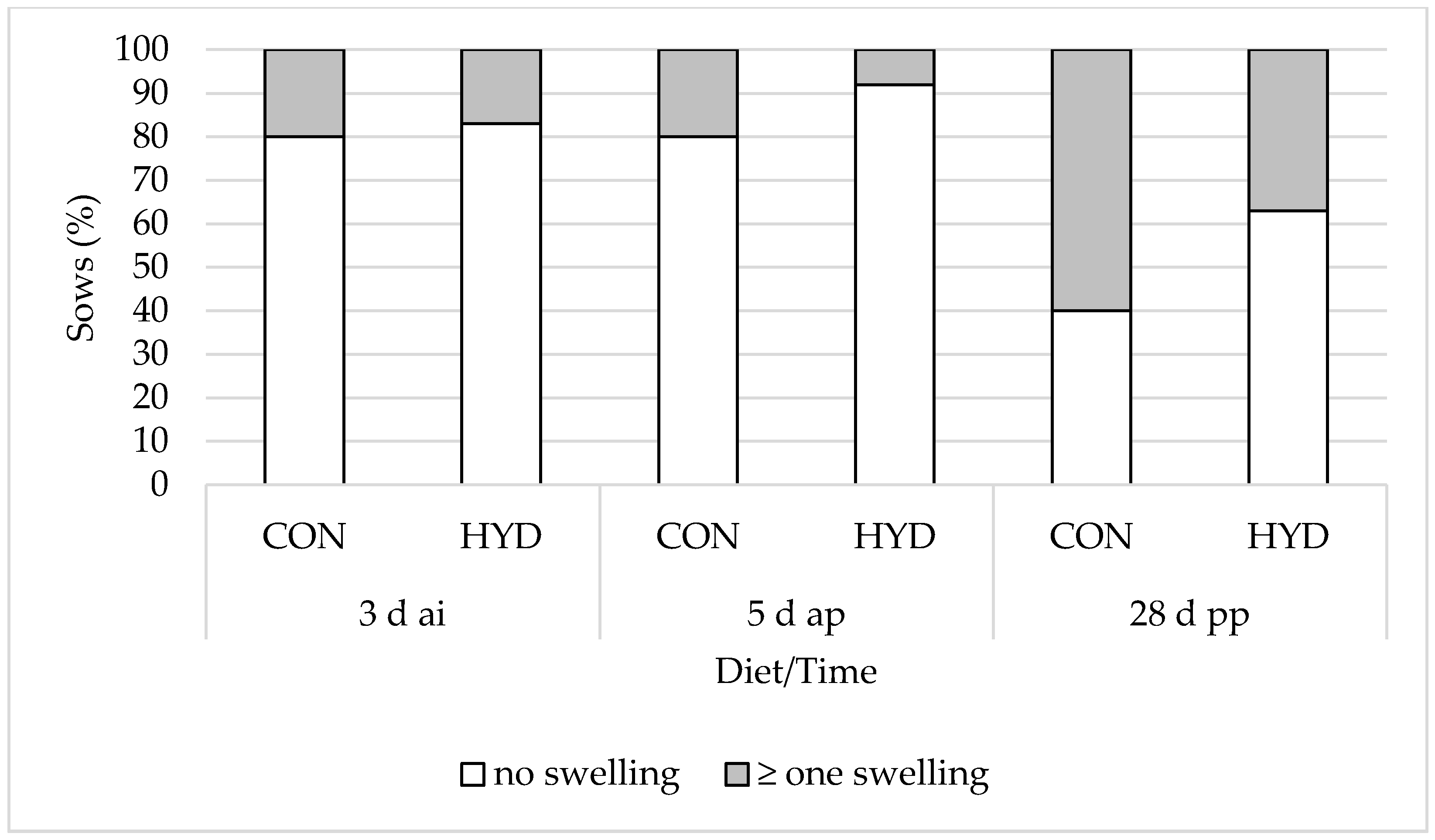
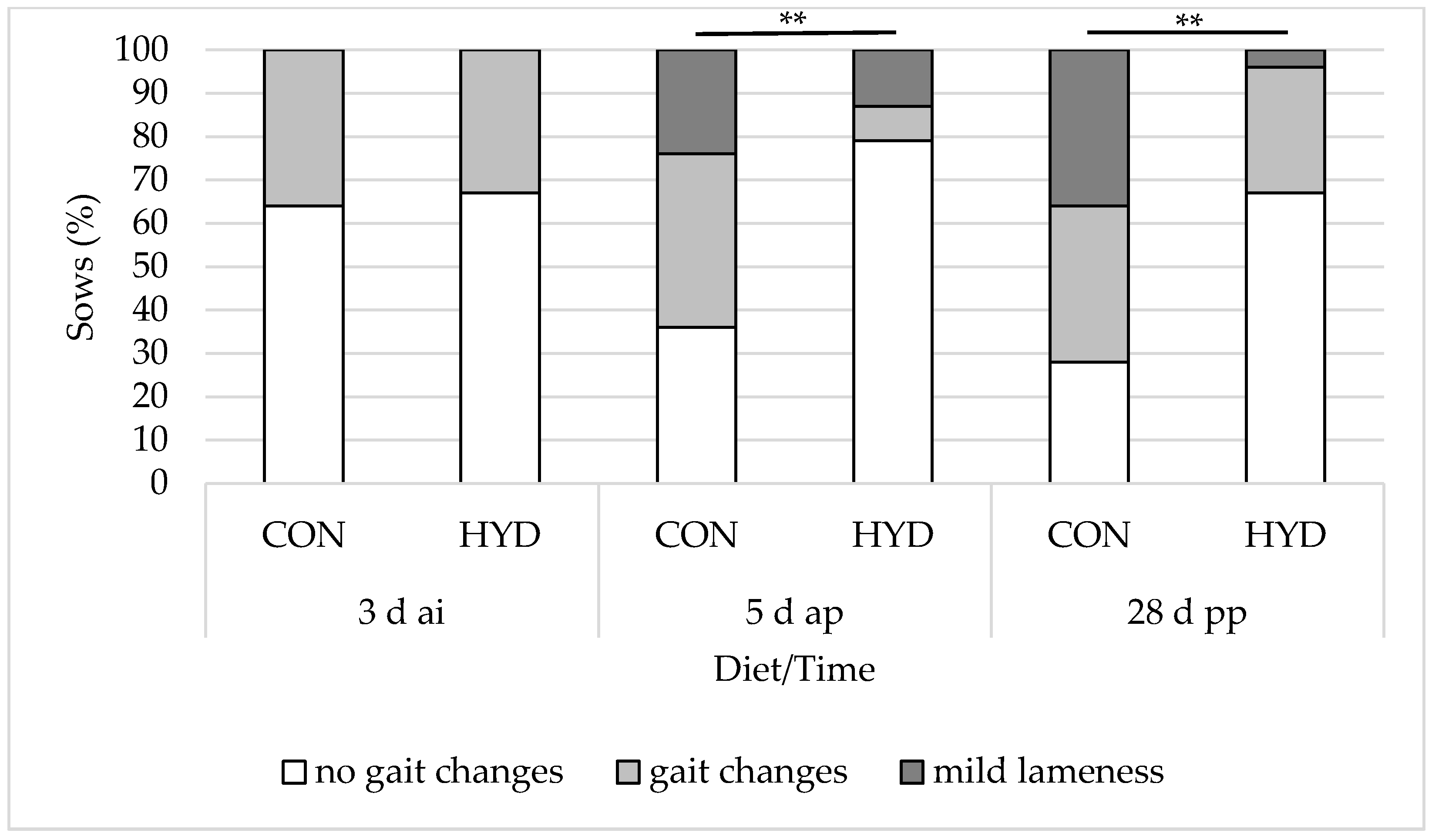
3.3. Welfare Parameters
3.4. Reproduction and Birth Parameters
4. Discussion
4.1. Vitamin D Status, Plasma Minerals and Bone Metabolism
4.2. Body Condition and Composition
4.3. Musculoskeletal System
4.4. Reproductive Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, J.; Madson, D.M.; Ensley, S.; Goff, J.P.; Sparks, C.; Stevenson, G.W.; Crenshaw, T.; Wang, C.; Horst, R.L. Survey of serum vitamin D status across stages of swine production and evaluation of supplemental bulk vitamin D premixes used in swine diets. JSHAP 2015, 23, 28. Available online: http://lib.dr.iastate.edu/vdpam_pubs/75 (accessed on 22 May 2022).
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Meurer, M. Vitamin D metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Safety and efficacy of 25-hydroxycholecalciferol as a feed additive for poultry and pigs. EFSA J. 2009, 7, 969. [Google Scholar] [CrossRef]
- Cashman, K.D.; Seamans, K.M.; Lucey, A.J.; Stöcklin, E.; Weber, P.; Kiely, M.; Hill, T.R. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am. J. Clin. Nutr. 2012, 95, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Bar, A.; Sharvit, M.; Noff, D.; Edelstein, S.; Hurwitz, S. Absorption and Excretion of Cholecalciferol and of 25-Hydroxycholecalciferol and Metabolites in Birds. J. Nutr. 1980, 110, 1930–1934. [Google Scholar] [CrossRef]
- Flohr, J.R.; Woodworth, J.C.; Bergstrom, J.R.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M. Evaluating the impact of maternal vitamin D supplementation: I. Sow performance, serum vitamin metabolites, and neonatal muscle characteristics. J. Anim. Sci. 2016, 94, 4629–4642. [Google Scholar] [CrossRef] [Green Version]
- Thayer, M.T.; Nelssen, J.L.; Langemeier, A.J.; Morton, J.M.; Gonzalez, J.M.; Kruger, S.R.; Ou, Z.; Makowski, A.J.; Bergstrom, J.R. The effects of maternal dietary supplementation of cholecalciferol (vitamin D3) and 25-OHD3 on sow and progeny performance. Transl. Anim. Sci. 2019, 3, 692–708. [Google Scholar] [CrossRef]
- Weber, G.M.; Witschi, A.K.; Wenk, C.; Martens, H. Triennial Growth Symposium—Effects of dietary 25-hydroxycholecalciferol and cholecalciferol on blood vitamin D and mineral status, bone turnover, milk composition, and reproductive performance of sows. J. Anim. Sci. 2014, 92, 899–909. [Google Scholar] [CrossRef]
- O’Doherty, J.V.; Gahan, D.A.; O’Shea, C.; Callan, J.J.; Pierce, K.M. Effects of phytase and 25-hydroxyvitamin D3 inclusions on the performance, mineral balance and bone parameters of grower–finisher pigs fed low-phosphorus diets. Animal 2010, 4, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. JANA 2003, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.V.; van Loon, L.J.; De Groot, L.C.P.G.M. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur. J. Clin. Nutr. 2013, 67, 1050–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos. Int. 2002, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Vitamin D and pregnancy: Skeletal effects, nonskeletal effects, and birth outcomes. Calcif. Tissue Int. 2013, 92, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I. Energiehaushalt. In Tierernährung, 14th ed.; Kirchgessner, M., Ed.; DLG-Verlag: Frankfurt am Main, Germany, 2014; pp. 135–174. [Google Scholar]
- Naumann, C.; Bassler, R. Methoden der landwirtschaftlichen Forschungs- und Untersuchungsanstalt. In Biochemische Untersuchung von Futtermitteln; VDLUFA: Darmstadt, Germany, 2012. [Google Scholar]
- Li, Y.Z.; Wang, L.H.; Johnston, L.J. Sorting by parity to reduce aggression toward first-parity sows in group-gestation housing systems. J. Anim. Sci. 2012, 90, 4514–4522. [Google Scholar] [CrossRef]
- Roongsitthichai, A.; Tummaruk, P. Importance of backfat thickness to reproductive performance in female pigs. Thai J. Vet. Med. 2014, 44, 171–178. [Google Scholar]
- Welfare Quality®. Welfare Quality® Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Grégoire, J.; Bergeron, R.; d’Allaire, S.; Meunier-Salaün, M.C.; Devillers, N. Assessment of lameness in sows using gait, footprints, postural behaviour and foot lesion analysis. Animal 2013, 7, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Main, D.C.; Clegg, J.; Spatz, A.; Green, L.E. Repeatability of a lameness scoring system for finishing pigs. Vet. Rec. 2000, 147, 574–576. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Vanderhaeghe, C.; Janssens, G.P.; Dewulf, J.; Maes, D.G. Risk factors associated with postpartum dysgalactia syndrome in sows. Vet. J. 2010, 184, 167–171. [Google Scholar] [CrossRef]
- Kemper, N. Update on postpartum dysgalactia syndrome in sows. J. Anim. Sci. 2020, 98, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.S.; Jakobsen, J.; Nielsen, J.P. Vitamin D Levels in Sows from Five Danish Outdoor Herds. Animals 2022, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Madson, D.M.; Ensley, S.M.; Gauger, P.C.; Schwartz, K.J.; Stevenson, G.W.; Cooper, V.L.; Janke, B.H.; Burrough, E.R.; Goff, J.P.; Horst, R.L. Rickets: Case series and diagnostic review of hypovitaminosis D in swine. J. Vet. Diagn. Investig. 2012, 24, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, J.D.; Hines, E.A.; Starkey, J.D.; Starkey, C.W.; Chung, T.K. Feeding 25-hydroxycholecalciferol improves gilt reproductive performance and fetal vitamin D status. J. Anim. Sci. 2012, 90, 3783–3788. [Google Scholar] [CrossRef]
- Heaney, R.P. The case for improving vitamin D status. J. Steroid Biochem. Mol. Biol. 2007, 103, 635–641. [Google Scholar] [CrossRef]
- Bouillon, R.; Rosen, C. The IOM-Endocrine Society Controversy on Recommended Vitamin D Targets: In Support of the IOM Position. In Vitamin D; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- DeLuca, H.F. Triennial Growth Symposium—vitamin D: Bones and beyond. J. Anim. Sci. 2014, 92, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Giesemann, M.A.; Lewis, A.J.; Miller, P.S.; Akhter, M.P. Effects of the reproductive cycle and age on calcium and phosphorus metabolism and bone integrity of sows. J. Anim. Sci. 1998, 76, 796–807. [Google Scholar] [CrossRef]
- Kirk, R.K.; Svensmark, B.; Ellegaard, L.P.; Jensen, H.E. Locomotive disorders associated with sow mortality in Danish pig herds. J. Vet. Med. A. 2005, 52, 423–428. [Google Scholar] [CrossRef]
- Kremer, R.; Campbell, P.P.; Reinhardt, T.; Gilsanz, V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J. Clin. Endocrinol. Metab. 2009, 94, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef] [Green Version]
- Prokoski, K.; Bittencourt, L.C.; Teixeira, L.V.; Bortoluzzi, C.; Vanroo, E.; Palma, S.; Fernandes, J.M. Classic and Non-Classic Effects of the Duration of Supplementation of 25-Hydroxicholecalciferol in Broiler Chicken Diets. Animals 2021, 11, 2971. [Google Scholar] [CrossRef] [PubMed]
- Vignale, K.; Greene, E.S.; Caldas, J.V.; England, J.A.; Boonsinchai, N.; Sodsee, P.; Pollock, E.D.; Dridi, S.; Coon, C.N. 25-Hydroxycholecalciferol Enhances Male Broiler Breast Meat Yield through the mTOR Pathway. J. Nutr. 2015, 145, 855–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioja-Lang, F.C.; Seddon, Y.M.; Brown, J.A. Shoulder lesions in sows: A review of their causes, prevention, and treatment. J. Swine Health Prod. 2018, 26, 101–107. [Google Scholar]
- Friedrich, L.; Krieter, J.; Kemper, N.; Czycholl, I. Test–retest reliability of the Welfare Quality Assessment protocol for pigs applied to sows and piglets. Part 2. Assessment of the principles good feeding, good housing, and good health. J. Anim. Sci. 2019, 97, 1143–1157. [Google Scholar] [CrossRef]
- Pluym, L.M.; van Nuffel, A.; van Weyenberg, S.; Maes, D. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal 2013, 7, 1174–1181. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, M.; Peltoniemi, O.; Valros, A. Impact of lameness and claw lesions in sows on welfare, health and production. Livest. Sci. 2013, 156, 2–9. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D₃ supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013, 98, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.-K.; Mittlmeier, T.; Vollmar, B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef]
- Iolascon, G.; Moretti, A.; Paoletta, M.; Liguori, S.; Di Munno, O. Muscle Regeneration and Function in Sports: A Focus on Vitamin D. Medicina 2021, 57, 1015. [Google Scholar] [CrossRef]
- Merhi, Z.O.; Seifer, D.B.; Weedon, J.; Adeyemi, O.; Holman, S.; Anastos, K.; Golub, E.T.; Young, M.; Karim, R.; Greenblatt, R.; et al. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil. Steril. 2012, 98, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, B.; Da Silva, C.L.A.; Soede, N.M. Recent advances in pig reproduction: Focus on impact of genetic selection for female fertility. Reprod. Dom. Anim. 2018, 53, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltoniemi, O.A.T.; Björkman, S.; Oliviero, C. Parturition effects on reproductive health in the gilt and sow. Reprod. Dom. Anim. 2016, 51, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bärebring, L.; Bullarbo, M.; Glantz, A.; Hulthén, L.; Ellis, J.; Jagner, Å.; Schoenmakers, I.; Winkvist, A.; Augustin, H. Trajectory of vitamin D status during pregnancy in relation to neonatal birth size and fetal survival: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 51. [Google Scholar] [CrossRef]
| Ingredient (%) | Gestation | Lactation |
|---|---|---|
| Barley | 40.2 | 20.0 |
| Wheat bran | 21.8 | 11.0 |
| Wheat | 15.0 | 33.5 |
| Corn | - | 10.0 |
| Molasses pulp | 4.0 | - |
| Sunflower extraction meal | 3.3 | - |
| Sunflower extraction meal (hydrothermal pressure) | 3.0 | 3.0 |
| Sugar beet molasses | 3.0 | - |
| Wheat, broken down | 2.5 | 2.5 |
| Calcium carbonate | 1.5 | 1.6 |
| Barley, broken down | 1.3 | 1.3 |
| Corn, broken down | 1.3 | 1.3 |
| Sunflower extraction meal (steam-heated) | 1.0 | 11.2 |
| Sodium chloride | 0.7 | 0.5 |
| Vegetable fatty acids (sunflower, palm, rape kernel) | 0.2 | 1.7 |
| Calcium sodium phosphate | - | 0.35 |
| Soybean oil | - | 0.50 |
| Palm oil | 0.01 | 0.02 |
| Minor components * | 1.19 * | 1.53 # |
| Parameter | Unit | Gestation | Lactation | ||
|---|---|---|---|---|---|
| CON | HYD | CON | HYD | ||
| Crude protein | % as-fed basis | 13.6 | 13.3 | 15.8 | 15.6 |
| Crude fat | 2.6 | 2.7 | 4.7 | 4.4 | |
| Crude fiber | 6.3 | 6.6 | 4.1 | 3.6 | |
| Crude ash | 5.4 | 5.3 | 5.1 | 4.7 | |
| Starch | 36.4 | 35.6 | 39.9 | 41.6 | |
| Calcium | 0.67 | 0.66 | 0.77 | 0.65 | |
| Phosphorus | 0.48 | 0.49 | 0.47 | 0.45 | |
| Metabolizable energy * | MJ/kg as-fed basis | 11.9 | 11.8 | 13.3 | 13.5 |
| Vitamin D Source | Gestation | Lactation |
|---|---|---|
| CON (vitamin D3, μg/kg) | 45 | 38 |
| HYD (25-OHD3, μg/kg) | 43 | 35 |
| Parameter | CON | HYD | p-Value Group | p-Value Time | p-Value Interaction |
|---|---|---|---|---|---|
| 25-OHD3, ng/mL | |||||
| 2 d pp | 24.5 a ± 4.9 | 46.5 b ± 10.1 | <0.001 | <0.001 | <0.001 |
| 24 d pp | 37.7 a ± 7.7 | 73.2 b ± 10.5 | |||
| Ca, mmol/L | |||||
| 2 d pp | 2.31 ± 0.16 | 2.29 ± 0.16 | 0.56 | <0.001 | 0.87 |
| 24 d pp | 2.52 ± 0.15 | 2.48 ± 0.21 | |||
| Pi, mmol/L | |||||
| 2 d pp | 2.03 ± 0.22 | 2.04 ± 0.24 | 0.11 | <0.001 | 0.11 |
| 24 d pp | 1.47 ± 0.15 | 1.68 ± 0.33 | |||
| CrossLaps, ng/mL | |||||
| 2 d pp | 0.66 ± 0.24 | 0.71 ± 0.33 | 0.41 | <0.001 | 0.83 |
| 24 d pp | 1.24 ± 0.50 | 1.33 ± 0.34 | |||
| Osteocalcin, ng/mL | |||||
| 2 d pp | 60.9 ± 34.3 | 70.1 ± 38.3 | 0.29 | <0.001 | 0.59 |
| 24 d pp | 109 ± 23.6 | 130 ± 80.5 | |||
| Osteocalcin/CrossLaps, ratio | |||||
| 2 d pp | 100 ± 60.1 | 129 ± 104 | 0.38 | 0.53 | 0.50 |
| 24 d pp | 101 ± 39.7 | 105 ± 76.3 |
| Parameter | CON | HYD | p-Value |
|---|---|---|---|
| ADFI (gestation), kg 1 | 3.15 ± 0.06 | 3.16 ± 0.07 | 0.73 |
| ADFI (lactation), kg | 5.74 ± 0.49 | 5.74 ± 0.45 | 0.94 |
| BW, kg | |||
| 3 d ai | 219.1 ± 27.5 | 217.5 ± 25.2 | 0.83 |
| 5 d ap | 292.3 ± 20.2 | 286.4 ± 20.4 | 0.10 |
| 28 d pp | 239.3 ± 24.9 | 236.5 ± 23.7 | 0.59 |
| BW gain (gestation), kg | 73.2 ± 14.2 | 68.9 ± 11.8 | 0.10 |
| BW loss (lactation), kg | 53.0 ± 11.1 | 49.9 ± 8.9 | 0.20 |
| BW loss (lactation) C, kg | 26.4 ± 9.3 | 24.9 ± 9.7 | 0.49 |
| BF, mm | |||
| 3 d ai | 13.4 ± 2.8 | 13.2 ± 2.9 | 0.78 |
| 5 d ap | 18.5 a ± 3.7 | 16.9 b ± 3.6 | <0.01 |
| 28 d pp | 14.2 ± 2.5 | 14.0 ± 3.3 | 0.93 |
| BF gain (gestation), mm | 5.1 a ± 1.7 | 3.8 b ± 1.3 | <0.01 |
| BF loss (lactation), mm | 4.2 a ± 1.9 | 2.9 b ± 1.4 | <0.01 |
| BCS, Score | |||
| 3 d ai | 2.9 ± 0.8 | 2.9 ± 1.0 | 0.50 |
| 5 d ap | 3.4 ± 0.5 | 3.3 ± 0.6 | 0.30 |
| 28 d pp | 2.8 ± 0.6 | 2.9 ± 0.5 | 0.43 |
| BCS gain (gestation), score | 0.5 ± 1.0 | 0.4 ± 0.9 | 0.50 |
| BCS loss (lactation), score | 0.6 ± 0.7 | 0.5 ± 0.7 | 0.41 |
| Parameter | n1/n2 | CON | HYD | p-Value |
|---|---|---|---|---|
| 3 d ai | 25/24 | 50.9 ± 69.7 | 44.2 ± 60.8 | 0.72 |
| 5 d ap | 25/23 | 22.7 a ± 7.9 | 29.0 b ± 11.4 | 0.03 |
| 28 d pp | 23/22 | 17.6 ± 7.6 | 19.0 ± 7.0 | 0.55 |
| Parameter | n1/n2 | CON | HYD | p-Value |
|---|---|---|---|---|
| Number of piglets, n | ||||
| Total born | 25/22 | 19.2 ± 3.1 | 19.3 ± 3.0 | 0.90 |
| Born alive | 25/22 | 18.0 ± 2.7 | 17.5 ± 2.6 | 0.48 |
| Born dead | 25/22 | 1.2 ± 1.5 | 1.8 ± 1.9 | 0.19 |
| Born mummified | 25/22 | 0.5 ± 0.7 | 0.4 ± 1.1 | 0.68 |
| Stillborn rate (%) | 25/22 | 5.6 ± 6.8 | 8.9 ± 8.7 | 0.15 |
| After cross-foster | 25/24 | 17.3 ± 2.7 | 16.9 ± 1.8 | 0.54 |
| Middle of lactation period | 25/24 | 14.2 ± 1.5 | 13.5 ± 1.3 | 0.06 |
| Weaned | 25/24 | 14.0 a ± 1.4 | 13.0 b ± 1.5 | 0.03 |
| Suckling piglet losses | 25/24 | 3.3 ± 3.7 | 3.9 ± 2.6 | 0.55 |
| Litter weight, kg (WLWV) | ||||
| Total born | 25/22 | 26.6 ± 3.9 (0.10) | 26.2 ± 3.5 (0.11) | 0.49 (0.69) |
| Born alive | 25/22 | 25.4 ± 3.5 (0.09) | 24.1 ± 3.6 (0.10) | 0.30 (0.34) |
| Born dead | 25/22 | 2.0 ± 1.6 (0.15) | 2.7 ± 1.8 (0.05) | 0.29 (0.07) |
| After cross-foster | 25/24 | 24.5 ± 3.3 (0.06) | 23.0 ± 3.5 (0.08) | 0.14 (0.22) |
| Middle of lactation period, 14 d pp | 25/24 | 50.5 ± 8.3 (0.52) | 49.4 ± 9.2 (0.54) | 0.67 (0.85) |
| Weaning, 28 d pp | 25/24 | 95.9 ± 11.3 (1.78) | 92.4 ± 14.0 (1.75) | 0.34 (0.91) |
| Litter weight gain, kg | ||||
| 1–14 d pp 3 | 25/24 | 26.0 ± 8.4 | 26.4 ± 8.3 | 0.88 |
| 15–28 d pp | 25/24 | 45.4 ± 5.3 | 43.0 ± 6.5 | 0.16 |
| 1–28 d pp 3 | 25/24 | 71.4 ± 11.7 | 69.4 ± 13.4 | 0.57 |
| Prestarter intake (g) 4 | 25/24 | 17.1 ± 7.0 | 16.7 ± 6.9 | 0.86 |
| Parameter | CON | HYD | p-Value |
|---|---|---|---|
| Farrowing duration, min | 288 ± 112 | 334 ± 173 | 0.34 |
| Birth interval, min | 16 ± 6 | 19 ± 11 | 0.28 |
| Placenta expulsion duration, min | 228 ± 221 | 147 ± 94 | 0.07 |
| Farrowing assistance, % | 22 | 9 | 0.41 |
| Body temperature (1–3 d pp), °C | 39.0 ± 0.3 | 39.0 ± 0.3 | 0.63 |
| <39.6 °C, % | 65 | 68 | 0.50 |
| 39.6–39.9 °C, % | 13 | 23 | |
| ≥40.0 °C, % | 22 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lütke-Dörhoff, M.; Schulz, J.; Westendarp, H.; Visscher, C.; Wilkens, M.R. Comparative Study of the Effects of Two Dietary Sources of Vitamin D on the Bone Metabolism, Welfare and Birth Progress of Sows Fed Protein- and Phosphorus-Reduced Diets. Animals 2022, 12, 1678. https://doi.org/10.3390/ani12131678
Lütke-Dörhoff M, Schulz J, Westendarp H, Visscher C, Wilkens MR. Comparative Study of the Effects of Two Dietary Sources of Vitamin D on the Bone Metabolism, Welfare and Birth Progress of Sows Fed Protein- and Phosphorus-Reduced Diets. Animals. 2022; 12(13):1678. https://doi.org/10.3390/ani12131678
Chicago/Turabian StyleLütke-Dörhoff, Michael, Jochen Schulz, Heiner Westendarp, Christian Visscher, and Mirja R. Wilkens. 2022. "Comparative Study of the Effects of Two Dietary Sources of Vitamin D on the Bone Metabolism, Welfare and Birth Progress of Sows Fed Protein- and Phosphorus-Reduced Diets" Animals 12, no. 13: 1678. https://doi.org/10.3390/ani12131678
APA StyleLütke-Dörhoff, M., Schulz, J., Westendarp, H., Visscher, C., & Wilkens, M. R. (2022). Comparative Study of the Effects of Two Dietary Sources of Vitamin D on the Bone Metabolism, Welfare and Birth Progress of Sows Fed Protein- and Phosphorus-Reduced Diets. Animals, 12(13), 1678. https://doi.org/10.3390/ani12131678






