Epidemiology, Diagnosis, and Prevention of Sparganosis in Asia
Abstract
:Simple Summary
Abstract
1. Introduction
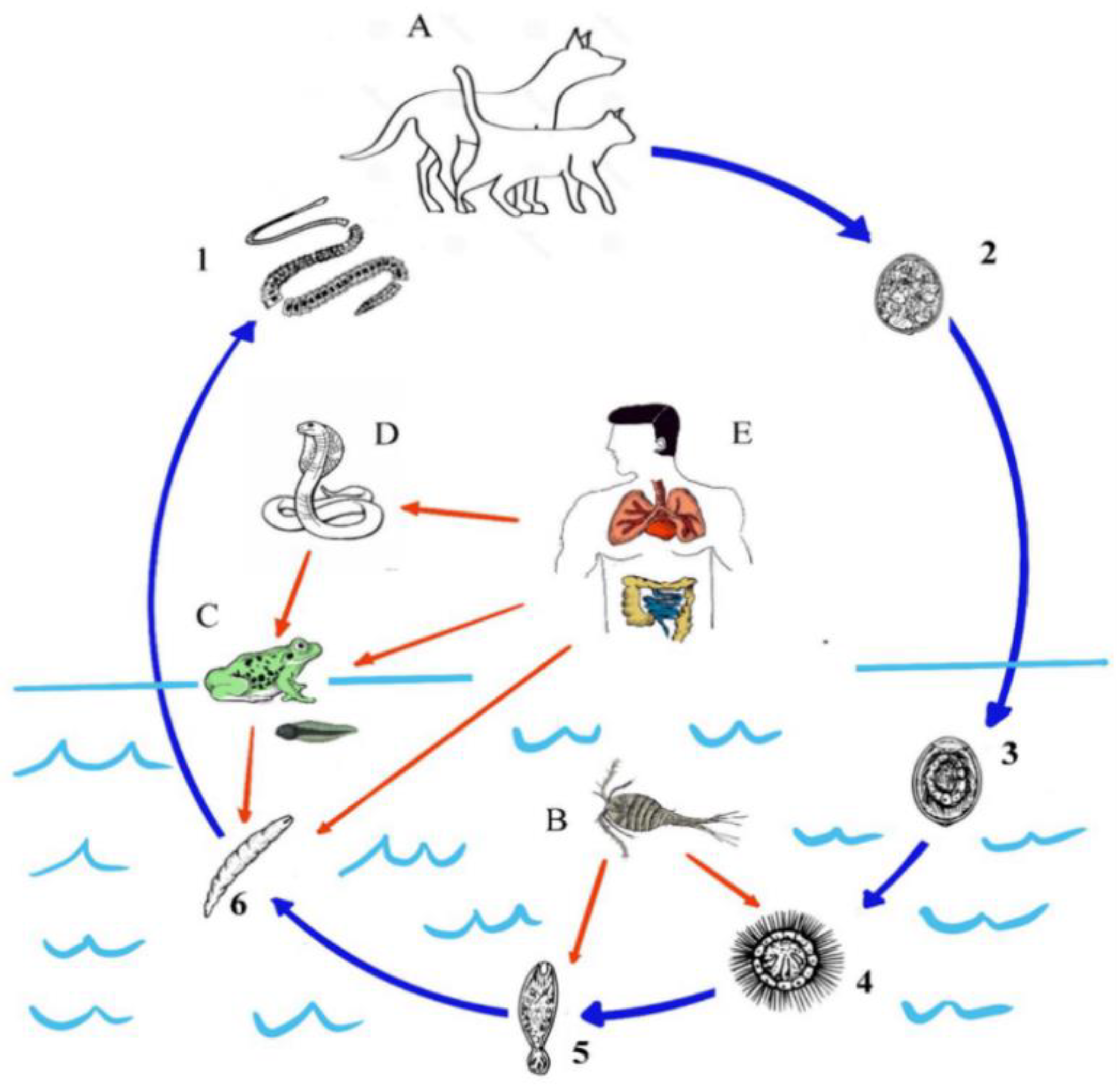
2. Lifecycle
3. Pathogenesis
4. Clinical Features
4.1. Proliferative Sparganosis
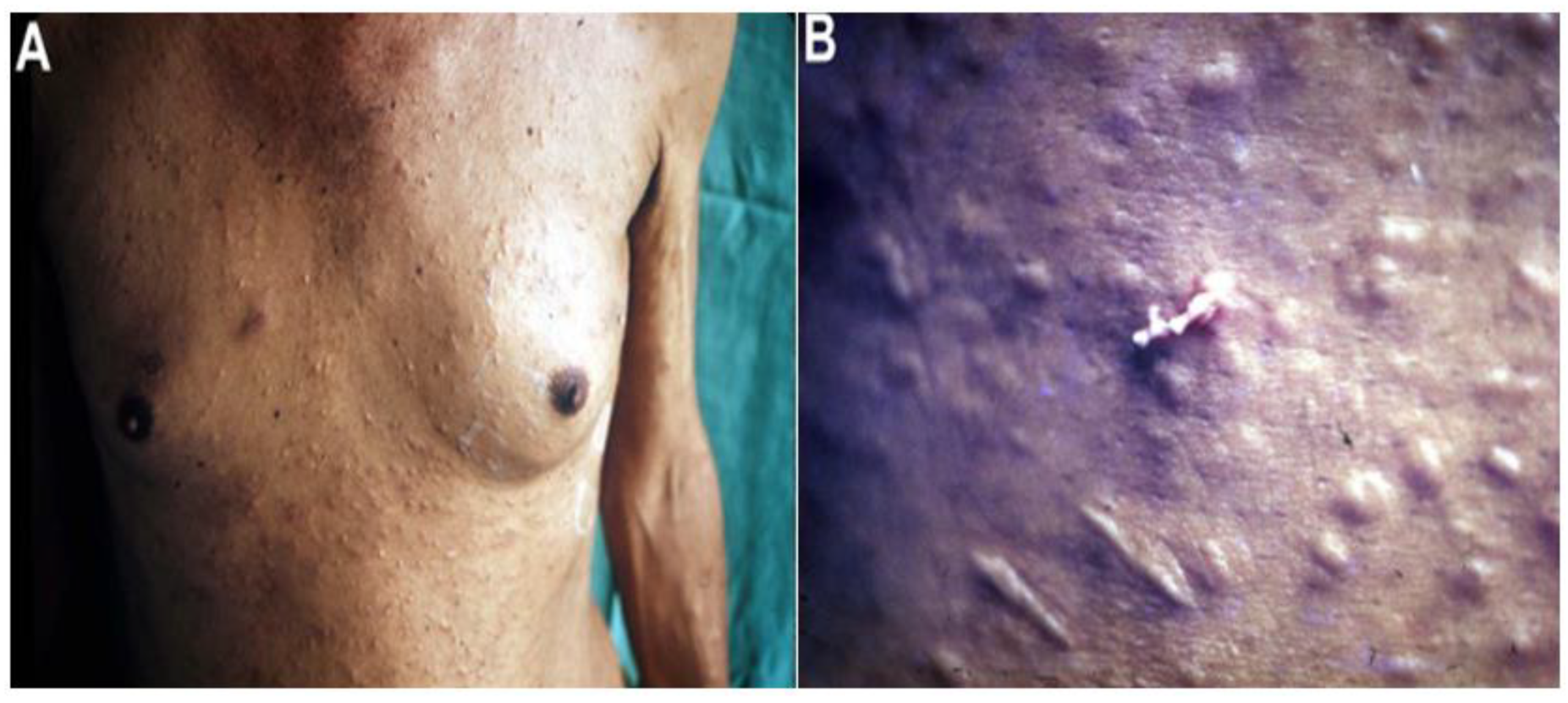
4.2. Nonproliferative Sparganosis
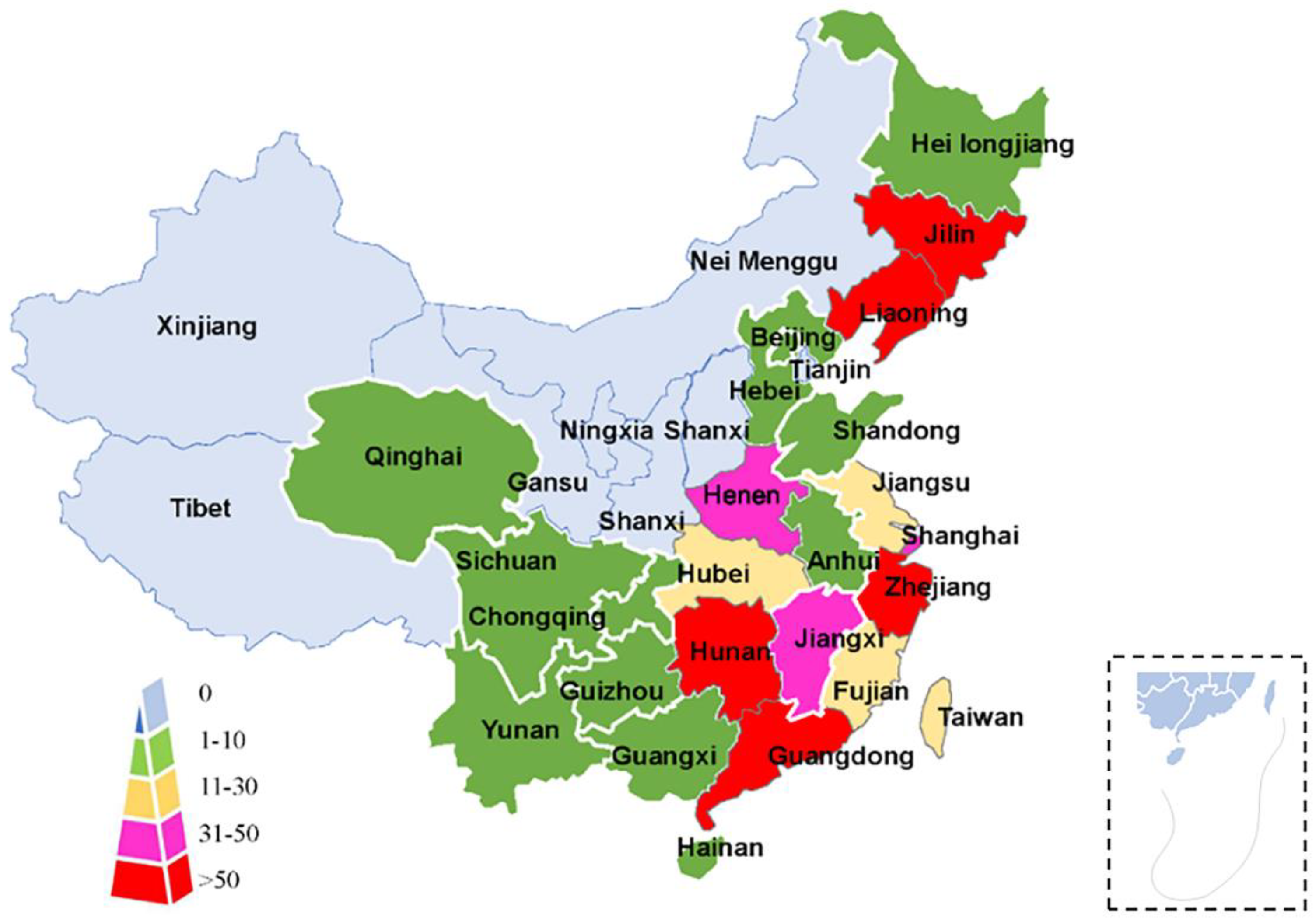
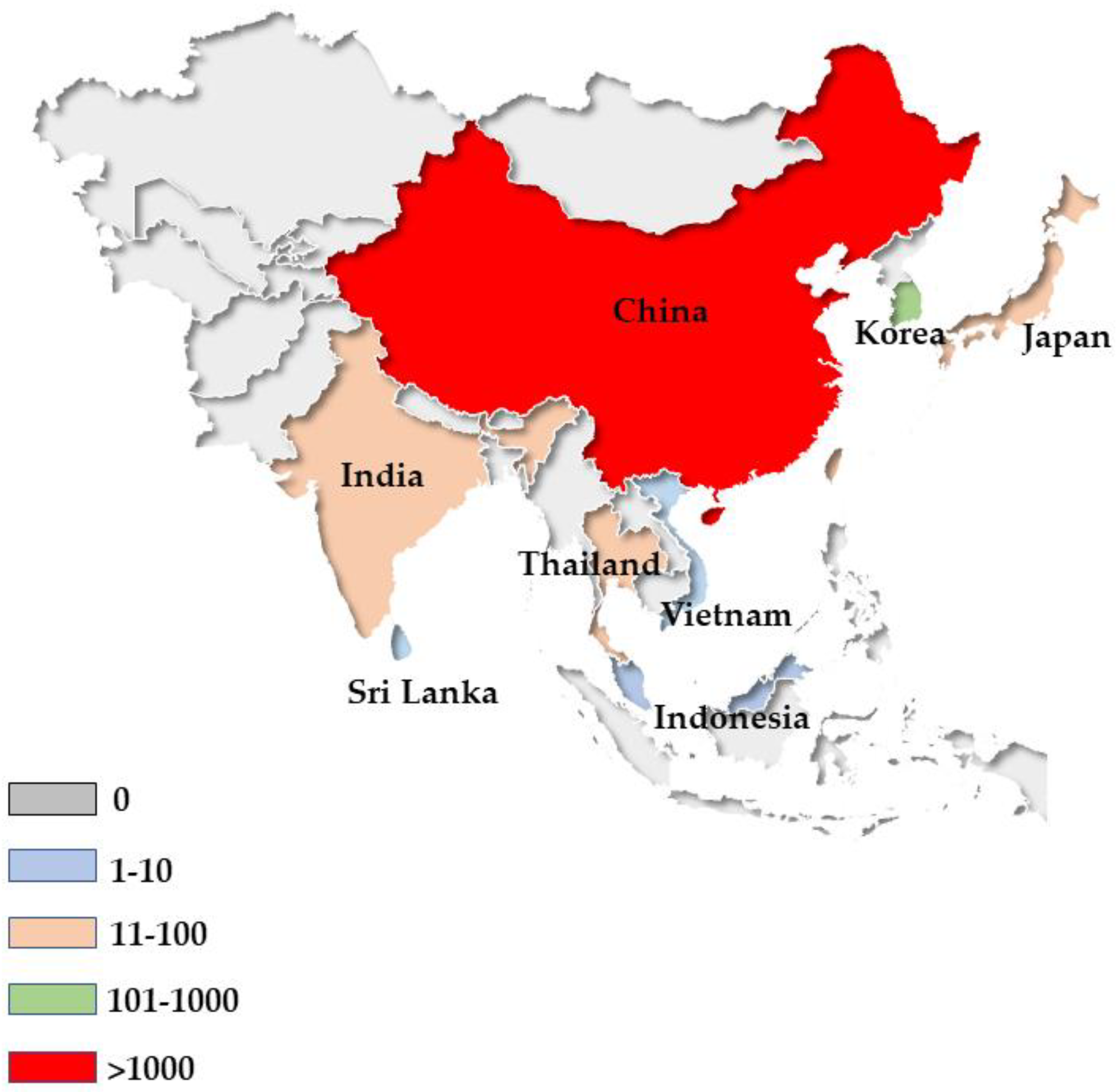
5. Epidemiology
5.1. S. erinaceieuropaei Infection
5.2. Spirometra Decipiens Infection
6. Diagnosis
6.1. Clinical Diagnosis
6.2. Laboratory Diagnosis
6.3. Molecular Diagnosis
7. Control (Including Treatment and Prevention)
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Duan, J.Y.; Shi, Y.L.; Jiang, P.; Zeng, J.; Wang, Z.Q.; Cui, J. Comparative mitochondrial genomics among Spirometra (Cestoda: Diphyllobothriidae) and the molecular phylogeny of related tapeworms. Mol. Phylogenet. Evol. 2017, 117, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, R.; Kołodziej-Sobocińska, M.; Brabec, J.; Młocicki, D.; Sałamatin, R.; Scholz, T. Sparganosis (Spirometra) in Europe in the Molecular Era. Clin. Infect. Dis. 2021, 72, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, R.; Scholz, T. Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth; Special Publication No. 25; Natural History Museum, University of Kansas: Lawrence, KS, USA, 2017; pp. 167–189. [Google Scholar]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Sohn, W.M.; Eom, K.S. Molecular Detection of Spirometra decipiens in the United States. Korean J. Parasitol. 2016, 54, 503–507. [Google Scholar] [CrossRef]
- Anantaphruti, M.T.; Nawa, Y.; Vanvanitchai, Y. Human sparganosis in Thailand: An overview. Acta Trop. 2011, 118, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Song, H.Q.; Li, C.; Lin, H.Y.; Xie, W.T.; Lin, R.Q.; Zhu, X.Q. Sparganosis in mainland China. Int. J. Infect. Dis. 2011, 15, e154–e156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Ahn, C.S.; Sohn, W.M.; Nawa, Y.; Kong, Y. Human Sparganosis in Korea. J Korean Med. Sci. 2018, 33, e273. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.L.; Thiele, E.A.; Yembo, G.E.; Yibi, M.S.; Cama, V.A.; Ruiz-Tiben, E. Thirty-seven human cases of Sparganosis from Ethiopia and South Sudan caused by Spirometra spp. Am. J. Trop. Med. Hyg. 2015, 93, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Liu, D.Y.; Zhang, L.Y. One case of sparganosis in a young child. Chin. J. Parasitol. Parasit. Dis. 2002, 20, 357. [Google Scholar]
- Song, W.J.; Yang, J. The treatment and nursing of a case of sparganosis in a child. Med. J. Chin. People’s Health 2014, 26, 127–128. [Google Scholar]
- Liu, Y.X.; Xu, X.Z.; Tong, D.S. One case of sparganosis in infant. Chin. J. Schistosomiasis Control. 2013, 25, 3. [Google Scholar]
- Yang, Y.; Tang, C.S.; Yao, X.P.; Sun, Y.H.; Yu, Y.L. A case of multiple serous cavity effusion caused by infection of sparganum was reviewed. Mod. Pract. Med. 2015, 27, 1478–1479, 1538. [Google Scholar]
- Bennett, H.M.; Mok, H.P.; Gkrania-Klotsas, E.; Tsai, I.J.; Stanley, E.J.; Antoun, N.M.; Coghlan, A.; Harsha, B.; Traini, A.; Ribeiro, D.M.; et al. The genome of the sparganosis tapeworm Spirometra erinaceieuropaei isolated from the biopsy of a migrating brain lesion. Genome Biol. 2014, 15, 510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.W.; Wang, Z.D.; Zhao, G.H.; Zhu, X.Q. Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 2015, 15, 1226–1235. [Google Scholar] [CrossRef]
- Kikuchi, T.; Maruyama, H. Human proliferative sparganosis update. Parasitol. Int. 2020, 75, 102036. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.N.; Wang, Z.Q.; Zhang, X.; Jiang, P.; Qi, X.; Liu, R.D.; Zhang, Z.F.; Cui, J. Characterization of Spirometra erinaceieuropaei Plerocercoid Cysteine Protease and Potential Application for Serodiagnosis of Sparganosis. PLoS Negl. Trop. Dis. 2015, 9, e0003807. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Chung, Y.B.; Kong, Y. Component proteins and protease activities in excretory-secretory product of sparganum. Korean J. Parasitol. 1992, 30, 227–230. [Google Scholar] [CrossRef]
- Liu, L.N.; Cui, J.; Zhang, X.; Wei, T.; Jiang, P.; Wang, Z.Q. Analysis of structures, functions, and epitopes of cysteine protease from Spirometra erinaceieuropaei Spargana. Biomed. Res. Int. 2013, 2013, 198250. [Google Scholar] [CrossRef] [Green Version]
- Bracaglia, G.; Ranno, S.; Mancinelli, L.; Santoro, M.; Cerroni, L.; Massone, C.; Sangueza, O.; Bravo, F.G.; Diociaiuti, A.; Nicastri, E.; et al. A waterborn zoonotic helminthiase in an Italian diver: A case report of a cutaneous Sparganum infection and review of European cases. Pathog. Glob. Health 2015, 109, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Lin, O.S.; Yeh, K.T. Subcutaneous sparganosis-a case report and a review of human sparganosis in Taiwan. Kaohsiung J. Med. Sci. 1999, 15, 567–571. [Google Scholar]
- Carlson, A.L.; Pruetpongpun, N.; Buppajarntham, A.; Damronglerd, P.; Anderson, N.W.; Apisarnthanarak, A. The Brief Case: Central Nervous System Sparganosis in a 53-Year-Old Thai Man. J. Clin. Microbiol. 2017, 55, 352–355. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Wang, M.; Chen, X.; Tuo, H.Z. A case report: 1-year follow-up of cerebral Spirometra mansoni with a stroke-like onset. BMC Neurol. 2019, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Appannanavar, S.; Bhatti, H.S.; Verma, S. Sparganosis of liver: A rare entity and review of literature. BMJ Case Rep. 2012, 2012, bcr2012006790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.B.; Gao, B.L.; Liu, J.M.; Xu, J.F. Pulmonary Spirometra mansoni: A case report from a non-endemic region. J. Thorac. Dis. 2014, 6, E120–E124. [Google Scholar] [PubMed]
- Chen, X.; Bai, J.; Wang, J.; Cheng, K.; Shen, C.; Yao, H.; Tang, B.; Qian, J. Sparganosis presenting as pericardial effusion and lung lesions. Intern. Med. 2015, 54, 1135–1139. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Baek, D.H.; Lee, B.E.; Kim, G.H.; Song, G.A.; Park, D.Y. Endoscopic resection of sparganosis presenting as colon submucosal tumor: A case report. World J. Gastroenterol. 2016, 22, 4776–4780. [Google Scholar] [CrossRef]
- Oh, M.Y.; Kim, K.E.; Kim, M.J.; Chu, A.; Lee, J.Y.; Park, J.H.; Kim, J.; Hwang, K.T. Breast Sparganosis Presenting with a Painless Breast Lump: Report of Two Cases. Korean J. Parasitol. 2019, 57, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Nath, R.; Gogoi, R. Ocular sparganosis from Assam. Trop Parasitol. 2015, 5, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Hu, J.; Sun, G.; Ding, B.; Feng, L. Orbital sparganosis in an 8-year boy: A case report. BMC Ophthalmol. 2018, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Tong, D.S. Epidemiological investigation and research status of Spirometra mannii in China. Chin. J. School Dr. 2018, 32, 395–439. [Google Scholar]
- Wu, Y.L.; Zhu, S.H.; Dong, H. Preliminary investigation on parasite infection of king snake and red chain snake in Shanghai. J Shanghai Norm. Univ. 2013, 42, 629–634. [Google Scholar]
- Cai, Y.C.; Zhang, Y.N.; Li, H. A case of sparganosis of the brain in a child. Chin. J. Parasitol. Parasit. Dis. 2015, 33, 227. [Google Scholar]
- Chen, Y.L.; Wang, R.B.; Kou, L.F. A case of Spirometra erinaceieuropaei of tapeworm dimagenia mandii and literature review. J. Trop. Dis. Parasitol. 2018, 16, 53–54. [Google Scholar]
- He, H.P.; Chai, X.P.; Yang, G.F. Clinical analysis of 15 cases of Spirometra erinaceieuropaei. Chin. Trop. Med. 2019, 19, 1197–1200. [Google Scholar]
- Shen, M.Q.; Mao, J.Q. A case of Sparganosis with measles was reported. Zhejiang J. Prev. Med. 2019, 27, 933–934. [Google Scholar]
- Fan, X.Y. A case of lungmann’s Sparganosis. Chin. J. Rural. Med. Pharm. 2017, 23, 69–70. [Google Scholar]
- Duan, X.; Xiang, H.J.; Xia, L. Ultrasonographic manifestations of pericardial effusion caused by sparganum in 1 case. J. Ultrasound Clin. Med. 2016, 18, 103–107. [Google Scholar]
- Song, Z.C.; Chen, Y.Q.; Huang, X.H.; Wei, K.Y.; Zhang, L.; Yin, G.W.; Huang, Z.J. Molecular identification and genetic analysis of sparganum from snakes in Fujian province. Progress Vet. Med. 2021, 42, 37–45. [Google Scholar]
- Wang, Y.; Pan, Y.H. Nursing care after removal of live worms from a child with cerebral infection of Spirometra erinaceieuropaei. Nurs. Rehabil. J. 2017, 16, 186–188. [Google Scholar]
- Qian, Q.M. One case of Spirometra erinaceieuropaei after drowning. Zhejiang Med. J. 2018, 40, 2599–2600. [Google Scholar]
- Zhang, L.L.; Wu, B.; Feng, Y. Pathogen identification and epidemiological analysis of adult tapeworms infected with Taenia mandii in human. Chin. J. Parasitol. Parasit. Dis. 2018, 36, 199–201. [Google Scholar]
- Liang, Y.; Li, Z.; Tao, J.X. Clinical radiology. J. Clin. Radiol. 2015, 34, 311–312. [Google Scholar]
- Tan, Z.G.; Chen, G.; Di, H.H. Visceral sparganosis was misdiagnosed as tuberculous pericarditis. J. Int. Intensive Med. 2015, 21, 154–158. [Google Scholar]
- Wang, N.; Huang, M. A case of treatment for sparganosis of mammary gland. J. Pract. Med. 2016, 32, 2970. [Google Scholar]
- Ding, H.F.; Yao, Y.; Lin, K. Subcutaneous infection of Spirometra erinaceieuropaei in human shoulder: A case report. J. Huanggang Polytech. 2018, 20, 104–105. [Google Scholar]
- Xiong, Y.B.; Wang, H.M.; Shao, Q. A case of sparganosis of brain and literature review. Chin. J. Clinic. Neurosurg. 2018, 23, 119–122. [Google Scholar]
- Liu, D.X.; Qin, J.H.; Xu, N. A case of Sparganosis erinaceieuropaei was reported. J Hubei Univ. Sci. Technol. 2018, 32, 363–364, 369. [Google Scholar]
- Xiao, B.; Chen, W.X.; Yang, Z.R. Double infection of Schistosoma japonicum with Sparganosis erinaceieuropaei. Guangxi Med. J. 2019, 41, 1313–1315. [Google Scholar]
- Huang, M.; Wang, N. One case of Sparganosis erinaceieuropaei infection with metacercosis. J. Hubei Univ. Sci. Technol. 2019, 33, 80–81, 93. [Google Scholar]
- Liu, D.; Zhu, W.; Zhou, A.Y.; Luo, J. Ultrasonography was used to diagnose 1 case of subcutaneous recurrence of sparganosis. Chin. Assoc. Ultrasound Med. Eng. 2016, 13, 584. [Google Scholar]
- Luo, Q.P.; Feng, C.Y.; Xia, Y.W. One case of Sparganosis. Lab. Med. Clin. 2016, 13, 3583. [Google Scholar]
- Shi, Q.; Yu, X.H. A case of infection of Spirometra erinaceieuropaei of Manchuria breast. Chin. J. Clin. Lab. Sci. 2019, 8, 200. [Google Scholar]
- Hou, Z.; Li, W.; An, N.; Li, S.Y. Two cases of Spirometra erinaceieuropaei in nervous system. Chin. J. Clin. Neurosurg. 2017, 22, 122. [Google Scholar]
- Wu, D.Y.; Tang, D.J.; Zhang, Y.; He, B.L.; Wang, Y.; Tan, R.J. A case of hypodermic schistosomiasis. Chin. J. Schistosomiasis Control. 2021, 33, 439–441. [Google Scholar]
- Tan, J.L.; Wu, J.; Fu, D.G. Characteristics and surgical treatment strategies of Sparganosis. Chin. J. Minim. Invasive Neurosurg. 2018, 23, 502–505. [Google Scholar]
- Jiang, S.F.; Hong, D.D.; Xie, D. One case of Sparganosis. Guangxi Med. J. 2018, 40, 1504–1505. [Google Scholar]
- Meng, H.; Jin, L.S. One case of Sparganosis of Manchuria. J. Med. Sci. Yanbian Univ. 2019, 42, 147–148. [Google Scholar]
- Pu, Y.L.; Jin, C.Z.; Sung-ja, L. Diagnosis of Cercariae montesiae in mammary gland with automatic total volume imaging. Chin. J. Ultrasound Med. 2018, 34, 480. [Google Scholar]
- Wang, L. Clinical characteristics of 24 cases of Sparganosis. Chin. Zoological. Societ. 2016, 2016, 51. [Google Scholar]
- Tsai, M.D.; Chang, C.N.; Ho, Y.S.; Wang, A.D. Cerebral sparganosis diagnosed and treated with stereotactic techniques. Report of two cases. J. Neurosurg. 1993, 78, 129–132. [Google Scholar] [CrossRef]
- Ho, T.; Lin, M.; Yu, W.; Lai, P.; Sheu, S.; Bee, Y. Ocular sparganosis mimicking an orbital idiopathic inflammatory syndrome. Orbit 2013, 32, 395–398. [Google Scholar] [CrossRef]
- Miyadera, H.; Kokaze, A.; Kuramochi, T.; Kita, K.; Machinami, R.; Noya, O.; Kojima, S. Phylogenetic identification of Spirometra proliferum as a pseudophyllidean cestode by the sequence analyses on mitochondrial COI and nuclear sdhB genes. Parasitol. Int. 2001, 50, 93–104. [Google Scholar] [CrossRef]
- Tappe, D.; Berger, L.; Haeupler, A.; Muntau, B.; Racz, P.; Harder, Y.; Poppert, S. Case report: Molecular diagnosis of subcutaneous Spirometra erinaceieuropaei sparganosis in a japanese immigrant. Am. J. Trop. Med. Hyg. 2013, 88, 198–202. [Google Scholar] [CrossRef]
- Tang, T.H.; Wong, S.S.; Lai, C.K.; Poon, R.W.; Chan, H.S.; Wu, T.C.; Wu, A.K. Molecular identification of Spirometra erinaceieuropaei tapeworm in cases of human sparganosis, Hong Kong. Emerg. Infect. Dis. 2017, 23, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Y.; Ikeuchi, H.; Yuda, K. Case of Sparganum mansoni infection in man and distribution of Diphyllobothrium erinacei in cats and dogs in Miyagi Prefecture. Med. Biol. 1970, 80, 121–124. [Google Scholar]
- Chiba, T.; Yasukochi, Y.; Moroi, Y.; Furue, M. A case of Sparganosis mansoni in the thigh: Serological validation of cure following surgery. Iran. J. Parasitol. 2012, 7, 103–106. [Google Scholar] [PubMed]
- Boonyasiri, A.; Cheunsuchon, P.; Suputtamongkol, Y.; Yamasaki, H.; Sanpool, O.; Maleewong, W.; Intapan, P.M. Nine human sparganosis cases in Thailand with molecular identification of causative parasite species. Am. J. Trop. Med. Hyg. 2014, 91, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, S.H.; Kim, M.J.; Jung, M.; Ko, B.H. Sparganosis of the breast and lower extremities: Sonographic appearance. J. Clin. Ultrasound 2014, 42, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, T.W.; Hong, S.M. Intramuscular sparganosis in the gastrocnemius muscle: A case report. Korean J. Parasitol. 2014, 52, 69–73. [Google Scholar] [CrossRef]
- Park, W.H.; Shin, T.Y.; Yoon, S.M. A case report of testicular sparganosis misdiagnosed as testicular tumor. J. Korean Med. Sci. 2014, 29, 1018–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarukawa, S.; Kawanabe, T.; Yagasaki, A.; Shimizu, A.; Shimada, S. Case of subcutaneous sparganosis: Use of imaging in definitive preoperative diagnosis. J. Dermatol. 2007, 34, 654–657. [Google Scholar] [CrossRef]
- Pampiglione, S.; Fioravanti, M.L.; Rivasi, F. Human sparganosis in Italy. Case report and review of the European cases. APMIS 2003, 111, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B. Modern Parasitology: Release 1; People’s Military Medical Publishing House: Beijing, China, 2002; pp. 715–721. [Google Scholar]
- Sun, Z.F.; Cui, C.S. Investigation on the infection of S. mansonoides in frog in Shangrao area. Chin. J. Vet. Sci. 1992, 2, 182, 187–188. [Google Scholar]
- Yang, G.B.; Zheng, J.G.; Chen, Z.G. Investigation of natural infection of the frog species in Ya’an city with Spirometra erinaceieuropaei. Chin. J. Parasitol. Parasit. Dis. 1992, 1, 36. [Google Scholar]
- Song, C.S.; Zhao, M.Y.; Chen, S.W.; Xu, J.S.; Li, G.; Zhang, S.H. An Investigation of Natural Infection of Schizothora mansii in Changxing County, Zhejiang Province. Chin. J. Parasitol. Parasit. Dis. 1995, 2, 81. [Google Scholar]
- Cui, J.; Jiang, P.; Qi, X.; Xi, X.M.; Li, N.; Wang, M.M.; Wang, Z.Q. Survey on the infection of frog Manchurian head maggot in part of Henan Province. Chin. J. Pathogen Bio. 2012, 7, 787–788, 803. [Google Scholar]
- Li, J.H.; Zhou, D.X.; Wang, T.; He, X.; Sheng, X.F.; Liu, W.; Liu, Y. Investigation on sparganum infection of Rana nigromaculata in Chenzhou city, Hunan province. Hunan J. Anim. Sci. Vet. Med. 2012, 3, 30–31. [Google Scholar]
- Zhang, X.; Cui, J.; Wei, T.; Li, L.Y.; Jiang, J.; Lu, J.C.; Jiang, P.; Liu, L.N.; Wang, Z.Q. Survey and genetic variation of Spirometra erinaceieuropaei sparganum in frogs and snakes from Guangxi of southern China. Trop. Biomed. 2014, 31, 862–870. [Google Scholar]
- Zhang, X.; Hong, X.; Liu, S.N.; Jiang, P.; Zhao, S.C.; Sun, C.X.; Wang, Z.Q.; Cui, J. Large-scale survey of a neglected agent of sparganosis Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae) in wild frogs in China. PLoS Negl. Trop. Dis. 2020, 14, e0008019. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.D.; Xiao, J.J.; Wang, F. Research overview and prevention and control measures of Cercariasis mansonii in Frogs in China. Guangdong For. Sci. Technol. 2013, 29, 62–67. [Google Scholar]
- Liu, W.; Zhao, G.H.; Tan, M.Y.; Zeng, D.L.; Wang, K.Z.; Yuan, Z.G.; Lin, R.Q.; Zhu, X.Q.; Liu, Y. Survey of Spirometra erinaceieuropaei spargana infection in the frog Rana nigromaculata of the Hunan Province of China. Vet. Parasitol. 2010, 173, 152–156. [Google Scholar] [CrossRef]
- Jiang, H.T.; Chen, Y.; Wu, Z.J. Investigation on the infection of Mannius cercariae in frog and snake in parts of Guizhou province. J. Guizhou Norm. Univ. 2008, 1, 5–6, 65. [Google Scholar]
- Deng, Y.; Liu, C.J.; Chen, W.Q. Investigation on the infection status of the frog Manchuria streptococcus in Henan province. Chin. J. Schistosomiasis Control. 2012, 24, 82–84. [Google Scholar]
- Yang, G.D.; Wang, F.M.; Gong, S.P. Analysis on the infection of Spirometra erinaceieuropaei in snakes in Guangdong province. Guangdong For. Sci. Technol. 2015, 31, 80–83. [Google Scholar]
- Zhou, Q.G.; Quan, C.Y.; Zeng, Y. Investigation on infection of frog and snake in Nanning city, Guangxi. Prog. Vet. Med. 2013, 34, 126–128. [Google Scholar]
- Ho, S.Y.; Hwang, K.I.; Seo, B.S. On the Sparganum Mansoni infection In Some Korean terrestrial snakes. Kisaengchunghak Chapchi 1973, 11, 87–94. [Google Scholar]
- Zhang, Y. Investigation on the Status of Serpentine Industry and Infection Rate of Cercariae in Guizhou Province. Master’s Thesis, Guizhou Medical University, Guiyang, China, 2018. [Google Scholar]
- Xu, W.M.; Tang, Y.; Wang, J. Investigation on the infection of Manchuria cercariae in frog and snake in Hangzhou. Dis. Surveill. 2009, 24, 612–613. [Google Scholar]
- Huang, W.D.; Liu, Y.Q.; Hui, Z. Preliminary investigation on infection of Cercariae in Zhanjiang snake. J. Guangdong Med. Univ. 1990, 3, 178–179. [Google Scholar]
- Zhang, T.F. Investigation on the infection of the cercariae in Sichuan wild animal. Chin. J. Parasitol. Parasit. Dis. 2002, 5, 67. [Google Scholar]
- Lu, Y.; Chen, J.X.; Li, H. Infection of the cercariae in Shanghai Zoo. Chin. J. Parasitol. Parasit. Dis. 2018, 36, 593–596. [Google Scholar]
- Zhang, H.F.; Wang, G.H.; Bei, L.V. Investigation on infection and population knowledge, attitude and behavior of Manchuria cercariae in Tongxiang, Zhejiang Province. Dis. Surveill. 2018, 33, 172–174. [Google Scholar]
- Yu, M.F.; Zheng, C.J.; Lu, M. Investigation on the infection of Plercariae mansonii in Rana serrata in Kaihua County. Chin. J. Health Lab. 2021, 31, 2939–2941, 2945. [Google Scholar]
- Liu, W.; Tan, L.; Huang, Y.; Li, W.C.; Liu, Y.S.; Yang, L.C. Prevalence and molecular characterization of Spirometra erinaceieuropaei spargana in snakes in Hunan Province, China. J. Helminthol. 2020, 94, e131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.L.; Liu, X.; Zhang, S.S. Harm and control of the split-headed spider on the white-browed viper. Spec. Prod. Res. 1990, 4, 29–30. [Google Scholar]
- Dai, R.S.; Li, Z.Y.; Li, F.; Liu, D.X.; Liu, W.; Liu, G.H.; He, S.W.; Tan, M.Y.; Lin, R.Q.; Liu, Y.; et al. Severe infection of adult dogs with helminths in Hunan Province, China poses significant public health concerns. Vet. Parasitol. 2009, 160, 348–350. [Google Scholar] [CrossRef]
- Li, M.Z.; Zhang, T.F.; Yang, M.L.; Jie, D.X.; Tang, R.S.; Lei, Z.X.; Li, S.X.; Du, L.T.; Cai, W.M. Schizocephaluscrucianus found in ducks. Chin. Vet. Sci. Technol. 1986, 6, 63. [Google Scholar]
- Dybing, N.A.; Fleming, P.A.; Adams, P.J. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int. J. Parasitol. Parasites Wildl. 2013, 2, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Bauchet, A.L.; Joubert, C.; Helies, J.M.; Lacour, S.A.; Bosquet, N.; Le Grand, R.; Guillot, J.; Lachapelle, F. Disseminated sparganosis in a cynomolgus macaque (Macaca fascicularis). J. Comp. Pathol. 2013, 148, 294–297. [Google Scholar] [CrossRef]
- Zhao, H.G. Infection of Cercariae mansonii by hedgehogs. Chin. J. Parasitol. Parasite. Dis. 1994, 4, 299. [Google Scholar]
- Guo, A.J.; Liu, K.; Gong, W.; Luo, X.N.; Yan, H.B.; Zhao, S.B.; Hu, S.N.; Jia, W.Z. Molecular identification of Diphyllobothrium latum and a brief review of diphyllobothriosis in China. Acta Parasitol. 2012, 57, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Kim, K.H.; Huh, S.; Sohn, W.M.; Chai, J.Y.; Eom, K.S. Human infections with Spirometra decipiens plerocercoids identified by morphologic and genetic analyses in Korea. Korean J. Parasitol. 2015, 53, 299–305. [Google Scholar] [CrossRef]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Kim, K.H.; Sohn, W.M.; Eom, K.S. Genetic identification of Spirometra decipiens plerocercoids in terrestrial snakes from Korea and China. Korean J. Parasitol. 2016, 54, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Kang, Y.; Bia, M.M.; Lee, S.H.; Sohn, W.M.; Hong, S.J.; Chai, J.Y.; et al. Genetic and morphologic identification of Spirometra ranarum in Myanmar. Korean J. Parasitol. 2018, 56, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.K.; Kim, K.H.; Sohn, W.M.; Eom, K.S. Differential diagnosis of human sparganosis using multiplex PCR. Korean J. Parasitol. 2018, 56, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.K.; Huh, S.; Sohn, W.M.; Chai, J.Y.; Eom, K.S. Molecular Genetic Findings of Spirometra decipiens and S. ranarum in Korea. Korean J. Parasitol. 2018, 56, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Eom, K.S. Spirometradecipiens (Cestoda: Diphyllobothriidae) Collected in A Heavily Infected Stray Cat from the Republic of Korea. Korean J. Parasitol. 2018, 56, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.M.; Kim, D.J.; Chang, M.Y.; Kim, Y.J.; Kim, J.H.; You, J.K. Axillary sparganosis, changes in ultrasound images over six months: A case report. Radiol. Case Rep. 2019, 15, 177–180. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, L.; Ding, X.; Wu, J.; Chen, Y. Cerebral sparganosis presenting with atypical postcontrast magnetic resonance imaging findings: A case report and literature review. BMC Infect. Dis. 2019, 19, 748. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Kim, J.T.; Kim, M.K.; Chang, Y.J.; Eom, K.; Park, J.G.; Lee, K.M.; Choe, K.H.; An, J.Y. Eosinophilic pleuritis due to sparganum: A case report. Korean J. Parasitol. 2014, 52, 541–543. [Google Scholar] [CrossRef]
- Lin, Q.; Ouyang, J.S.; Li, J.M.; Yang, L.; Li, Y.P.; Chen, C.S. Eosinophilic pleural effusion due to Spirometra mansoni spargana: A case report and review of the literature. Int. J. Infect. Dis. 2015, 34, 96–98. [Google Scholar] [CrossRef] [Green Version]
- Kavana, N.; Sonaimuthu, P.; Kasanga, C.; Kassuku, A.; Al-Mekhlafi, H.M.; Fong, M.Y.; Khan, M.B.; Mahmud, R.; Lau, Y.L. Seroprevalence of Sparganosis in Rural Communities of Northern Tanzania. Am. J. Trop. Med. Hyg. 2016, 95, 874–876. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, G.H.; Li, F.; He, D.S.; Wang, T.; Sheng, X.F.; Zeng, D.L.; Yang, F.F.; Liu, Y. Sequence variability in three mitochondrial DNA regions of Spirometra erinaceieuropaei spargana of human and animal health significance. J. Helminthol. 2012, 86, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.J.; Liu, G.H.; Liao, C.Y.; Zeng, D.L.; Yang, F.F.; Liu, W. Study on the Polymorphism of Partial Sequence of Mitochondrial Nad1 Gene in Hedgehog Palace Tadpoles. Chin. Anim. Husb. Vet. Med. 2011, 38, 92–94. [Google Scholar]
- Dai, R.S.; Liu, G.H.; Song, H.Q.; Lin, R.Q.; Yuan, Z.G.; Li, M.W.; Huang, S.Y.; Liu, W.; Zhu, X.Q. Sequence variability in two mitochondrial DNA regions and internal transcribed spacer among three cestodes infecting animals and humans from China. J. Helminthol. 2012, 86, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.K.; Deng, X.Z.; Liu, T.B.; Tan, L.; Liu, W. Analysis of phylogenetic relationship of Schizophyllum spp. Based on nad5 gene. Chin. J. Prev. Vet. Med. 2015, 37, 725–727. [Google Scholar]
- Hou, Q.H.; Tan, L.; Deng, X.Z.; Liu, W. Analysis of phylogenetic relationship of Schizophyllum spp. Based on prrns gene sequence. J. Economic. Anima 2016, 20, 74–77. [Google Scholar]
- Jin, Y.C.; Liu, J.H.; Kong, X.X.; Tan, L.; Liu, W. Analysis of phylogenetic relationship of Schizochydra serrata based on ITS gene. Chin. J. Prev. Vet. Med. 2018, 40, 167–169. [Google Scholar]
- Tan, L.; Wang, A.; Zhou, X.; Liu, X.; Kong, X.X.; Liu, W. Analysis of the phylogenetic relationship of Schizophyllum spp. based on ITS gene. Chin. J. Vet. Med. 2017, 37, 1924–1927. [Google Scholar]
- Tan, L.; Wang, A.B.; Kong, X.X.; Liang, X.; He, J.L.; Li, J.; Hu, D.; Liu, W. Study on Polymorphisms of Mitochondrial pnad1 Gene Sequences in Different Species of Snake-headed Salamander from Hunan Province. Chin. Parasitol. Parasit. Mag. 2019, 37, 448–452. [Google Scholar]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Cui, J.; Jiang, P.; Fu, G.M.; Zhong, K.; Zhang, Z.F.; Wang, Z.Q. Characterisation of the relationship between Spirometra erinaceieuropaei and Diphyllobothrium species using complete cytb and cox1 genes. Infect. Genet. Evol. 2015, 35, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, J.; Liu, L.N.; Jiang, P.; Wang, H.; Qi, X.; Wu, X.Q.; Wang, Z.Q. Genetic structure analysis of Spirometra erinaceieuropaei isolates from central and southern China. PLoS ONE 2015, 10, e0119295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Král’ová, I.; Hanzelová, V.; Scholz, T.; Gerdeaux, D.; Spakulová, M. A comparison of the internal transcribed spacer of the ribosomal DNA for Eubothrium crassum and Eubothrium salvelini (Cestoda: Pseudophyllidea), parasites of salmonid fish. Int. J. Parasitol. 2001, 31, 93–96. [Google Scholar] [CrossRef]
- Zhu, X.; Chilton, N.B.; Jacobs, D.E.; Boes, J.; Gasser, R.B. Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int. J. Parasitol. 1999, 29, 469–478. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Stojak, J.; Kondzior, E.; Ruczyńska, I.; Wójcik, J.M. Genetic diversity of two mitochondrial DNA genes in Spirometra erinaceieuropaei (Cestoda: Diphyllobothridae) from Poland. J. Zool. Syst. Evol. Res. 2019, 57, 764–777. [Google Scholar] [CrossRef]
| Province | Brain and Spine | Eye | Breast and Abdomen | Lung | Limbs | Other Positions | References |
|---|---|---|---|---|---|---|---|
| Anhui | 1 | 1 | [32,33] | ||||
| Hunan | 9 | 3 | 2 | 1(Ovary) | [34] | ||
| Zhejiang | 1 | 4 | 2 | 1 | 1(Serum) | [12,35,36,37,38,39,40,41] | |
| Hubei | 2 | 4 | 2 | 1(Thyroid) | [42,43,44,45,46,47,48,49] | ||
| Jiangxi | 2 | 1(Urethra) | [50,51,52] | ||||
| Chongqing | 2 | 1 | [53,54] | ||||
| Guangdong | 79 | [55] | |||||
| Guangxi | 1 | [56] | |||||
| Jilin | 1 | 1 | [57,58] | ||||
| Beijing | 24 | [59] | |||||
| Total | 118 | 1 | 16 | 6 | 2 | 4 |
| Province | No. Positive/No. Tested (Prevalence, %) | Intensity of Infection | References |
|---|---|---|---|
| Guizhou | 47/204 (23) | 1–125 | [83,88] |
| Jiangsu | 3/3 (100.00) | 2–99 | [89] |
| Guangxi | 35/158 (22.2) 3/6 (50) | 1–208 | [79,86] |
| Guangdong | 62/177 (35) | 1–44 | [85,90] |
| Sichuan | 5/16 (31.3) | 1–371 | [91] |
| Shanghai | 45/49 (91.8) | 1–294 | [30,92] |
| Zhejiang | 5/6 (83.3) 5/10 (50.0) | 1–143 | [93,94] |
| Hunan | 344/375 (91.7) | 1–70 | [95] |
| Jilin | 134/435 (30.8) | — | [96] |
| Species | No. Positive/No. Tested (Prevalence, %) | Intensity of Infection |
|---|---|---|
| Elaphe carinata | 91/189 (48.1) | 1~294 |
| Elaphe mandarins | 0/2 (0) | 0 |
| Zaocys dhumnades | 25/45 (55.5) | 1~371 |
| Elaphe taeniura | 8/37 (21.6) | 1~125 |
| Ptyas korros | 15/49 (30.6) | 1~125 |
| Elaphe radiata | 1/21 (4.8) | 1~125 |
| Naja atra | 320/363 (55) | 1~208 |
| Ptyas mucosus | 36/114 (31.5) | 1~208 |
| Lycodon rufozonnatum | 31/53 (58.4) | 0~44 |
| Bungarus multicinctus | 7/48 (14.6) | 0~44 |
| Bungarus fasciatus | 2/22 (9.1) | 1~43 |
| Tryptelytrops albolabris | 1/4 (25) | 1~63 |
| Protobothrops jerdonii | 3/5 (60) | 1~67 |
| Other Species | 0/4 (0) | 0 |
| Total | 542/981 (55.2) | 1~371 |
| Genes | Primers | Sequence (5′-3′) | References |
|---|---|---|---|
| cox1 | spcox1f | 5′-GTA TTG AAG GAA TTA GTT AGG TTA-3′ | [104] |
| spcox1r | 5′-CAA CCC AAT TAA ATT AAG TTC CAC-3′ | ||
| cox1 | Se/Sd-7963F | 5ʹ-ACG TGG TTT GTG GTG GCT CAT TTT-3ʹ | [103] |
| Sd8584R | 5ʹ-GTA TCA AGT TGG TTA GGA AGT TAA-3ʹ | ||
| cox1 | p1f | 5′-TGG TTT TTT GGA CAT CCT GAA -3′ | [107] |
| p1r | 5′-ATC ACA TAA TGA AAG TGA GCC-3′ | ||
| rRNA | rRNA F | 5′GAT TTT GTA AAT CAG GGG GTA-3′ | [107] |
| rRNA R | 5′-AAT TTA TGC GAT TCA CCT TAA-3′ | ||
| nad4 | Se/Sd-1800F | 5′-TAT TTT CGG TTG GTG CTG TAG-3′ | [108] |
| Sd-2317R | 5′-TCC TCC CCC CAC ACG ACA AAA-3′ | ||
| lrDNA | Se/Sd-7955F | 5′-ACG TGG TTT GTG GTG GCT CAT TTT-3′ | [108] |
| Sd-8567R | 5′-TTA TTA ACT TCC TAA CCA ACT TGAT AC-3′ |
| Genes | Primers | Sequence (5′-3′) | References |
|---|---|---|---|
| cox1 | JB3 | 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′ | [82] |
| JB4.5 | 5′-AAAGAAAGAACATAATGAAAATG-3′ | ||
| cox1 | JB3 | 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′ | [122] |
| JB4.5 | 5′-AAAGAAAGAACATAATGAAAATG-3′ | ||
| cox1 | JB3 | 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′ | [95] |
| JB4.5 | 5′-AAAGAAAGAACATAATGAAAATG-3′ | ||
| cox1 | Cox1-F | 5′-TAGACTAAGTGTTTCAAAACACTA-3′ | [123] |
| Cox1-R | 5′-ATAGCATGATCGAAAAGG-3′ | ||
| cox1 | Cox1-F | 5′-TAGACTAAGTGTTTCAAAACACTA-3′ | [124] |
| Cox1-R | 5′-ATAGCATGATGCAAAAGG-3′ | ||
| cox1 | Spi-CO1F | 5′-GACTAAGTGTTTTCAAAACACTAAGTG-3′ | [105] |
| Spi-CO1R | 5′-CAC CCT ACC CCT GAT TTA CAA AAT-3′ | ||
| cox1 | Se658-F | 5′-TTTGATCCTTTGGGTGGTGG-3′ | [67] |
| Se1124-R | 5′-ACCACAAACCACGTGTCATG-3′ | ||
| cox1 | cox1-F | 5′-CGGCTTTTTTTGATCCTTTGGGTGG-3′ | [64] |
| cox1- R | 5′-GTATCATATGAACAACCTAATTTAC-3′ | ||
| cox1 | 12STaen-aFF | 5′-CAC AGT GCC AGC ATC YGC GGT-3′ | [63] |
| 12STaeniaRR | 5′-GAG GGT GAC GGG CGG TGT GTA C-3′ | ||
| cox1 | JB3 | 5′-TTTTTTGGGCATCCTGAGGTTTAT-3′ | [64] |
| JB4.5 | 5′-AAAGAAAGAACATAATGAAAATG-3′ | ||
| cox3 | Secox3F | 5′-GGGTGTCATTTCTTCCTATTTTTAA-3′ | [120] |
| Secox3R | 5′-AAATGTCAATACCAAGTAACTAAAG-3′ | ||
| cytb | Cob-F | 5′-TGATAGTATTAAACTGGC-3′ | [123] |
| Cob-R | 5′-TCAACAGTTGAAACAACCA-3′ | ||
| cytb | Cob-F | 5′-TGATAGTATTAAACTGGC-3′ | [124] |
| Cob-R | 5′-TCAACAGTTGAAACAACCA-3′ | ||
| nad1 | Nad1u | 5′-ATAAGGTGGGGGTGATGGGGTTG-3′ | [121] |
| Nad1d | 5′-ATAAAAAATAAAAGATGAAAGGG-3′ | ||
| nad1 | Nad1u | 5′-ATAAGGTGGGGGTGATGGGGTTG-3′ | [121] |
| Nad1d | 5′-ATAAAAAATAAAAGATGAAAGGG-3′ | ||
| nad1 | Spi-ND1F | 5′-GGA GAATATTGGTTTGTCTAACCA-3′ | [105] |
| Spi-ND1R | 5′-CCTTCTTAACGTTAACAGCATTAC GAT- 3′ | ||
| pnad1 | Senad1F | 5′-ATAAGGTGGGGGTGATGGGGTTG-3′ | [120] |
| Senad1R | 5′-ATAAAAAATAAAAGATGAAAGGG-3′ | ||
| nad4 | ND4F | 5′-GAGTCTCCTTATTCTGAGCG-3′ | [13] |
| ND4R | 5′-ATAGTAGTAGGAAATGAACA-3′ | ||
| pnad4 | Senad4F | 5′-TTTTTTCCTTGGGTTAAGATTAA-3′ | [120] |
| Senad4R | 5′-GCTACTACCCTCAAAAGACTCAC-3′ | ||
| nad5 | SCND5F | 5′-TCATACTGGGTCTATCAGGTGTT-3′ | [122] |
| SCND5R | 5′-ACAGCAAAGTTAGGGGGTAATAGGT-3′ | ||
| ITS | BD1 | 5′-GTCGTAACAAGGTTTCCG-3′ | [125] |
| BD2 | 5′-TATGCTTAAATTCAGCGGGT-3′ | ||
| ITS | NC5 | 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′ | [126] |
| NC2 | 5′-TTAGTTTCTTTTCCTCCGCT-3′ | ||
| ITS | NC5 | 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′ | [119] |
| NC2 | 5′-TTAGTTTCTTTTCCTCCGCT-3′ | ||
| ITS | NC5 | 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′ | [120] |
| NC2 | 5′-TTAGTTTCTTTTCCTCCGCT-3′ | ||
| ITS | BD1 | 5′-GTCGTAACAAGGTTTCCG-3′ | [13] |
| BD2 | 5′-TATGCTTAAATTCAGCGGGT-3′ | ||
| 28s | 28S-F | 5′-CACCGAAGC CTGCGGTA-3′ | [63] |
| 28S-R | 5′-GAAGGTCGACCTGGTGAA-3′ | ||
| prrnS | SCRRNSF | 5′-TAGTTTGGCAGTGAGTTATTCCG-3′ | [118] |
| SCRRNSR | 5′-GGCTACCTTGTTACGACT-TACCTCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Gong, T.; Chen, S.; Liu, Q.; Zhou, H.; He, J.; Wu, Y.; Li, F.; Liu, Y. Epidemiology, Diagnosis, and Prevention of Sparganosis in Asia. Animals 2022, 12, 1578. https://doi.org/10.3390/ani12121578
Liu W, Gong T, Chen S, Liu Q, Zhou H, He J, Wu Y, Li F, Liu Y. Epidemiology, Diagnosis, and Prevention of Sparganosis in Asia. Animals. 2022; 12(12):1578. https://doi.org/10.3390/ani12121578
Chicago/Turabian StyleLiu, Wei, Tengfang Gong, Shuyu Chen, Quan Liu, Haoying Zhou, Junlin He, Yong Wu, Fen Li, and Yisong Liu. 2022. "Epidemiology, Diagnosis, and Prevention of Sparganosis in Asia" Animals 12, no. 12: 1578. https://doi.org/10.3390/ani12121578
APA StyleLiu, W., Gong, T., Chen, S., Liu, Q., Zhou, H., He, J., Wu, Y., Li, F., & Liu, Y. (2022). Epidemiology, Diagnosis, and Prevention of Sparganosis in Asia. Animals, 12(12), 1578. https://doi.org/10.3390/ani12121578








