Simple Summary
The objective of this study was to investigate, for the first time in Greece, the prevalence of ESBL producers in swine populations and to correlate their occurrence with risk factors. A total of 214 fecal samples were collected from the farms from December 2019 to April 2021. A subset of 78 (78/214, 36.5%) ESBL producers were identified as Escherichia coli (E. coli, 88.5%), Klebsiella pneumoniae spp. pneumoniae (K. pneumoniae, 3.8%), Proteus mirabilis (P. mirabilis, 5.1%), Enterobacter cloacae complex (E. cloacae complex, 1.3%) and Salmonella enterica spp. diarizonae (S. enterica spp. diarizonae, 1.3%). CTX-M, SHV and TEM genes were detected along with genes conferring resistance to fluoroquinolones, aminoglycosides, sulfonamides, trimethoprim, macrolides and colistin. This study displayed high antimicrobial resistance rates in the Greek swine industry, and our results are alarming for both human and animal health.
Abstract
This study aimed to estimate the prevalence of extended-spectrum β-lactamase-producing (ESBL) bacteria in swine. Thus, 214 fecal samples were collected from suckling and weaned piglets from 34 farms in Greece (out of an overall population of about 14,300 sows). A subset of 78 (36.5%) ESBL producers were identified as E. coli (69/78, 88.5%), K. pneumoniae spp. pneumoniae (3.8%), P. mirabilis (5.1%), E. cloacae complex (1.3%) and S. enterica spp. diarizonae (1.3%). Resistance to at least one class of non-β-lactam antibiotics was detected in 78 isolates. Among the E. coli strains, resistance was identified with regard to aminoglycosides (n = 31), fluoroquinolones (n = 49), tetracycline (n = 26) and trimethoprim/sulfamethoxazole (n = 46). Of the three K. pneumoniae spp. pneumoniae, two displayed resistances to aminoglycosides and all were resistant to fluoroquinolones, tetracyclines and trimethoprim/sulfamethoxazole. As for the four P. mirabilis isolates, three had a resistant phenotype for aminoglycosides and all were resistant to imipenem, fluoroquinolones, tetracyclines and trimethoprim/sulfamethoxazole. Molecular characterization of the isolates revealed the presence of CTX-M, SHV and TEM genes, as well as of genes conferring resistance to fluoroquinolones, aminoglycosides, sulfonamides, trimethoprim, macrolides and colistin. High levels of antimicrobial resistance (AMR) were demonstrated in Greek swine herds posing a concern for the efficacy of treatments at the farm level as well as for public health.
1. Introduction
By the 1950s, antibiotics were being regularly used in industrial livestock production to secure animals’ health and improve their productivity [1]. Antibiotics are typically used in farms, not only for treatment purposes, but also for regulating the spread of infections (metaphylaxis), inhibiting infections (prophylaxis), especially in high-stress periods (weaning stage, post-vaccination period, after farrowing), and improving feed intake and growth performance [2]. Subtherapeutic doses of antimicrobials were administered to livestock for decades to prevent diseases and/or to enhance growth [3,4], a strategy that promoted the development and spread of antimicrobial resistant strains. Nowadays, the use of antibiotics (e.g., amoxicillin) is included at routine metaphylaxis programs. For instance, injectable amoxicillin can be administered postpartum for metaphylaxis of postpartum dysgalactia syndrome (PPDS) or in weaning feed for the prevention and metaphylaxis of post-weaning diarrhea (PWD) [5,6].
Antimicrobial resistance (AMR) in food-producing animals has drawn global attention, as approximately 70% of the overall antibiotic consumption in Europe is related to the animal sector [7,8]. A major outcome of AMR dissemination is non-effective treatments of livestock, which are further associated with decreased productivity and economic losses due to increased treatment costs [9]. Considering the estimated continuous rise in the global demand for animal derived products, consumption of antimicrobials by livestock is anticipated to increase by two-thirds over the next years [10]. In fact, antimicrobial consumption is estimated to be greater in pigs in comparison to chicken and cattle production systems [10] and a greater possibility of AMR has already been reported in pigs than in chicken and other food animals, or aquaculture [11,12,13]. Furthermore, AMR has been demonstrated in wild boars, a wildlife species that can act as a source of zoonotic pathogens causing human diseases, such as colibacillosis, salmonellosis, yersiniosis and listeriosis [14,15]. AMR in domestic and wild animals poses a hazard for human health by introducing resistant pathogens into the food chain and by triggering horizontal transfer of resistance determinants to other bacteria [16].
Extended spectrum β-lactamase-producing bacteria (ESBL) display resistance to the commonly used beta-lactam antimicrobial agents, including third generation cephalosporins, such as ceftriaxone, ceftazidime and ceftiofur [17]. β-lactams, namely penicillins, carbapenems, monobactams and cephalosporins, constitute 60% (by weight) of all antibiotics used worldwide, and are among the most extensively prescribed antibiotic classes in human medicine [18,19]. Due to excessive usage of β-lactams in both humans and animals, an increased spread of ESBL-producing bacteria has been observed, threatening personnel in the swine industry, and consequently posing a threat to human health [20]. ESBLs are widespread in Enterobacteriaceae, especially in E. coli and Salmonella spp., while ESBL-producing E. coli has been reported in food animals worldwide [21,22]. Previous reports have associated human ESBL carriage with exposure to ESBL-producing Enterobacteriaceae of livestock origin, raising concerns about the possible transfer of ESBL producers through the food chain, which could jeopardize public health [23,24,25,26,27,28].
Reviewing previous literature revealed the scarcity of published data about ESBL-producing Enterobacteriaceae from pig farms in Greece. Thus, the objective of this study was to report, for the first time in Greece, the prevalence of ESBL producers in pig herds, to phenotypically and molecularly identify their antimicrobial resistance patterns and to investigate potential factors that could promote the development of AMR.
2. Materials and Methods
2.1. Ethics
All procedures were performed according to the ethical standards in the Helsinki Declaration of 1975, as revised in 2000, as well as the national law, and after receiving approval (number 96/19.12.2019) from the Institutional Animal Use Ethics Committee of the Faculty of Veterinary Science, University of Thessaly.
2.2. Study Design
The current cross-sectional study was conducted in different regions of Greece for two years (between 2019–2021) and included 34 pig farms. The farms had an overall population of about 14,300 sows, which represented approximately 24% of the entire capacity of the Greek swine production. Farms were in northern (n = 4), central (n = 13), western (n = 10) and southern (n = 7) Greece. Generally, central and western Greece are the regions with the highest pig density (more than 50% of total pig population of Greece). The classifications of pig farms according to their geographic origin and capacity are presented in Table 1.

Table 1.
Geographic origin and capacity of study’s farms.
Farmers or managers consented to participating in the study. The inclusion and exclusion criteria for farm selection are shown in Table 2. These criteria were met by all participating herds in order to include intensive farms that employ common practices of the Greek swine production system, while taking preventive measures for disease control.

Table 2.
Criteria for farms selection.
In addition, data concerning sow age and prior administration of antimicrobials for the last six months were collected. The most used classes of antibiotics were penicillins, collistin, cephalosporins, quinolones, pleuromoutilins (PLMs), macrolides, tetracyclines and trimethoprim/sulfonamides; these were used according to the manufacturers’ instructions with respect to duration of therapy and dosage.
2.3. Sample Collection
A total of 214 fecal samples were collected from 73 suckling and 141 weaning piglets of 34 farms (Table 1). Samples were obtained directly from the rectum by using swabs with Amies transport medium (Transwab®, Amies, UK) and were transferred within a day to the Laboratory of Microbiology and Parasitology (Faculty of Veterinary Medicine, Karditsa, Greece).
2.4. Isolation and Identification of Extended Spectrum Cephalosporin Resistant (ESCR) Strains
For the detection of ESCR isolates, fecal swabs were directly streaked on ESBL selective media (CHROMID® ESBL, BioMérieux, Marcy l’Etoile, France) and the plates were incubated aerobically at 37 °C for 24–48 h. Subcultures were grown on both MacConkey agar and 5% sheep blood agar until pure cultures were obtained. Bacterial species identification was carried out using the automated Vitek-2 system (BioMérieux. Marcy l’Etoile, France), according to the manufacturer’s instructions.
2.5. Isolation and Identification of Salmonella spp.
Isolation of Salmonella spp. was conducted, according to ISO 6579-1:2017. Initially, swabs were agitated and squeezed into sterilized tubes containing 9 mL Buffered Peptone Water (BPW). Subsequently, Modified Semisolid Rappaport-Vassiliadis (MSRV) agar, Xylose Lysine Deoxycholate (XLD) agar, and Salmonella Shigella (SS) agar were used as selective media under the recommended conditions. All presumptive Salmonella colonies were identified as to species using the Vitek-2 system.
2.6. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing of all the obtained strains was performed by the Vitek-2 system. The AST-GN96 card was used to determine the minimum inhibitory concentration (MIC) of the following antimicrobial classes: penicillins (ampicillin-AMP, amoxicillin/clavulanic acid-AMC, ticarcillin/clavulanic acid-TCC), cephalosporins (cefalexin-CEX, cefalotin-CF, cefoperazone-CEP, ceftiofur-CEF, cefquinome-CEQ), carbapenems (imipenem-IMI), aminoglycosides (gentamicin-GEN, neomycin-NEO), quinolones (flumequine-FLU, enrofloxacin-ENR, marbofloxacin-MRX), tetracyclines (tetracycline-TET), amphenicols (florfenicol-FLO), polymyxin B-PL and sulfonamides (trimethoprim/sulfamethoxazole-SXT).
2.7. Phenotypic Confirmation of ESBL Production
All the isolates that presented resistance to 3rd (CEP, CEF)-generation cephalosporins were screened via the double disk synergy test (DDST) or a combination disk test (CDT) for ESBL production, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [30]. In brief, antibiotic disks containing cefotaxime (CTX) (30 μg), ceftazidime (CAZ) (30 μg), cefepime (CPM) (30 μg) and AMC (20 μg/10 μg) were applied at a distance of 20 mm (center to center) on Mueller Hinton agar previously inoculated with an 0.5 McFarland inoculum of the isolate to be tested. After incubation, any enhanced zone of inhibition between cephalosporin disks and the AMC disk or a ‘’keyhole’’ formation in the direction of the disk containing clavulanic acid were considered as evidence for the presence of an ESBL-producing strain. In cases of ambiguous results, a combination disk test was also applied, using CTX and CAZ disks (30 µg each), alone and in combination with clavulanic acid (10 µg). A difference of ≥5 mm in zone diameter between the test using the disks alone and that using the disks combined with clavulanic acid antimicrobial agents was interpreted as ESBL production.
2.8. Antibacterial Resistance Genes of ESBL-Producing Enterobacteriaceae
Isolates that were found to be positive in the DDST or the CDT were characterized using the DNA microarray-based assay CarbaResist from InterArray (FZMB GmbH, Bad Langensalza, Germany). Primer and probe sequences have previously been described in detail [31]. In addition, probes for the detection of the colistin resistance gene family mcr were included on the present microarray (see Supplementary File S1). Protocols and procedures were conducted in accordance with the manufacturer’s instructions (https://www.inter-array.com/Further-Genotyping-Kits, accessed on 10 May 2022). In brief, bacteria were grown overnight on Columbia blood agar. Bacteria were harvested and genomic DNA was extracted using the Qiagen blood and tissue kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. The DNA was used in a multiplexed primer elongation incorporating biotin-16-dUTP. Amplicons were stringently hybridized to the microarray, washed and incubated with a horseradish-peroxidase-streptavidin conjugate. Hybridizations were detected by adding a precipitating dye.
2.9. Statistical Analysis
Principal component analysis (PCA) and hierarchical clustering were used to explore the patterns of antimicrobial co-resistance among the isolated Enterobacteriaceae species and identify clusters of co-resistance [32]. Subsequently, the prevalence of AMR by species was estimated within a Bayesian estimation framework [33].
Logistic regression models were used to assess whether (a) the presence of ESBL-producing strains and (b) antimicrobial resistance to a certain type of antibiotic are associated with a series of candidate variables. Candidate variables for both (a) and (b) were herd size, sow age and administration of antibiotics (penicillins, collistin, cephalosporins, quinolones, pleuromoutilins (PLMs), macrolides, tetracyclines and trimethoprim/sulfonamides) according to the manufacturers’ instructions. All candidate variables were initially screened, one-by-one, with a significance level of 0.25. For the shortlisted variables, collinearity analyses were conducted to identify pairs of collinear variables. For each pair of collinear variables, one was excluded from further analyses. The variable that was retained was the one more strongly associated with the outcome. Variables with p < 0.25 were then tested in the final model and were subsequently reduced by backwards elimination, until only significant (p < 0.05) variables remained.
All analyses were performed in R program [34]. For PCA, we used the prcomp built-in functions, and for Bayesian prevalence estimation, the runjags package [35] and figures were built with the ggplot2 package [36]. For logistic regression, the glmer function was used [37].
3. Results
3.1. Isolation and Identification of ESBL-Producing Enterobacteriaceae
A total of 98 ESCR strains were recovered by selective cultivation from 95 of the 214 (44.4%) swine samples tested. Additionally, five Salmonella spp. isolates were retrieved from an equal number of samples (2.3%).
Seventy-eight (36.5%) isolates presented resistance to 3rd generation cephalosporins and were phenotypically confirmed to produce ESBL. ESBL producers were identified as E. coli (n = 69), K. pneumoniae spp. pneumoniae (n = 3), P. mirabilis (n = 4), E. cloacae complex (n = 1) and S. enterica subsp. diarizonae (n = 1). The results are summarized in Table 3.

Table 3.
Isolation and identification of ESBL-producing bacteria from pig samples.
3.2. Antimicrobial Resistance Phenotype and Genotype of the ESBL-Producing Enterobacteriaceae
All ESBL isolates (n = 78) presented resistance to AMP and to all the cephalosporins tested, apart from four E. coli strains, which were susceptible to CEQ, and the isolate of the E. cloacae complex, which was susceptible to CEP. Resistances to AMC (33.3%) and TCC (26.9%) were also detected, while diminished susceptibility to imipenem was identified only in the four P. mirabilis isolates (5.1%). ESBL phenotypes are illustrated as percentages in Figure 1. Three E. coli isolates could not be retrieved after storage in −80 °C, and were thus not genotypically characterized. Of the remaining 66 E. coli isolates, ESBL genes were detected in 65. In particular, blaCTX-M1/15 was detected in 52 isolates (78.8%), blaCTX-M9 in six (9.1%), blaCTX-M8 in six (9.1%), blaSHV in three (4.5%) and blaTEM in 38 (57.6%), alone (n = 5) or in combination with other variants (n = 33). The K. pneumoniae isolates were found to harbor blaCTX-M1/15 (n = 2), blaSHV (n = 2), blaTEM (n = 1) and blaCTX-M9 (n = 1), while the P. mirabilis harbored blaCTX-M9 (n = 3), blaCTX-M8 (n = 1) and blaTEM (n = 3). Finally, the S. enterica harbored blaTEM, whereas no ESBL genes were detected in the E. cloacae.
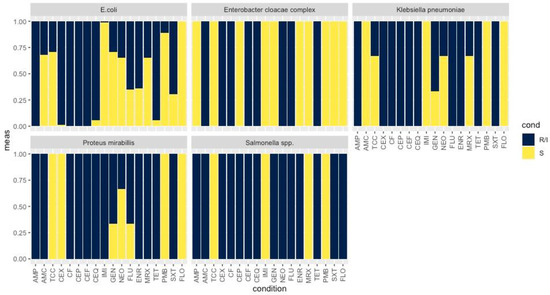
Figure 1.
Bar plot showing the percentage of AMR of E. coli, E. cloacae complex, K. pneumoniae, P. mirabilis and Salmonella spp. to a range of antibiotics; AMP, AMC, TCC, CEX, CF, CEP, CEF, CEQ, IMI, GEN, NEO, FLU, ENR, MRX, TET, SXT and FLO. The values varied from 0.00 to 1.00; 0.00 indicates resistance and 1.00 susceptibility. R/I: Resistance/Intermediate results; S: Susceptibility; AMP: ampicillin, AMC: amoxicillin/clavulanic acid, TCC: ticarcillin/clavulanic acid, CEX: cefalexin, CF: cefalotin, CEP: cefoperazone, CEF: ceftiofur, CEQ: cefquinome, IMI: imipenem, GEN: gentamicin, NEO: neomycin, FLU: flumequine, ENR: enrofloxacin, MRX: marbofloxacin, TET: tetracycline, FLO: florfenicol, PMB: polymyxin B, SXT: trimethoprim/sulfamethoxazole (SXT).
The AmpC gene blaACT was detected in 18 E. coli (27.3%) and the blaCMY in one. Moreover, 21 isolates (31.8%) possessed blaOXA-1 and one blaOXA-60.
The detailed antimicrobial resistance phenotype and genotype of the ESBL isolates is reported in Supplementary File S1.
3.3. Antimicrobial Resistance Phenotype and Genotype of ESBL-Producing Enterobacteriaceae to non β-lactam Antibiotics
All 78 ESBL-producing isolates displayed resistance to at least one class of non β-lactam antibiotics. Among the E. coli strains, resistances were reported for fluoroquinolones (FLU; n = 45, ENR; n = 42, MRX; n = 22), aminoglycosides (GEN; n = 19, NEO; n = 24), TET (n = 26) and SXT (n = 46) (Figure 1, Figure 2 and Figure 3).
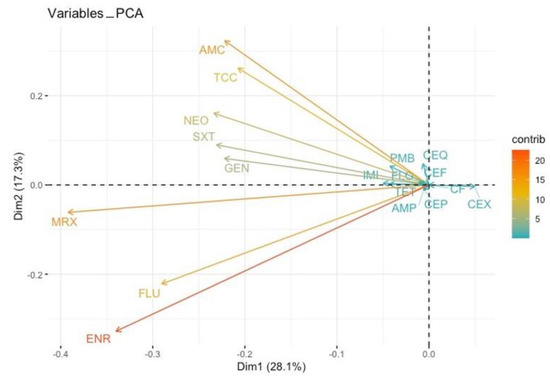
Figure 2.
Principal component analysis (PCA) on co-resistances. Antibiotics are represented by vectors (arrows). Two vectors of antibiotics pointing in the same direction is an indication of a positive correlation between them; when we observe AMR in one antibiotic, we are expecting the development of AMR in the other one. An angle of 180 degrees between the vectors of two antibiotics is an indication of a negative correlation between them; when we observe AMR in one, we are not expecting the development of AMR in the other. A 90-degree angle between the vectors of two antibiotics indicates no relationship between them toward the developing AMR. The longer the vectors, the greater the intensity of this relationship. AMP: ampicillin, AMC: amoxicillin/clavulanic acid, TCC: ticarcillin/clavulanic acid, CEX: cefalexin, CF: cefalotin, CEP: cefoperazone, CEF: ceftiofur, CEQ: cefquinome, IMI: imipenem, GEN: gentamicin, NEO: neomycin, FLU: flumequine, ENR: enrofloxacin, MRX: marbofloxacin, TET: tetracycline, FLO: florfenicol, PMB: polymyxin B, SXT: trimethoprim/sulfamethoxazole (SXT).
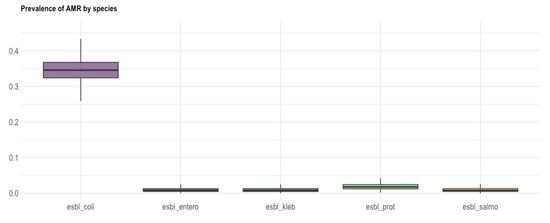
Figure 3.
Box and whisker plot showing the prevalence of AMR (y axis) of ESBL-producing E. coli, E. cloacae complex, K. pneumoniae, P. mirabilis and Salmonella spp. (x-axis). The bold line shows the median value, while the length of the box represents the interquartile range.
Regarding E. coli, resistance genes were detected for fluoroquinolones in 35 isolates (qnrA, qnrB, qnrS), for aminoglycosides in 59 (aadA1, aadA2, aadA4, aphA, rmtA, rmtC, aac(6′)-Ib, aac(3′)-Iva), for sulfonamides in 51 (sul1, sul2, sul3), for trimethoprim in 58 (dfrA1, dfrA12, dfrA13, dfrA14, dfrA15, dfrA17, dfrA19, dfrA5, dfrA7), for macrolides in 16 (mph, mrx) and for colistin in seven (mcr-1/mcr-2, mcr-4, mcr-8). Additionally, the intl1, intl2 and tnpISEcp1 genes associated with mobile elements were identified in 20, seven and 30 E. coli isolates, respectively.
K. pneumoniae spp. pneumoniae isolates (n = 3) were resistant to fluoroquinolone (ENR; n = 3, FLU; n = 3, MRX; n = 1), TET and SXT, and two of the isolates displayed resistances to aminoglycosides (GEN; n = 2, NEO; n = 1) (Figure 1 and Figure 3). All three isolates harbored genes conferring resistance to fluoroquinolones (qnrS), sulfonamides (sul1, sul2) and trimethoprim (dfrA14, dfrA7, dfrA17, dfrA1), while two also possessed aminoglycoside-resistance genes (aadA1, aphA). Genes encoding mobile elements (intl1, intl2, tnpISEcp1) as well as the oqxAB multidrug efflux pump (oqxA, oqxB) were additionally detected in the three isolates.
P. mirabilis isolates (n = 4) were resistant to fluoroquinolones (ENR; n = 4, FLU; n = 3, MRX; n = 3), TET and SXT, while a resistant phenotype for aminoglycosides (GEN; n = 3, NEO; n = 1) was observed in three of them (Figure 1 and Figure 3). Antibacterial resistance genes revealed the presence of genes conferring resistance to sulfonamide (sul1, sul2) and trimethoprim (dfrA1, dfrA5, dfrA17) in all four of them, as well as of aminoglycoside-resistance genes in three (aadA1, aadA2, aphA, aac(6′)-Ib, aac(3′)-Iva). Furthermore, three of the strains harbored intl2.
The isolate of the E. cloacae complex presented intermediate resistance for NEO and TET, was resistant to FLU (Figure 1 and Figure 3) and was only found to harbor dfrA5.
Finally, the S. enterica spp. diarizonae exhibited resistance to fluoroquinolones (FLU, ENR), TET, SXT, aminoglycosides (GEN, NEO) and FLO (Figure 1 and Figure 3). The isolate carried resistance determinants against fluoroquinolones (qnrS), sulfonamides (sul2, sul3), trimethoprim (dfrA12, dfrA17) and aminoglycosides (aadA1, aadA2, aadA4, aphA), as well as the tnpISEcp1.
The antimicrobial resistance phenotype and genotype of the ESBL isolates are detailed in Supplementary File S2. The resistance genes detected among the ESBL-producing Enterobacteriaceae are presented in Table 4.

Table 4.
Resistance genes detected among the ESBL-producing Enterobacteriaceae.
3.4. Logistic Regression Analysis Results
Logistic regression analysis demonstrated that the presence of ESBL-producing E. coli strains was negatively associated with prior administration of PMLs, and that the occurrence of ESBL-producing P. mirabilis was associated with herd size. Further associations concerning ESBL-producing Enterobacteriaceae were not recognized by the model. The results are presented in Table 5.

Table 5.
Associations of ESBL-producing Enterobacteriaceae with herd characteristics and administration of antibiotics.
The reported antimicrobial resistances of this study were not related to the sows’ ages. The development of resistances to AMP and AMC, as well as the diminished susceptibility to IMI, were positively associated with the farm size. By examining whether previous administration of antibiotics led to the development of AMR, we recognized a series of positive associations between (a) GEN resistance and previous administration of quinolones, (b) NEO resistance and previous usage of TETs, (c) MRX resistance and prior application of TETs and (d) ENR resistance and prior administration of cephalosporins. Finally, the previous application of PLMs was negatively associated with the development of resistance to both GEN and NEO. The results are described in Table 6.

Table 6.
Association of AMR with herd characteristics and administration of antibiotics.
4. Discussion
The present study aimed to describe, for the first time, the frequency of ESBL-producing Enterobacteriaceae from 34 pig farms located in different geographical regions of Greece, and to characterize their AMR phenotype and genotype. To that end, we collected and tested 214 fecal samples from 73 suckling and 141 weaning piglets from herds that met certain inclusion criteria. The most commonly isolated ESBL producers were E. coli strains that presented co-resistance to at least one class of non β-lactam antibiotics. In addition, K. pneumoniae, P. mirabilis, E. cloacae complex and S. enterica subsp. diarizonae isolates were identified as ESBL producers. Notably, four P. mirabilis strains displayed diminished susceptibility to IMI.
Resistance to AMC was observed in ESBL-producing E. coli isolates (Figure 3). In the studied farms, injectable amoxicillin was used postpartum in sows as part of a routine program of metaphylaxis for PPDS, and it was also added in weaning feed for the prevention and metalphylaxis of PWD. Resistance to amoxicillin has not become a major problem to date, because it is usually combined with clavulanic acid, a highly effective ESBL inhibitor [5]. As this antimicrobial is routinely administered in pigs against respiratory (e.g., bacterial pneumonia), enteric (post weaning diarrhea) and urogenital (e.g., PPDS) diseases, its unnecessary usage could further promote the selection of ESBL-producing bacteria. Therefore, laboratory diagnosis based on bacterial culture and sensitivity testing is mandatory prior to its application [5]. The observed AMC resistance of E. coli isolates retrieved from the piglets could be also associated with the administration of amoxicillin in sows postpartum, considering that vertical transmission of resistant bacteria from sows to piglets has been already documented [38]. However, future studies, including investigation of related risk factors (e.g., farm capacity and management, previous and current treatments with antibiotics, age, and treatment groups) will help us understand the development of AMR under field conditions.
ESBL-producing E. coli strains presented co-resistances to fluoroquinolones (n = 49), aminoglycosides (n = 31), TETs (n = 26) and SXT (n = 46), confirming previously reported antimicrobial resistance patterns of ESBL-producing E. coli [39,40,41,42]. In fact, the occurrence of strains resistant to AMP, AMC, SXT and TET [43,44], and the occurrence of ESBL producers resistant to at least one more class of non β-lactam antibiotics, have been formerly described in Greece [45]. We also observed increased resistance levels with regard to fluoroquinolones, which are widely used in swine clinical practice as first-choice agents for individual injectable treatment for respiratory, enteric and urogenital disease. This finding could be attributed to the extensive use of this agent at farm level and should be considered by veterinarians and farmers, especially in acute clinical cases that demand rapid treatment processes.
The occurrence of four P. mirabilis ESBL-producing isolates was reported and all strains presented reduced susceptibility to IMI. At first, this was an alarming finding, considering the prohibition of the use of IMI in livestock [46] and the reported carbapenemase resistance in humans [47,48]. As these isolates were not found to harbor a carbapenemase gene, the reported IMI resistance could be explained by pore mutations or mutations affecting a penicillin binding protein [49]. Furthermore, P. mirabilis isolates were also resistant to fluoroquinolones, TETs and SXT, while three of them had a resistant phenotype for aminoglycosides. This bacterial species is considered to be the most common etiological agent of PPDS in sows, causing severe economic losses in swine industry, whereas fluoroquinolones, TET and SXT are recommended as first-choice antimicrobial treatments [6]. Our results reveal a potential risk for treatment failure in field cases of PPDS, further emphasizing the importance of etiological diagnosis and sensitivity testing prior to the use of antibiotics by swine practitioners.
An ESBL-producing S. enterica subsp. diarizonae isolate was also noticed, presenting co-resistance to aminoglycosides, fluoroquinolones (FLU, ENR), TETs, SXT and FLO. Serovars of ESBL-producing S. enterica of poultry origin, co-resistant to aminoglycosides, TETs and SXT have previously been reported in Greece [50]. Herein, we identified a multidrug-resistant serovar in pigs that is usually isolated from humans [51], and thus, our results underline that AMR in livestock could pose, through the food chain, a serious threat to public health.
Molecular characterization of the ESBL isolates revealed the predominance of CTX-M type genes, mainly those of group 1, which is in accordance with preexisting literature about pigs [52,53,54]. Interestingly, various other genes conferring resistance to fluoroquinolones, aminoglycosides, sulfonamides, trimethoprim, macrolides and colistin were detected in the present study. This finding underlines the wide dissemination of AMR determinants among animals farmed for human consumption, which may have been facilitated by the detected mobile genetic elements [55]. The co-occurrence of mcr variants is especially noteworthy given the importance of colistin as a last-resort therapeutic option against multidrug-resistant strains in clinical settings. Colistin is commonly used in pigs to prevent and control the clinical outcomes of E. coli infection, including neonatal diarrhea, post-weaning diarrhea and edema disease [56]. Gene mcr-1, one of the most common colistin-resistance genes around the world, was detected among E. coli isolates from Chinese pigs at slaughter and retail meats, and its occurrence was speculated to be a result of colistin usage [57]. In Greece, multidrug-resistant mcr-1-positive ESBL-producing E. coli has previously been recovered on a dairy farm and was the etiological agent of mastitis [58].
Logistic regression analysis demonstrated a positive association between the herd size and the development of antimicrobial resistance to AMP, AMC and IMI, further confirming previous reports [59,60]. Specifically, it was more likely for AMR to appear in larger farms. A potential explanation for the observed AMP and AMC resistance could be provided by the routine administration of these antimicrobials, as infections in large herds are a more common phenomenon by reason of the higher swine density and the increased rates of gilt replacement from external sources [56]. On the contrary, diminished susceptibility to IMI cannot be ascribed to the usage of carbapenems, since this class of antibiotics is banned for swine due to the risk of compromising human treatments. In P. mirabilis, IMI resistance can be considered random, and it could be speculated that exposure to other beta-lactams might have been the driver (as the mechanism is unspecific).
At a national level, the usage of specific antimicrobials is strongly associated with the development of resistance towards these agents in commensal E. coli isolates in pigs, poultry and cattle [61]. We report a positive association between previous administration of TETs and the development of resistance to NEO and MRX, while increased GEN resistance was observed following quinolone administration. It is well-known that the use of different classes of antibiotics depends on the type of infections to be treated, and varies according to the pigs’ ages [1]. For instance, the gut microbiota is more exposed to orally administered tetracycline than to injected tetracycline, and thus an increase in AMR bacteria in the intestinal microbiome could be promoted by oral administration [62]. It is critical to expand our knowledge on the impact of the administration of different antibiotics in pigs, as increased resistance in bacteria may impair treatment efficacy and potentially lead to therapeutic failure.
Recent studies in Greece and worldwide have described the AMR profile of ESBL-producing E. coli strains isolated from livestock, including pigs [9,45,54]. Currently, as a wide dissemination of multidrug resistant bacteria in diverse ecosystems is observed, the establishment of an integrated antimicrobial surveillance system under the One Health approach is crucial for the protection of public health. The AMR profile of the ESBL-producing Enterobacteriaceae isolates presented in this study demonstrates the urgent need for the application of a monitoring program targeting preferentially multiple species.
5. Conclusions
We report a high percentage of ESBL-producing bacteria in the Greek swine industry, which is triggering alarm for veterinary practitioners and farmers as well as for public health authorities. The 78 ESBL producers (36.5%) were identified as E. coli (n = 69), K. pneumoniae (n = 3), P. mirabilis (n = 4), E. cloacae complex (n = 1) and S. enterica subsp. diarizonae (n = 1), and presented resistance to at least one class of non β-lactam antibiotics. CTX-M-1/15 enzymes were the most frequently observed ESBLs, followed by TEM and SHV types, and accompanied by several other resistance determinants for fluoroquinolones, aminoglycosides, sulfonamides, trimethoprim, macrolides, colistin and mobile genetic elements. As AMR increases the cost of animal production, antimicrobial usage must be prudent and based on laboratory diagnosis and antimicrobial sensitivity testing, in order to keep antibiotics as a therapeutic weapon in experts’ hands. The integration of a surveillance system for AMR monitoring under the One Health approach will contribute not only to the rational use of antibiotics, but also to the protection of public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12121560/s1, Supplementary File S1: Genes detected by the CarbaResist DNA microarray-based assay; Supplementary File S2: Phenotype and Genotype of the ESBL isolates.
Author Contributions
Conceptualization, N.T., V.G.P., Z.A., S.D.B., S.M., R.E., G.C. and C.B.; methodology, N.T., V.G.P., Z.A., S.D.B., P.K., D.G., D.K., R.E., V.S, G.C. and C.B.; software, C.D., S.D.B., P.K., D.G. and D.K.; validation, N.T., V.G.P., Z.A., S.D.B., M.S., S.M., R.E., D.C.C., P.K., V.S, G.C. and C.B.; formal analysis, N.T., V.G.P., Z.A., R.E., C.D.; S.D.B. and S.M.; investigation, N.T., Z.A., C.D., S.D.B., M.S. and D.C.C.; resources, N.T., D.G., D.K. and R.E.; data curation, N.T., Z.A. and S.D.B.; writing—original draft preparation, N.T. and Z.A.; writing—review and editing, N.T., V.G.P., Z.A., S.D.B., M.S., R.E., P.K., S.M., V.S. and C.B.; visualization, C.D., S.D.B. and P.K.; supervision, S.D.B., S.M., R.E., V.G.P., G.C. and C.B.; project administration, S.D.B., S.M., V.G.P., R.E., G.C. and C.B.; funding acquisition, S.D.B., S.M., R.E., M.S., D.C.C., V.S. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under the project “Novel technologies for surveillance and characterization of Extended-spectrum β-lactamase- and Carbapenemase-producing Enterobacteriaceae, in humans and animals (CARBATECH)”, of the Bilateral S&T Cooperation Program Greece–Germany 2017. The European Union and the General Secretariat for Research and Innovation, Ministry of Development & Investments co-funded the Greek side (T2DGE-0944). The Federal Ministry of Education and Research funded the German side (01EI1701 and 13GW0458D). This support is gratefully acknowledged.
Institutional Review Board Statement
All samples were obtained by noninvasive rectal swabs and no research on animals, as defined in the EU Ethics for Researchers document (European Commission, 2013, Ethics for Researchers-Facilitating Research Excellence in FP7, Luxembourg: Office for Official Publications of the European Communities, ISBN 978-92-79-28854-8), was carried out for this study. Handling and sampling of the animals complied with European and national legislation and the study protocol was approved by the academic board of the Veterinary Faculty of the University of Thessaly, Meeting 96/19.12.2019.
Data Availability Statement
All data generated for this study are presented within the manuscript and the supplementary files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of Antibiotic Use in Global Pig Production: A Systematic Review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Veterinary Drug Usage and Antimicrobial Resistance in Bacteria of Animal Origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Burch, D.G.S.; Sperling, D. Amoxicillin—Current Use in Swine Medicine. J. Vet. Pharmacol. Ther. 2018, 41, 356–368. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Alexopoulos, C.; Kyriakis, S.C. Latest Information in Relation to Postpartum Dysgalactia Syndrome of Sows. J. Hell. Vet. Med. Soc. 2007, 58, 61–75. [Google Scholar] [CrossRef][Green Version]
- ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4872 (accessed on 4 May 2022).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar] [CrossRef]
- Bengtsson, B.; Greko, C. Antibiotic Resistance—Consequences for Animal Health, Welfare, and Food Production. Ups. J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Nhung, N.T.; Cuong, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial Usage and Antimicrobial Resistance in Animal Production in Southeast Asia: A Review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef]
- Pollock, J.; Muwonge, A.; Hutchings, M.R.; Mainda, G.; Bronsvoort, B.M.; Gally, D.L.; Corbishley, A. Resistance to Change: AMR Gene Dynamics on a Commercial Pig Farm with High Antimicrobial Usage. Sci. Rep. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Pholwat, S.; Pongpan, T.; Chinli, R.; Rogawski McQuade, E.T.; Thaipisuttikul, I.; Ratanakorn, P.; Liu, J.; Taniuchi, M.; Houpt, E.R.; Foongladda, S. Antimicrobial Resistance in Swine Fecal Specimens Across Different Farm Management Systems. Front. Microbiol. 2020, 11, 1238. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Bogi, S.; Ebani, V.V.; Turini, L.; Nuvoloni, R.; Cerri, D.; Fratini, F.; Turchi, B. Pathotypes and Antimicrobial Susceptibility of Escherichia Coli Isolated from Wild Boar (Sus Scrofa) in Tuscany. Animals 2020, 10, 744. [Google Scholar] [CrossRef]
- Cilia, G.; Turchi, B.; Fratini, F.; Bilei, S.; Bossù, T.; De Marchis, M.L.; Cerri, D.; Pacini, M.I.; Bertelloni, F. Prevalence, Virulence and Antimicrobial Susceptibility of Salmonella Spp., Yersinia Enterocolitica and Listeria Monocytogenes in European Wild Boar (Sus Scrofa) Hunted in Tuscany (Central Italy). Pathogens 2021, 10, 93. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 2–4. [Google Scholar] [CrossRef]
- Lee, W.-C.; Yeh, K.-S. Characteristics of Extended-Spectrum β-Lactamase–Producing Escherichia Coli Isolated from Fecal Samples of Piglets with Diarrhea in Central and Southern Taiwan in 2015. BMC Vet. Res. 2017, 13, 66. [Google Scholar] [CrossRef]
- Livermore, D.M.; Woodford, N. The β-Lactamase Threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 2006, 14, 413–420. [Google Scholar] [CrossRef]
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Bergšpica, I.; Kaprou, G.; Alexa, E.A.; Prieto, M.; Alvarez-Ordóñez, A. Extended Spectrum β-Lactamase (ESBL) Producing Escherichia Coli in Pigs and Pork Meat in the European Union. Antibiotics 2020, 9, 678. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: An Emerging Public-Health Concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Smet, A.; Martel, A.; Persoons, D.; Dewulf, J.; Heyndrickx, M.; Herman, L.; Haesebrouck, F.; Butaye, P. Broad-Spectrum β-Lactamases among Enterobacteriaceae of Animal Origin: Molecular Aspects, Mobility and Impact on Public Health. FEMS Microbiol. Rev. 2010, 34, 295–316. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-Spectrum β-Lactamase-Producing and AmpC-Producing Escherichia Coli from Livestock and Companion Animals, and Their Putative Impact on Public Health: A Global Perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Jouini, A.; Vinué, L.; Slama, K.B.; Sáenz, Y.; Klibi, N.; Hammami, S.; Boudabous, A.; Torres, C. Characterization of CTX-M and SHV Extended-Spectrum β-Lactamases and Associated Resistance Genes in Escherichia Coli Strains of Food Samples in Tunisia. J. Antimicrob. Chemother. 2007, 60, 1137–1141. [Google Scholar] [CrossRef]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-Spectrum β-Lactamase Genes of Escherichia Coli in Chicken Meat and Humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch Patients, Retail Chicken Meat and Poultry Share the Same ESBL Genes, Plasmids and Strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef]
- Kola, A.; Kohler, C.; Pfeifer, Y.; Schwab, F.; Kühn, K.; Schulz, K.; Balau, V.; Breitbach, K.; Bast, A.; Witte, W.; et al. High Prevalence of Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae in Organic and Conventional Retail Chicken Meat, Germany. J. Antimicrob. Chemother. 2012, 67, 2631–2634. [Google Scholar] [CrossRef]
- Dohmen, W.; Bonten, M.J.M.; Bos, M.E.H.; van Marm, S.; Scharringa, J.; Wagenaar, J.A.; Heederik, D.J.J. Carriage of Extended-Spectrum β-Lactamases in Pig Farmers Is Associated with Occurrence in Pigs. Clin. Microbiol. Infect. 2015, 21, 917–923. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine (NRC): Eleventh Revised Edition; The National Academies Press (NAP): Washington, DC, USA, 2012. [Google Scholar]
- Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0; The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020; 77.
- Braun, S.D.; Jamil, B.; Syed, M.A.; Abbasi, S.A.; Weiß, D.; Slickers, P.; Monecke, S.; Engelmann, I.; Ehricht, R. Prevalence of Carbapenemase-Producing Organisms at the Kidney Center of Rawalpindi (Pakistan) and Evaluation of an Advanced Molecular Microarray-Based Carbapenemase Assay. Future Microbiol. 2018, 13, 1225–1246. [Google Scholar] [CrossRef]
- Butiuc-Keul, A.; Carpa, R.; Podar, D.; Szekeres, E.; Muntean, V.; Iordache, D.; Farkas, A. Antibiotic Resistance in Pseudomonas spp. Through the Urban Water Cycle. Curr. Microbiol. 2021, 78, 1227–1237. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Rubin, D.B. Bayesian Data Analysis; Chapman and Hall/CRC: New York, NY, USA, 1995; ISBN 978-0-429-25841-1. [Google Scholar]
- R Core Team (2020). European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 4 May 2022).
- Denwood, M.J. Runjags: An R Package Providing Interface Utilities, Model Templates, Parallel Computing Methods and Additional Distributions for MCMC Models in JAGS. J. Stat. Softw. 2016, 71, 1–25. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Lee, W.; Grimm, K.J. Generalized Linear Mixed-Effects Modeling Programs in R for Binary Outcomes. Struct. Equ. Modeling A Multidiscip. J. 2018, 25, 824–828. [Google Scholar] [CrossRef]
- Burow, E.; Rostalski, A.; Harlizius, J.; Gangl, A.; Simoneit, C.; Grobbel, M.; Kollas, C.; Tenhagen, B.-A.; Käsbohrer, A. Antibiotic Resistance in Escherichia Coli from Pigs from Birth to Slaughter and Its Association with Antibiotic Treatment. Prev. Vet. Med. 2019, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Wang, L.; Peng, Q.; Li, Y.; Zhou, H.; Li, Q. Molecular Characterization of Extended-Spectrum β-Lactamase-Producing Multidrug Resistant Escherichia Coli from Swine in Northwest China. Front. Microbiol. 2018, 9, 1756. [Google Scholar] [CrossRef]
- Aguirre, L.; Vidal, A.; Seminati, C.; Tello, M.; Redondo, N.; Darwich, L.; Martín, M. Antimicrobial Resistance Profile and Prevalence of Extended-Spectrum Beta-Lactamases (ESBL), AmpC Beta-Lactamases and Colistin Resistance (Mcr) Genes in Escherichia Coli from Swine between 1999 and 2018. Porc. Health Manag. 2020, 6, 8. [Google Scholar] [CrossRef]
- Tamta, S.; Kumar, O.R.V.; Singh, S.V.; Pruthvishree, B.S.; Karthikeyan, R.; Rupner, R.; Sinha, D.K.; Singh, B.R. Antimicrobial Resistance Pattern of Extended-Spectrum β-Lactamase-Producing Escherichia Coli Isolated from Fecal Samples of Piglets and Pig Farm Workers of Selected Organized Farms of India. Vet. World 2020, 13, 360–363. [Google Scholar] [CrossRef]
- Lee, S.; An, J.-U.; Guk, J.-H.; Song, H.; Yi, S.; Kim, W.-H.; Cho, S. Prevalence, Characteristics and Clonal Distribution of Extended-Spectrum β-Lactamase- and AmpC β-Lactamase-Producing Escherichia Coli Following the Swine Production Stages, and Potential Risks to Humans. Front. Microbiol. 2021, 12, 710747. [Google Scholar] [CrossRef]
- Valiakos, G.; Vontas, A.; Tsokana, C.N.; Giannakopoulos, A.; Chatzopoulos, D.; Billinis, C. Resistance in Escherichia Coli Strains Isolated from Pig Faecal Samples and Pig Farm Workers, Greece. Am. J. Anim. Vet. Sci. 2016, 11, 142–144. [Google Scholar] [CrossRef][Green Version]
- Papadopoulos, D.; Papadopoulos, T.; Papageorgiou, K.; Sergelidis, D.; Adamopoulou, M.; Kritas, S.K.; Petridou, E. Antimicrobial Resistance Rates in Commensal Escherichia Coli Isolates from Healthy Pigs in Greek Swine Farms. J. Hell. Vet. Med. Soc. 2021, 72, 2909–2916. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia Coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Poirel, L.; Stephan, R.; Perreten, V.; Nordmann, P. The Carbapenemase Threat in the Animal World: The Wrong Culprit. J. Antimicrob. Chemother. 2014, 69, 2007–2008. [Google Scholar] [CrossRef]
- Scientific Opinion on Carbapenem Resistance in Food Animal Ecosystems|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3501 (accessed on 4 May 2022).
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between Antibiotic Consumption and the Rate of Carbapenem-Resistant Gram-Negative Bacteria from China Based on 153 Tertiary Hospitals Data in 2014. Antimicrob. Resist. Infect. Control. 2018, 7, 137. [Google Scholar] [CrossRef]
- Villar, H.E.; Danel, F.; Livermore, D.M. Permeability to Carbapenems of Proteus Mirabilis Mutants Selected for Resistance to Imipenem or Other Beta-Lactams. J. Antimicrob. Chemother. 1997, 40, 365–370. [Google Scholar] [CrossRef][Green Version]
- Politi, L.; Tassios, P.T.; Lambiri, M.; Kansouzidou, A.; Pasiotou, M.; Vatopoulos, A.C.; Mellou, K.; Legakis, N.J.; Tzouvelekis, L.S. Repeated Occurrence of Diverse Extended-Spectrum β-Lactamases in Minor Serotypes of Food-Borne Salmonella Enterica Subsp. Enterica. J. Clin. Microbiol. 2005, 43, 3453–3456. [Google Scholar] [CrossRef]
- Rubira, I.; Figueras, L.P.; Jiménez, J.C.; de Arcaute, M.R.; Ruiz, H.; Ventura, J.A.; Lacasta, D. Salmonella Enterica Subsp. Diarizonae Serotype 61:K:1:5:(7) a Host Adapted to Sheep; IntechOpen: London, UK, 2021; ISBN ISBN 978-1-83969-018-1. [Google Scholar]
- Bernreiter-Hofer, T.; Schwarz, L.; Müller, E.; Cabal-Rosel, A.; Korus, M.; Misic, D.; Frankenfeld, K.; Abraham, K.; Grünzweil, O.; Weiss, A.; et al. The Pheno- and Genotypic Characterization of Porcine Escherichia Coli Isolates. Microorganisms 2021, 9, 1676. [Google Scholar] [CrossRef]
- Balázs, B.; Nagy, J.B.; Tóth, Z.; Nagy, F.; Károlyi, S.; Turcsányi, I.; Bistyák, A.; Kálmán, A.; Sárközi, R.; Kardos, G. Occurrence of Escherichia Coli Producing Extended Spectrum β-Lactamases in Food-Producing Animals. Acta Vet. Hung. 2021, 69, 211–215. [Google Scholar] [CrossRef]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; Accogli, M.; Agnoletti, F.; Agodi, A.; Alborali, G.L.; Arghittu, M.; Auxilia, F.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia Coli from Extraintestinal Infections in Humans and from Food-Producing Animals in Italy: A ‘One Health’ Study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Madec, J.Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-Spectrum β-Lactamase/AmpC- and Carbapenemase-Producing Enterobacteriaceae in Animals: A Threat for Humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Mills, A.; Rushton, J.; Yeung, S. How antibiotics are used in pig farming: A mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob. Health 2020, 5, e001918. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Filioussis, G.; Kachrimanidou, M.; Christodoulopoulos, G.; Kyritsi, M.; Hadjichristodoulou, C.; Adamopoulou, M.; Tzivara, A.; Kritas, S.K.; Grinberg, A. Short communication: Bovine mastitis caused by a multidrug-resistant, mcr-1-positive (colistin-resistant), extended-spectrum β-lactamase-producing Escherichia coli clone on a Greek dairy farm. J. Dairy Sci. 2020, 103, 852–857. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Puister-Jansen, L.F.; van Asselt, E.D.; Burgers, S.L.G.E. Farm Factors Associated with the Use of Antibiotics in Pig Production. J. Anim. Sci. 2011, 89, 1922–1929. [Google Scholar] [CrossRef]
- Ström, G.; Halje, M.; Karlsson, D.; Jiwakanon, J.; Pringle, M.; Fernström, L.-L.; Magnusson, U. Antimicrobial Use and Antimicrobial Susceptibility in Escherichia Coli on Small- and Medium-Scale Pig Farms in North-Eastern Thailand. Antimicrob. Resist. Infect. Control. 2017, 6, 75. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between Veterinary Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: A Report on Seven Countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Ricker, N.; Trachsel, J.; Colgan, P.; Jones, J.; Choi, J.; Lee, J.; Coetzee, J.F.; Howe, A.; Brockmeier, S.L.; Loving, C.L.; et al. Toward Antibiotic Stewardship: Route of Antibiotic Administration Impacts the Microbiota and Resistance Gene Diversity in Swine Feces. Front. Vet. Sci. 2020, 7, 255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).