Fat Deposition and Fat Effects on Meat Quality—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Genetics or Breed Effect on Fat
2.1. Gender
2.2. Environment
2.3. Intramuscular Fat or Marbling
2.4. Development of Adipose Tissue
2.5. Nutritional Restriction
2.6. Endocrine Functions of Adipose Tissue
3. Conclusions and Directions for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, R.T.; Butterfield, R.M. Growth Patterns of Bovine Muscle, Fat and Bone. J. Anim. Sci. 1968, 27, 611–619. [Google Scholar] [CrossRef]
- Robelin, J. Growth of adipose tissues in cattle; partitioning between depots, chemical composition and cellularity. A review. Livest. Prod. Sci. 1986, 14, 349–364. [Google Scholar] [CrossRef]
- Robison, O.W. Growth patterns in swine. J. Anim. Sci. 1976, 42, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Trenkle, A.; Marple, D.N. Growth and development of meat animals. J. Anim. Sci. 1983, 57, 273–283. [Google Scholar]
- Owens, F.N.; Dubeski, P.; Hanson, C.F. Factors that alter the growth and development of ruminants. J. Anim. Sci. 1993, 71, 3138–3150. [Google Scholar] [CrossRef]
- Nürnberg, K.; Wegner, J.; Ender, K. Factors influencing fat composition in muscle and adipose tissue of farm animals. Livest. Prod. Sci. 1998, 56, 145–156. [Google Scholar] [CrossRef]
- Kempster, A.J. Fat partition and distribution in the carcasses of cattle, sheep and pigs: A review. Meat Sci. 1981, 5, 83–98. [Google Scholar] [CrossRef]
- Cole, J.W.; Ramsey, C.B.; Epley, R.H. Simplified Method for Predicting Pounds of Lean in Beef Carcasses. J. Anim. Sci. 1962, 21, 355–361. [Google Scholar] [CrossRef]
- Fourie, P.D.; Kirton, A.H.; Jury, K.E. Growth and development of sheep: II. Effect of breed and sex on the growth and carcass composition of the Southdown and Romney and their cross. N. Z. J. Agric. Res. 1970, 13, 753–770. [Google Scholar] [CrossRef]
- Berg, R.T.; Andersen, B.B.; Liboriussen, T. Growth of bovine tissues 1. Genetic influences on growth patterns of muscle, fat and bone in young bulls. Anim. Sci. 1978, 26, 245–258. [Google Scholar] [CrossRef]
- Butterfield, R.M.; Griffiths, D.A.; Thompson, J.M.; Zamora, J.; James, A.M. Changes in body composition relative to weight and maturity in large and small strains of Australian Merino rams 1. Muscle, bone and fat. Anim. Sci. 1983, 36, 29–37. [Google Scholar] [CrossRef]
- Butler-Hogg, B.W.; Francombe, M.A.; Dransfield, E. Carcass and meat quality of ram and ewe lambs. Anim. Sci. 1984, 39, 107–114. [Google Scholar] [CrossRef]
- Tess, M.W.; Dickerson, G.E.; Nienaber, J.A.; Ferrell, C.L. Growth, Development and Body Composition in Three Genetic Stocks of Swine. J. Anim. Sci. 1986, 62, 968–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiniou, N.; Noblet, J.; Dourmad, J.-Y. Effect of energy intake on the performance of different types of pig from 45 to 100 kg body weight. 2. Tissue gain. Anim. Sci. 1996, 63, 289–296. [Google Scholar] [CrossRef]
- Davies, A.S.; Pearson, G.; Carr, J.R. The carcass composition of male, castrated male and female pigs resulting from two levels of feeding. J. Agric. Sci. 1980, 95, 251–259. [Google Scholar] [CrossRef]
- Berg, R.T.; Walters, L.E. The meat animal: Changes and challenges. J. Anim. Sci. 1983, 57, 133–146. [Google Scholar]
- Pomeroy, R. Live Weight Growth in: Progress in Physiology of Farm Animal; Butterworth: London, UK, 1955. [Google Scholar]
- Davies, A.S.; Pryor, W.J. Growth changes in the distribution of dissectable and intramuscular fat in pigs. J. Agric. Sci. 1977, 89, 257–266. [Google Scholar] [CrossRef]
- Gotoh, T.; Albrecht, E.; Teuscher, F.; Kawabata, K.; Sakashita, K.; Iwamoto, H.; Wegner, J. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 2009, 82, 300–308. [Google Scholar] [CrossRef]
- Pethick, D.; Dunshea, F. The partitioning of fat in farm animals. In Proceeding of the Nutrition Society of Australia, Twentieth Annual Scientific Meeting, Sydney, Australia, 28 September–1 October 1996; pp. 3–4. [Google Scholar]
- Pena, R.N.; Noguera, J.L.; García-Santana, M.J.; González, E.; Tejeda, J.F.; Ros-Freixedes, R.; Ibáñez-Escriche, N. Five genomic regions have a major impact on fat composition in Iberian pigs. Sci. Rep. 2019, 9, 2031. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, F.; Combes, S.; Zened, A.; Meynadier, A. Rumen microbiota and dietary fat: A mutual shaping. J. Appl. Microbiol. 2017, 123, 782–797. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.T.N.; To, K.V.; Schilling, M.W. Fatty Acid Composition of Meat Animals as Flavor Precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M.; Hughes, S.I. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lanier, J.S.; Corl, B.A. Challenges in enriching milk fat with polyunsaturated fatty acids. J. Anim. Sci. Biotechnol. 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harfoot, C.G.; Hazlewood, G.P. Lipid metabolism in the rumen. In The Rumen Microbial Ecosystem; Springer: Dordrecht, The Netherlands, 1997; pp. 382–426. [Google Scholar] [CrossRef]
- Van Nevel, C.J.; Demeyer, I.D. Influence of pH on lipolysis and biohydrogenation of soybean oil by rumen contents in vitro. Reprod. Nutr. Dev. 1996, 36, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beam, T.; Jenkins, T.; Moate, P.; Kohn, R.; Palmquist, D. Effects of Amount and Source of Fat on the Rates of Lipolysis and Biohydrogenation of Fatty Acids in Ruminal Contents. J. Dairy Sci. 2000, 83, 2564–2573. [Google Scholar] [CrossRef]
- Nurnberg, K.; Grumback, S.; Papstein, H.; Matthew, H.; Ender, K.; Nurnberg, G. Fat composition of lamb. Fett/Lipid 1996, 98, 77–80. [Google Scholar]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.; Mosley, E.E. Board-Invited Review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewett, B.; Fursey, G.; Wood, J.; Harrington, G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 1998, 49, 329–341. [Google Scholar] [CrossRef]
- Teye, G.A.; Sheard, P.R.; Whittington, F.M.; Nute, G.R.; Stewart, A.; Wood, J.D. Influence of dietary oils and protein level on pork quality. 1. Effects on muscle fatty acid composition, carcass, meat and eating quality. Meat Sci. 2006, 73, 157–165. [Google Scholar] [CrossRef]
- Teye, G.A.; Wood, J.D.; Whittington, F.M.; Stewart, A.; Sheard, P.R. Influence of dietary oils and protein level on pork quality. 2. Effects on properties of fat and processing characteristics of bacon and frankfurter-style sausages. Meat Sci. 2006, 73, 166–177. [Google Scholar] [CrossRef]
- Smink, W.; Gerrits, W.J.J.; Hovenier, R.; Geelen, M.J.H.; Verstegen, M.W.A.; Beynen, A.C. Effect of dietary fat sources on fatty acid deposition and lipid metabolism in broiler chickens. Poult. Sci. 2010, 89, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Choi, N.-J.; Kurt, E.; Fisher, A.V.; Enser, M.; Wood, J.D. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 2001, 85, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.R.F.; Tweed, J.K.S.; Moloney, A.P.; Scollan, N.D. The effects of fish oil supplementation on rumen metabolism and the biohydrogenation of unsaturated fatty acids in beef steers given diets containing sunflower oil. Anim. Sci. 2005, 80, 361–367. [Google Scholar] [CrossRef]
- Manner, W.; Maxwell, R.J.; Williams, J.E. Effects of Dietary Regimen and Tissue Site on Bovine Fatty Acid Profiles. J. Anim. Sci. 1984, 59, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Terevinto, A.; Saadoun, A.; Cabrera, M.C. From the fatty acid content perspective, is it healthier to eat a hindquarter or a forequarter cut? Angus steers in pasture or concentrate systems. CyTA J. Food 2020, 18, 698–703. [Google Scholar] [CrossRef]
- Daley, A.C.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Alabiso, M.; Maniaci, G.; Giosuè, C.; Gaglio, R.; Francesca, N.; Di Grigoli, A.; Bonanno, A.; Portolano, B. Effect of muscle type and animal category on fatty acid composition of bresaola made from meat of Cinisara cattle: Preliminary investigation. CyTA J. Food 2020, 18, 734–741. [Google Scholar] [CrossRef]
- Kerth, C.R.; Miller, R.K. Beef flavor: A review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef]
- Taylor, C.S.; Murray, J.I.; Thonney, M.L. Breed and sex differences among equally mature sheep and goats 4. Carcass muscle, fat and bone. Anim. Sci. 1989, 49, 385–409. [Google Scholar] [CrossRef]
- Berg, R.T.; Jones, S.D.M.; Price, M.A.; Hardin, R.T.; Fukuhara, R.; Butterfield, R.M. Patterns of carcass fat deposition in heifers, steers and bulls. Can. J. Anim. Sci. 1979, 59, 359–366. [Google Scholar] [CrossRef]
- Maniaci, G.; Alabiso, M.; Francesca, N.; Giosuè, C.; Di Grigoli, A.; Corona, O.; Bonanno, A.; Cardamone, C.; Graci, G.; Portolano, B. Bresaola made from Cinisara cattle: Effect of muscle type and animal category on physicochemical and sensory traits. CyTA J. Food 2020, 18, 383–391. [Google Scholar] [CrossRef]
- Woodworth, J.; Bohrer, B.; Faccin, J. Characterizing the Differences between Barrow and Gilt Growth Performance, Carcass Composition, and Meat Quality. Available online: https://www.asi.k-state.edu/research-and-extension/swine/Characterizing%20barrow%20vs%20gilt%20performance%20differences.pdf (accessed on 26 April 2022).
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heng, J.; Tian, M.; Zhang, W.; Chen, F.; Guan, W.; Zhang, S. Maternal heat stress regulates the early fat deposition partly through modification of m6A RNA methylation in neonatal piglets. Cell Stress Chaperon- 2019, 24, 635–645. [Google Scholar] [CrossRef]
- Kouba, M.; Hermier, D.; Le Dividich, J. Influence of a high ambient temperature on lipid metabolism in the growing pig. J. Anim. Sci. 2001, 79, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.Y.; Wang, J.; Sun, P.; Bu, D.P. The effect of heat stress on the metabolism of dairy cows: Updates & review. Austin J. Nutr. Metab. 2016, 3, 1036. [Google Scholar]
- Lu, Q.; Wen, J.; Zhang, H. Effect of Chronic Heat Exposure on Fat Deposition and Meat Quality in Two Genetic Types of Chicken. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef]
- Soren, N.M. Nutritional Manipulations to Optimize Productivity During Environmental Stresses in Livestock. In Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 181–218. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Maltecca, C.; Bergamaschi, M.; Tiezzi, F. The interaction between microbiome and pig efficiency: A review. J. Anim. Breed. Genet. 2020, 137, 4–13. [Google Scholar] [CrossRef]
- Nishimura, T.; Hattori, A.; Takahashi, K. Structural changes in intramuscular connective tissue during the fattening of Japanese black cattle: Effect of marbling on beef tenderization. J. Anim. Sci. 1999, 77, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Harper, G.S.; Pethick, D.W. How might marbling begin? Austr. J. Exp. Agric. 2004, 44, 653–662. [Google Scholar] [CrossRef]
- Pearson, A.M. Desirability of Beef—Its Characteristics and Their Measurement. J. Anim. Sci. 1966, 25, 843–854. [Google Scholar] [CrossRef]
- Vierck, K.R.; Gonzalez, J.M.; Houser, T.A.; Boyle, E.A.E.; O’Quinn, T.G. Marbling Texture’s Effects on Beef Palatability. Meat Muscle Biol. 2018, 2, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Zhu, H.; Mao, Y.; Zhang, Y.; Zhu, L.; Cornforth, D.; Wang, R.; Meng, X.; Luo, X. Tenderness and sensory attributes of the longissimus lumborum muscles with different quality grades from Chinese fattened yellow crossbred steers. Meat Sci. 2016, 112, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, B.B.; Cooper, C.C.; Cassens, R.G.; Evans, G.; Bray, R.W. Influence of Marbling and Maturity on the Palatability of Beef Muscle. I. Chemical and Organoleptic Considerations. J. Anim. Sci. 1968, 27, 1532–1541. [Google Scholar] [CrossRef]
- Blumer, T.N. Relationship of Marbling to the Palatability of Beef. J. Anim. Sci. 1963, 22, 771–778. [Google Scholar] [CrossRef]
- Jeremiah, L.E. The influence of subcutaneous fat thickness and marbling on beef: Palatability and consumer acceptability. Food Res. Int. 1996, 29, 513–520. [Google Scholar] [CrossRef]
- Aalhus, J.L.; Janz, J.A.M.; Tong, A.K.W.; Jones, S.D.M.; Robertson, W.M. The influence of chilling rate and fat cover on beef quality. Can. J. Anim. Sci. 2001, 81, 321–330. [Google Scholar] [CrossRef]
- Smith, G.C.; Carpenter, Z.L.; Cross, H.R.; Murphey, C.E.; Abraham, H.C.; Savell, J.W.; Parrish, F.C., Jr.; Davis, G.W.; Berry, W. Relationship of USDA marbling groups to palatability of cooked beef. J. Food Qual. 1985, 7, 289–308. [Google Scholar] [CrossRef]
- Davis, G.W.; Smith, G.C.; Carpenter, Z.L.; Cross, H.R. Relationships of Quality Indicators to Palatability Attributes of Pork Loins. J. Anim. Sci. 1975, 41, 1305–1313. [Google Scholar] [CrossRef]
- Brewer, M.S.; Zhu, L.G.; McKeith, F.K. Marbling effects on quality characteristics of pork loin chops: Consumer purchase intent, visual and sensory characteristics. Meat Sci. 2001, 59, 153–163. [Google Scholar] [CrossRef]
- Cannata, S.; Engle, T.E.; Moeller, S.J.; Zerby, H.N.; Radunz, A.E.; Green, M.D.; Bass, P.; Belk, K.E. Effect of visual marbling on sensory properties and quality traits of pork loin. Meat Sci. 2010, 85, 428–434. [Google Scholar] [CrossRef]
- McBee, J.L., Jr.; Wiles, J.A. Influence of marbling and carcass grade on the physical and chemical characteristics of beef. J. Anim. Sci. 1967, 26, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Corbin, C.; O’Quinn, T.; Garmyn, A.; Legako, J.; Hunt, M.; Dinh, T.; Rathmann, R.; Brooks, J.; Miller, M. Sensory evaluation of tender beef strip loin steaks of varying marbling levels and quality treatments. Meat Sci. 2015, 100, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M. The effects of marbling on flavour and juiciness scores of cooked beef, after adjusting to a constant tenderness. Aust. J. Exp. Agric. 2004, 44, 645–652. [Google Scholar] [CrossRef]
- Iida, F.; Saitou, K.; Kawamura, T.; Yamaguchi, S.; Nishimura, T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim. Sci. J. 2014, 86, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, R.J.; Thompson, J.M. Meat standards and grading: A world view. Meat Sci. 2010, 86, 227–235. [Google Scholar] [CrossRef]
- Hale, D.S.; Goodson, K.; Savell, J.W. USDA Beef Quality and Yield Grades; Texas A&M AgriLife Extension Service: College Station, TX, USA, 2013. [Google Scholar]
- USDA. United States Standards for Grades of Carcass Beef. 2017. Available online: https://www.ams.usda.gov/sites/default/files/media/CarcassBeefStandard.pdf (accessed on 13 June 2022).
- Park, S.J.; Beak, S.-H.; Jung, D.J.; Yeob, K.S.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Baik, M.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle– A review. Asian-Aust. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [Green Version]
- Piao, M.Y.; Baik, M. Seasonal Variation in Carcass Characteristics of Korean Cattle Steers. Asian-Austr. J. Anim. Sci. 2015, 28, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, S.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- Bessa, R.; Santos-Silva, J.; Ribeiro, J.; Portugal, A. Reticulo-rumen biohydrogenation and the enrichment of ruminant edible products with linoleic acid conjugated isomers. Livest. Prod. Sci. 2000, 63, 201–211. [Google Scholar] [CrossRef]
- Lewis, S.L.; Tam, P.O. Definitive endoderm of the mouse embryo: Formation, cell fates and morphogenetic function. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Tzouanacou, E.; Wegener, A.; Wymeersch, F.J.; Wilson, V.; Nicolas, J.-F. Redefining the Progression of Lineage Segregations during Mammalian Embryogenesis by Clonal Analysis. Dev. Cell 2009, 17, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.G.C.; Berghella, L.; Coletta, M.; Lattanzi, L.; Zanchi, M.; Gabriella, M.; Ponzetto, C.; Cossu, G. Skeletal Myogenic Progenitors Originating from Embryonic Dorsal Aorta Coexpress Endothelial and Myogenic Markers and Contribute to Postnatal Muscle Growth and Regeneration. J. Cell Biol. 1999, 147, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasi, M.G.; Riminucci, M.; De Angelis, L.; Borello, U.; Berarducci, B.; Innocenzi, A.; Caprioli, A.; Sirabella, D.; Baiocchi, M.; De Maria, R.; et al. The meso-angioblast: A multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Dev. Dis. 2002, 179, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Young, H.E.; Steele, T.A.; Bray, R.A.; Hudson, J.; Floyd, J.A.; Hawkins, K.; Thomas, K.; Austin, T.; Edwards, C.; Cuzzourt, J.; et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec. Off. Publ. Am. Assoc. Anat. 2001, 264, 51–62. [Google Scholar] [CrossRef]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef] [Green Version]
- Rodeheffer, M.S.; Birsoy, K.; Friedman, J.M. Identification of White Adipocyte Progenitor Cells In Vivo. Cell 2008, 135, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.L.; Marshall, M.A.; McSkimming, C.C.; Harmon, D.B.; Garmey, J.C.; Oldham, S.N.; Hallowell, P.; McNamara, C.A. Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Mol. Metab. 2015, 4, 779–794. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Naslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Pires-Dasilva, A.; Sommer, R.J. The evolution of signalling pathways in animal development. Nat. Rev. Genet. 2003, 4, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.F.; Farmer, S. Hormonal Signaling and Transcriptional Control of Adipocyte Differentiation. J. Nutr. 2000, 130, 3116S–3121S. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yin, J.; Zhu, M.J. Cellular signaling pathways regulating the initial stage of adipogenesis and marbling of skeletal muscle. Meat Sci. 2010, 86, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C.; Cousin, W.; Plaisant, M.; Dani, C.; Peraldi, P. Hedgehog Signaling Alters Adipocyte Maturation of Human Mesenchymal Stem Cells. Stem Cells 2009, 26, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.; de Vries-Smits, L.; Barker, N.; Polderman, P.; Burgering, B.; Korswagen, H. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005, 308, 1181–1184. [Google Scholar] [CrossRef]
- Carter, M.E.; Brunet, A. FOXO transcription factors. Curr. Biol. 2007, 17, 113–114. [Google Scholar] [CrossRef] [Green Version]
- Darnell, J.E., Jr. STATs and Gene Regulation. Science 1997, 225, 1630–1635. [Google Scholar] [CrossRef]
- Garcés, C.; Hidalgo, M.J.R.; de Mora, J.F.; Park, C.; Miele, L.; Goldstein, J.; Bonvini, E.; Porras, A.; Laborda, J. Notch-1 Controls the Expression of Fatty Acid-activated Transcription Factors and Is Required for Adipogenesis. J. Biol. Chem. 1997, 272, 29729–29734. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.M.; Pan, Y.; Herreman, A.; Hadland, B.K.; De Strooper, B.; Kopan, R.; Huppert, S.S. Notch pathway is dispensable for adipocyte specification. Genesis 2004, 40, 40–44. [Google Scholar] [CrossRef]
- Ross, D.A.; Rao, P.K.; Kadesch, T. Dual Roles for the Notch Target Gene Hes-1 in the Differentiation of 3T3-L1 Preadipocytes. Mol. Cell. Biol. 2004, 24, 3505–3513. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Spotts, G.D.; Halvorsen, Y.-D.; Shih, H.-M.; Ellenberger, T.; Towle, H.C.; Spiegelman, B.M. Dual DNA binding specificity of ADD1/SREBP1 controlled by a single amino acid in the basic helix-loop-helix domain. Mol. Cell. Biol. 1995, 15, 2582–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seillet, C.; Belz, G.T. Terminal differentiation of dendritic cells in development and function of myeloid subsets. Adv. Immunol. 2013, 120, 185–210. [Google Scholar] [PubMed]
- Greenwood, P.L.; Cafe, L.M. Prenatal and pre-weaning growth and nutrition of cattle: Long-term consequences for beef production. Anim. Consort. 2007, 1, 1283–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, B.W.; Leddy, K.F.; Bond, J.; Rumsey, T.S.; Hammond, A.C. Effects of Silage Diets and Electrical Stimulation on the Palatability, Cooking and pH Characteristics of Beef Loin Steaks. J. Anim. Sci. 1988, 66, 892–900. [Google Scholar] [CrossRef]
- Vestergaard, M.; Oksbjerg, N.; Henckel, P. Influence of feeding intensity, grazing and finishing feeding on muscle fibre characteristics and meat colour of semitendinosus, longissimus dorsi and supraspinatus muscles of young bulls. Meat Sci. 2000, 54, 177–185. [Google Scholar] [CrossRef]
- Dıáz, M.T.; Velasco, S.; Caneque, V.; Lauzurica, S.; Ruiz De Huidobro, F.; Pérez, C.; González, J.; Manzanares, C. Use of concentrate or pasture for fattening lambs and its effect on carcass and meat quality. Small Rumin. Res. 2002, 43, 257–268. [Google Scholar] [CrossRef]
- Owens, F.N.; Gill, D.R.; Secrist, D.S.; Coleman, S.W. Review of some aspects of growth and development of feedlot cattle. J. Anim. Sci. 1995, 73, 3152–3172. [Google Scholar] [CrossRef]
- Kristensen, L.; Therkildsen, M.; Riis, B.; Sørensen, M.T.; Oksbjerg, N.; Purslow, P.P.; Ertbjerg, P. Dietary-induced changes of muscle growth rate in pigs: Effects on invivo and postmortem muscle proteolysis and meat quality. J. Anim. Sci. 2002, 80, 2862–2871. [Google Scholar] [CrossRef]
- Sainz, R.D.; De La Torre, F.; Oltjen, J.W. Compensatory growth and carcass quality in growth-restricted and refed beef steers. J. Anim. Sci. 1995, 73, 2971–2979. [Google Scholar] [CrossRef]
- Drouillard, J.S.; Klopfenstein, T.J.; Britton, A.R.; Bauer, M.L.; Gramlich, S.M.; Wester, T.J.; Ferrell, C.L. Growth, body composition, and visceral organ mass and metabolism in lambs during and after metabolizable protein or net energy restrictions. J. Anim. Sci. 1991, 69, 3357–3375. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.J.; Starkey, D.L.; Calkins, C.R.; Crouse, J.D. Myofibrillar protein turnover in feed-restricted and realimented beef cattle. J. Anim. Sci. 1990, 68, 2707–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therkildsen, M.; Riis, B.; Karlsson, A.; Kristensen, L.; Ertbjerg, P.; Purslow, P.; Oksbjerg, N.; Aaslyng, M.D. Compensatory growth response in pigs, muscle protein turn-over and meat texture: Effects of restriction/re-alimentation period. J. Anim. Sci. 2002, 75, 367–377. [Google Scholar] [CrossRef]
- Therkildsen, M.; Vestergaard, M.B.; Jensen, M.; Riis, B.; Karlsson, A.; Kristensen, L.; Ertbjerg, P.; Oksbjerg, N. Compensatory growth in slaughter pigs—In vitro muscle protein turnover at slaughter, circulating IGF-1, performance and carcass quality. Livest. Prod. Sci. 2004, 88, 63–75. [Google Scholar] [CrossRef]
- Thomas, G.B.; Mercer, J.E.; Karalis, T.; Rao, A.; Cummins, J.T.; Clarke, I.J. Effect of Restricted Feeding on the Concentrations of Growth Hormone (GH), Gonadotropins, and Prolactin (PRL) in Plasma, and on the Amounts of Messenger Ribonucleic Acid for GH, Gonadotropin Subunits, and PRL in Pituitary Glands of Adult Ovariectomized Ewes. Endocrinology 1990, 126, 1361–1367. [Google Scholar] [CrossRef]
- Maes, M.; Underwood, L.E.; Ketelslegers, J.-M. Plasma somatomedin-C in fasted and refed rats: Close relationship with changes in liver somatogenic but not lactogenic binding sites. J. Endocrinol. 1983, 97, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. Eur. Mol. Biol. Organ. Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Carroll, F.D.; Ellsworth, J.D.; Kroger, D. Compensatory Carcass Growth in Steers following Protein and Energy Restriction. J. Anim. Sci. 1963, 22, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Chay-Canul, A.J.; Ayala-Burgos, A.J.; Ku-Vera, J.C.; Magaña-Monforte, J.G.; Tedeschi, L.O. The effects of metabolizable energy intake on body fat depots of adult Pelibuey ewes fed roughage diets under tropical conditions. Trop. Anim. Heal. Prod. 2011, 43, 929–936. [Google Scholar] [CrossRef]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dfrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef]
- Catrysse, L.; van Loo, G. Adipose tissue macrophages and their polarization in health and obesity. Cell. Immun. 2018, 330, 114–119. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adipose tissue hormones. J. Endocrinol. Investig. 2002, 25, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. Endocrine abnormalities of obesity. Metabolism 1995, 44, 21–23. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-Reducing Effects of the Plasma Protein Encoded by the obese Gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Paracchini, V.; Pedotti, P.; Taioli, E. Genetics of Leptin and Obesity: A HuGE Review. Am. J. Epidemiol. 2005, 162, 101–114. [Google Scholar] [CrossRef]
- Siegrist-Kaiser, C.A.; Pauli, V.; Juge-Aubry, C.E.; Boss, O.; Pernin, A.; Chin, W.W.; Cusin, I.; Rohner-Jeanrenaud, F.; Burger, A.G.; Zapf, J.; et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Investig. 1997, 100, 2858–2864. [Google Scholar] [CrossRef] [Green Version]
- Gokulakrishnan, K.; Amutha, A.; Ranjani, H.; Bibin, S.Y.; Balakumar, M.; Pandey, G.K.; Anjana, R.M.; Ali, M.K.; Narayan, K.V.; Mohan, V. Relationship of Adipokines and Proinflammatory Cytokines Among Asian Indians With Obesity and Youth Onset Type 2 Diabetes. Endocr. Pr. 2015, 21, 1143–1151. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Spranger, J.; Kroke, A.; Mohlig, M.; Hoffman, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F.H. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, X.; Wang, L.; Liu, M.; Wetzel, M.D.; Guan, K.-L.; Dong, L.Q.; Liu, F. Adiponectin Sensitizes Insulin Signaling by Reducing p70 S6 Kinase-mediated Serine Phosphorylation of IRS-1. J. Biol. Chem. 2007, 282, 7991–7996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Stumvoll, M.; Vozarova, B.; Weyer, C.; Funahashi, T.; Matsuzawa, Y.; Bogardus, C.; Tataranni, P.A. Plasma Adiponectin and Endogenous Glucose Production in Humans. Diabetes Care 2003, 26, 3315–3319. [Google Scholar] [CrossRef] [Green Version]
- De Koster, J.; Urh, C.; Hostens, M.; Van den Broeck, W.; Sauerwein, H.; Opsomer, G. Relationship between serum adiponectin concentration, body condition score, and peripheral tissue insulin response of dairy cows during the dry period. Domest. Anim. Endocrinol. 2017, 59, 100–104. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Rajala, M.W.; Qi, Y.; Pathel, H.R.; Takahashi, N.; Bannerjee, R.; Pajvani, U.B.; Sinha, M.K.; Gingerich, R.L.; Scherer, P.E.; Ahima, R.S. Regulation of resistin expression in circulating levels in obesity, diabetes, and fasting. Diabetes 2004, 53, 1671–1679. [Google Scholar] [CrossRef] [Green Version]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef]
- Qi, Y.; Nie, Z.; Lee, Y.-S.; Singhal, N.S.; Scherer, P.E.; Lazar, M.A.; Ahima, R.S. Loss of Resistin Improved Glucose Homeostasis in Leptin Deficiency. Diabetes 2006, 55, 3083–3090. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, M.; Ramé, C.; Cognié, J.; Briant, E.; Elis, S.; Guillaume, D.; Dupont, J. Resistin in Dairy Cows: Plasma Concentrations during Early Lactation, Expression and Potential Role in Adipose Tissue. PLoS ONE 2014, 9, e93198. [Google Scholar] [CrossRef] [Green Version]

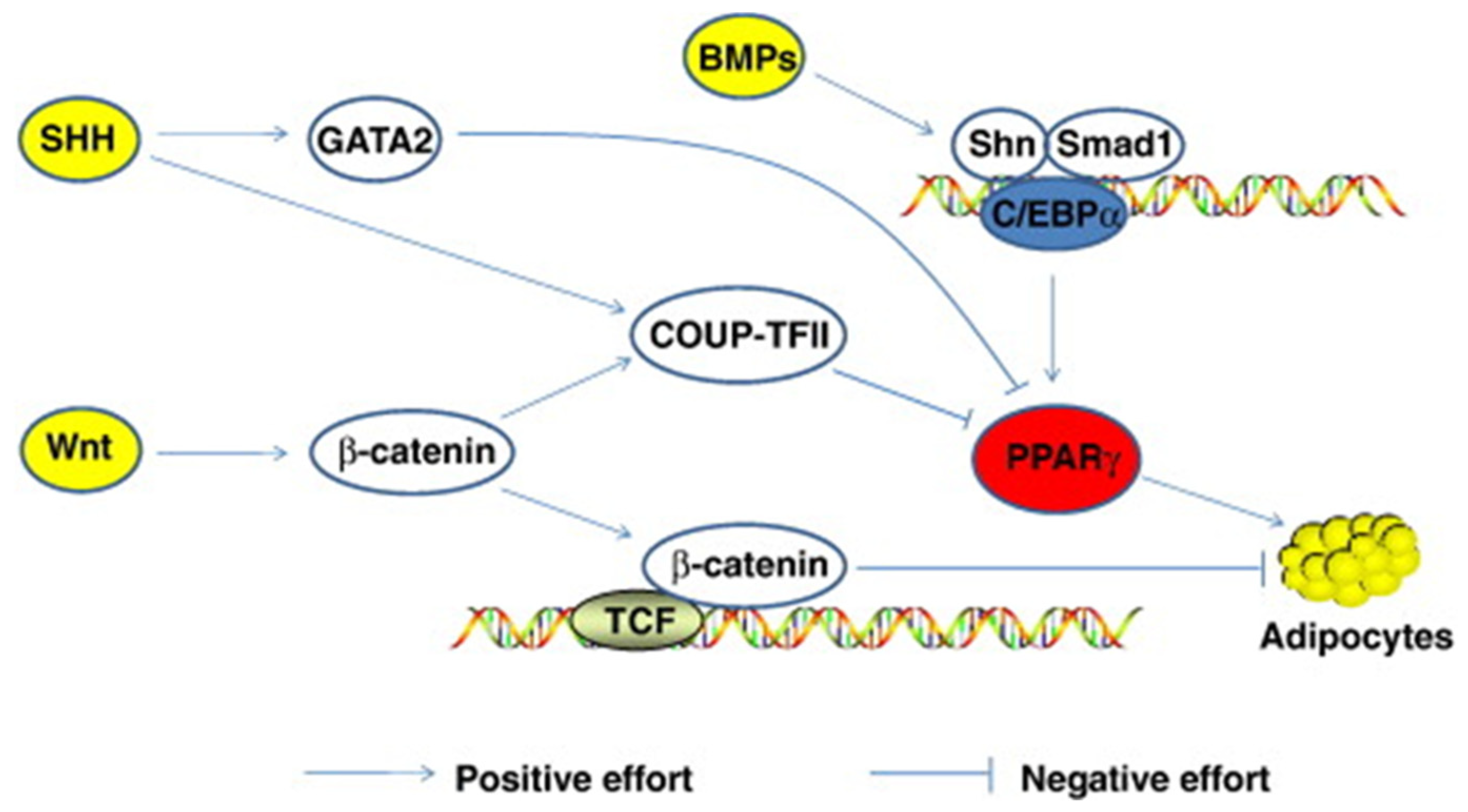
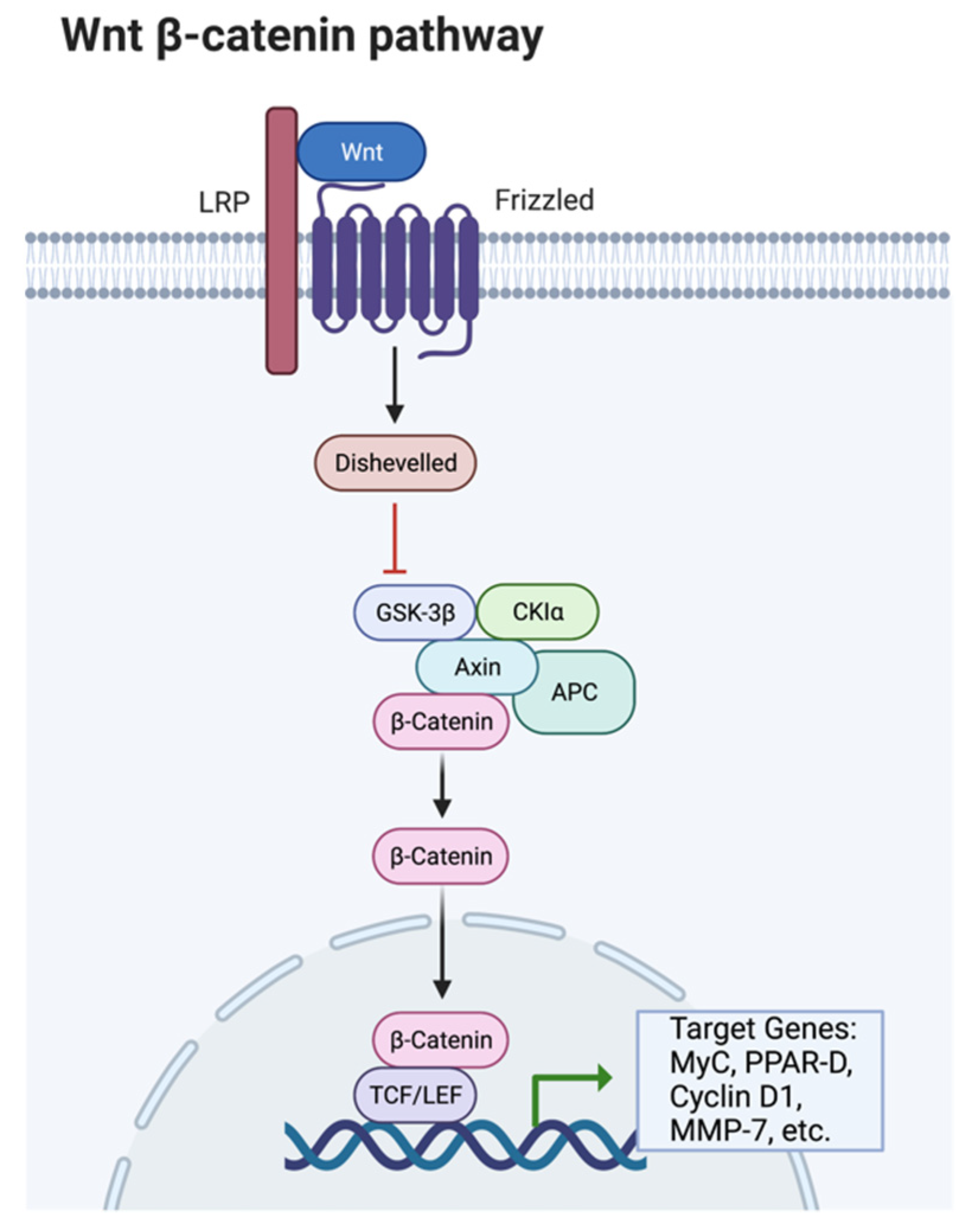
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality—A Review. Animals 2022, 12, 1550. https://doi.org/10.3390/ani12121550
Schumacher M, DelCurto-Wyffels H, Thomson J, Boles J. Fat Deposition and Fat Effects on Meat Quality—A Review. Animals. 2022; 12(12):1550. https://doi.org/10.3390/ani12121550
Chicago/Turabian StyleSchumacher, Madison, Hannah DelCurto-Wyffels, Jennifer Thomson, and Jane Boles. 2022. "Fat Deposition and Fat Effects on Meat Quality—A Review" Animals 12, no. 12: 1550. https://doi.org/10.3390/ani12121550
APA StyleSchumacher, M., DelCurto-Wyffels, H., Thomson, J., & Boles, J. (2022). Fat Deposition and Fat Effects on Meat Quality—A Review. Animals, 12(12), 1550. https://doi.org/10.3390/ani12121550






