Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. 1H-NMR Analysis
2.3. Statistical Analysis
3. Results
3.1. Characterization of Samples
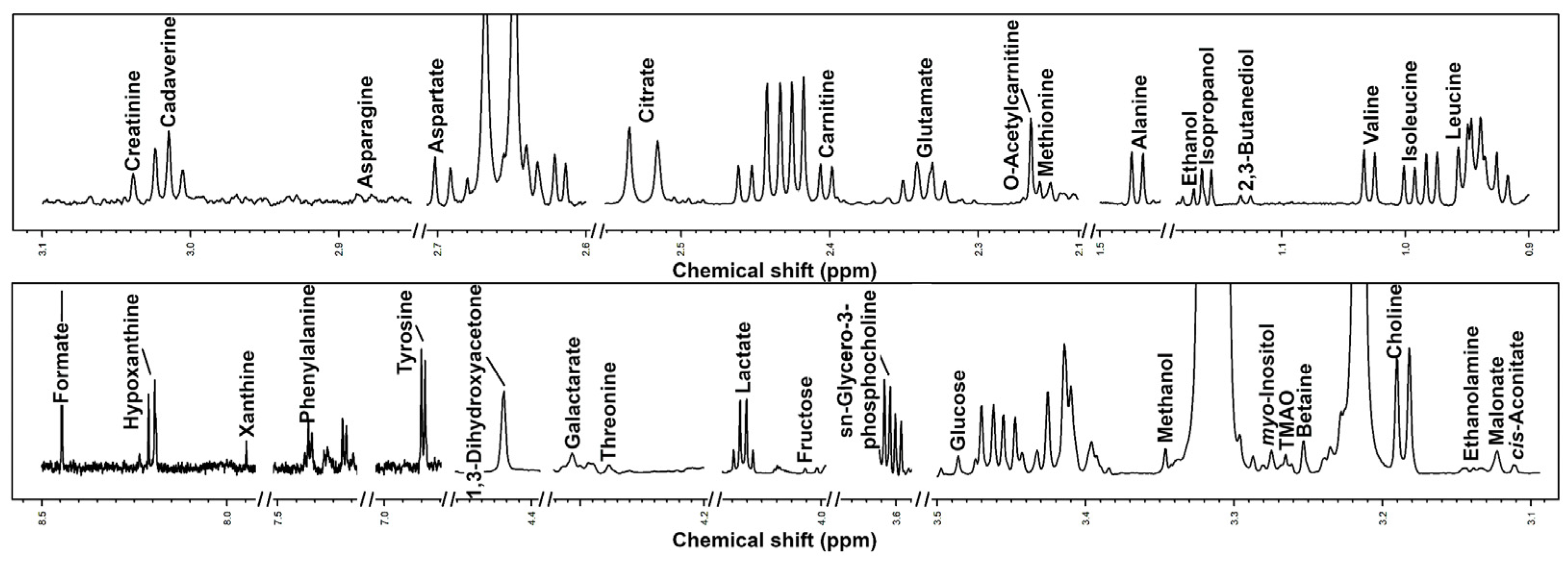
3.2. Characterization of Semen Plasma Metabolome of Giant Panda
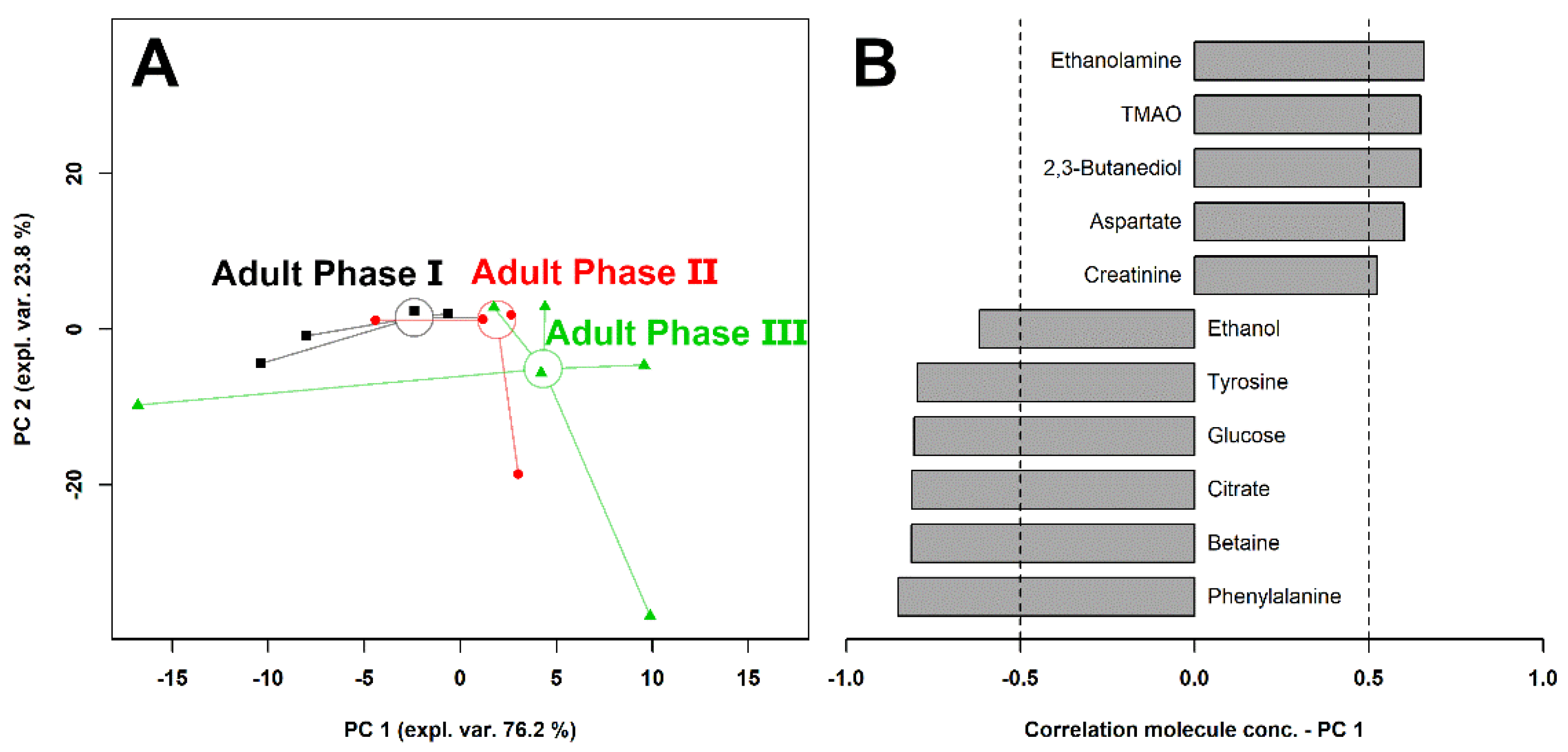
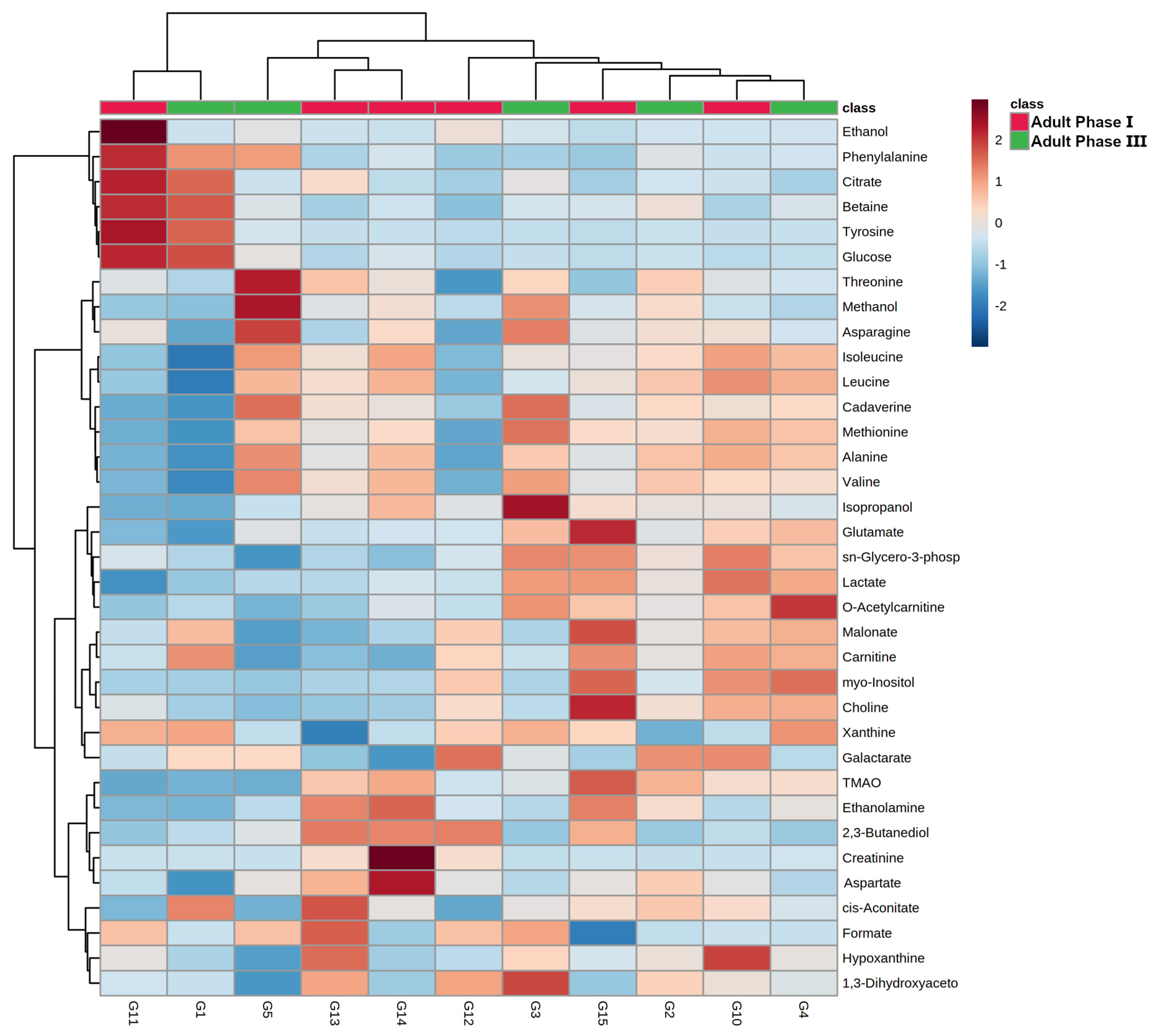
3.3. Metabolome’s Age-Related Features
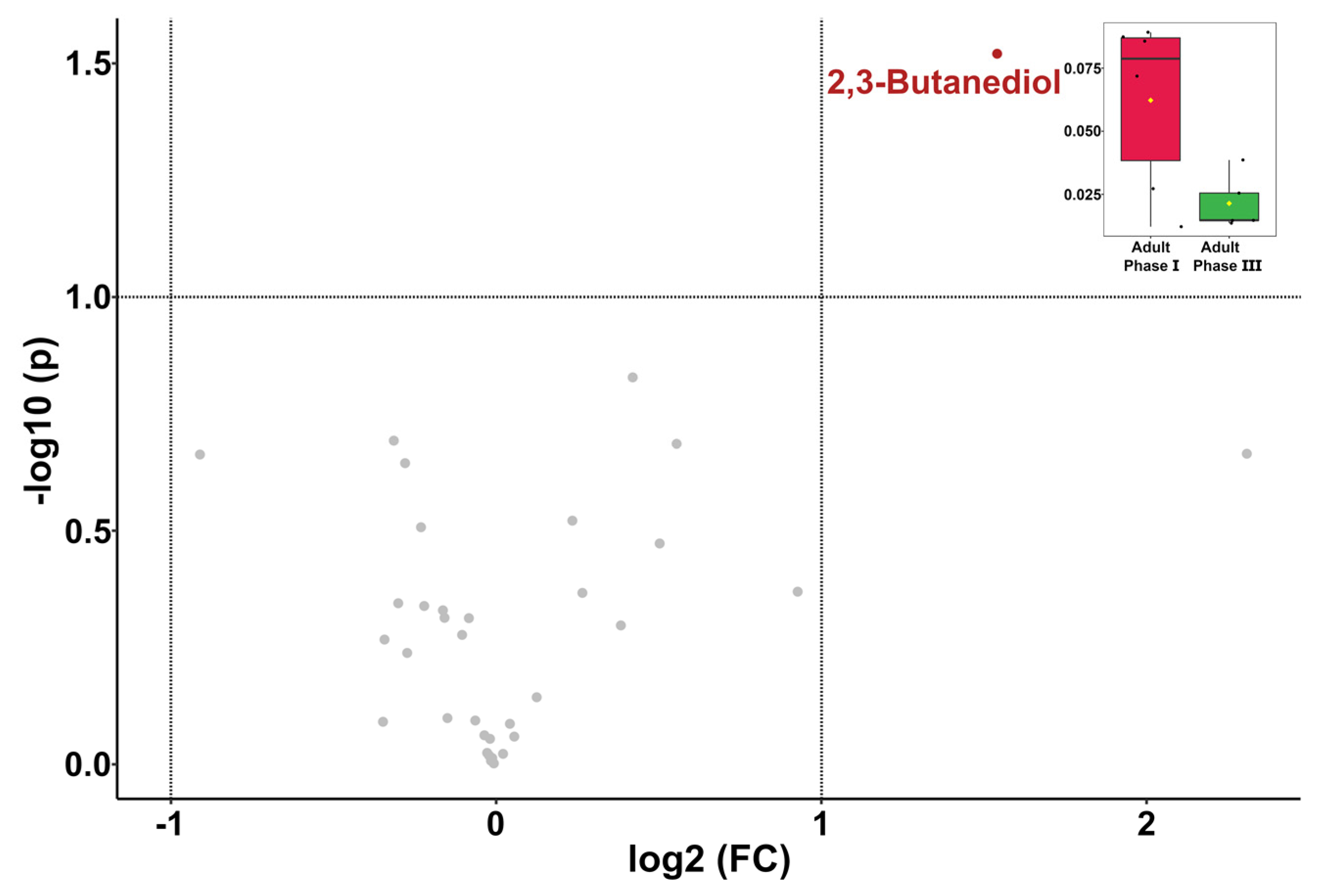
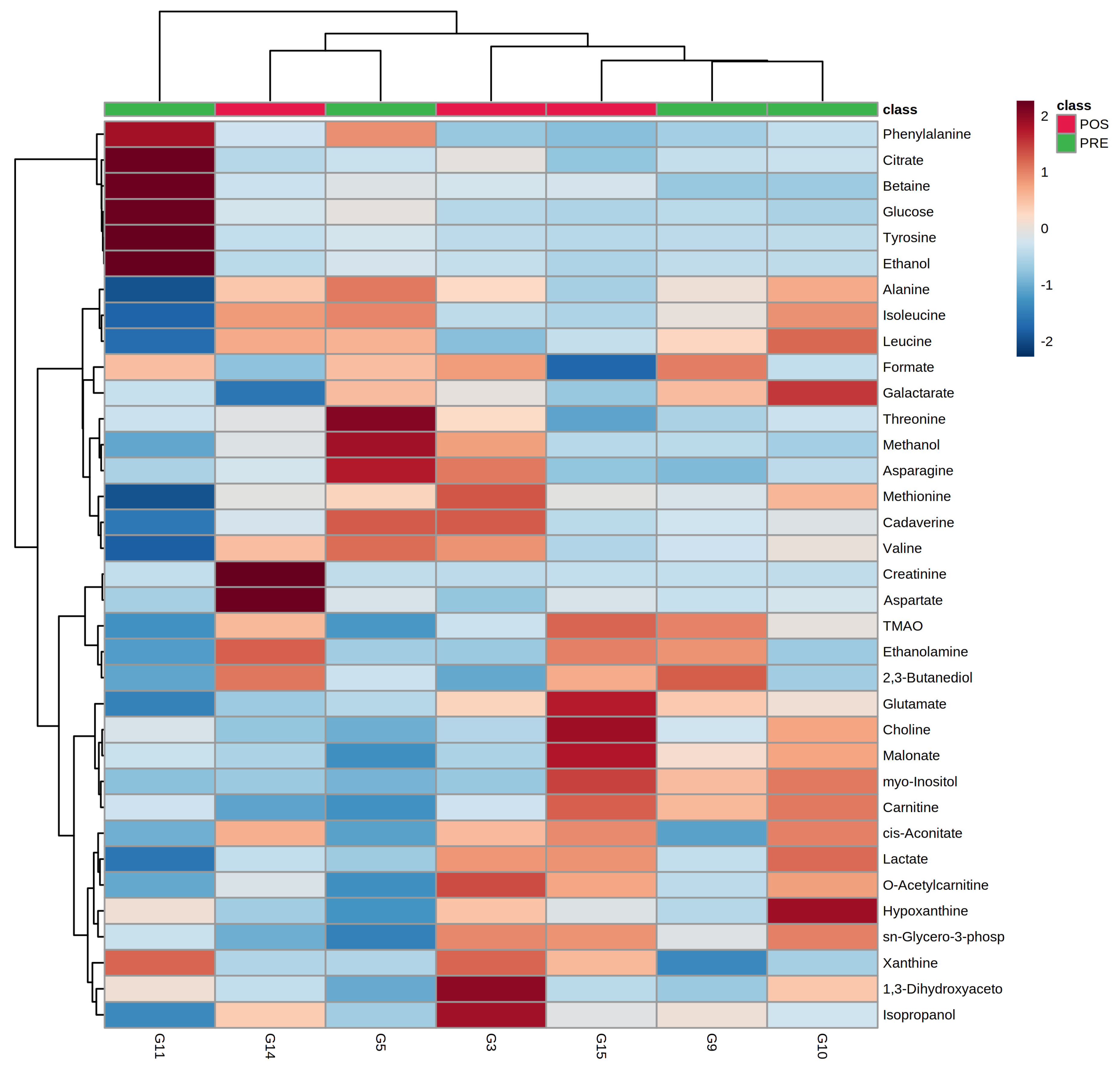
3.4. Giant Panda Seminal Plasma Metabolome Is Related to the Estrus Phases
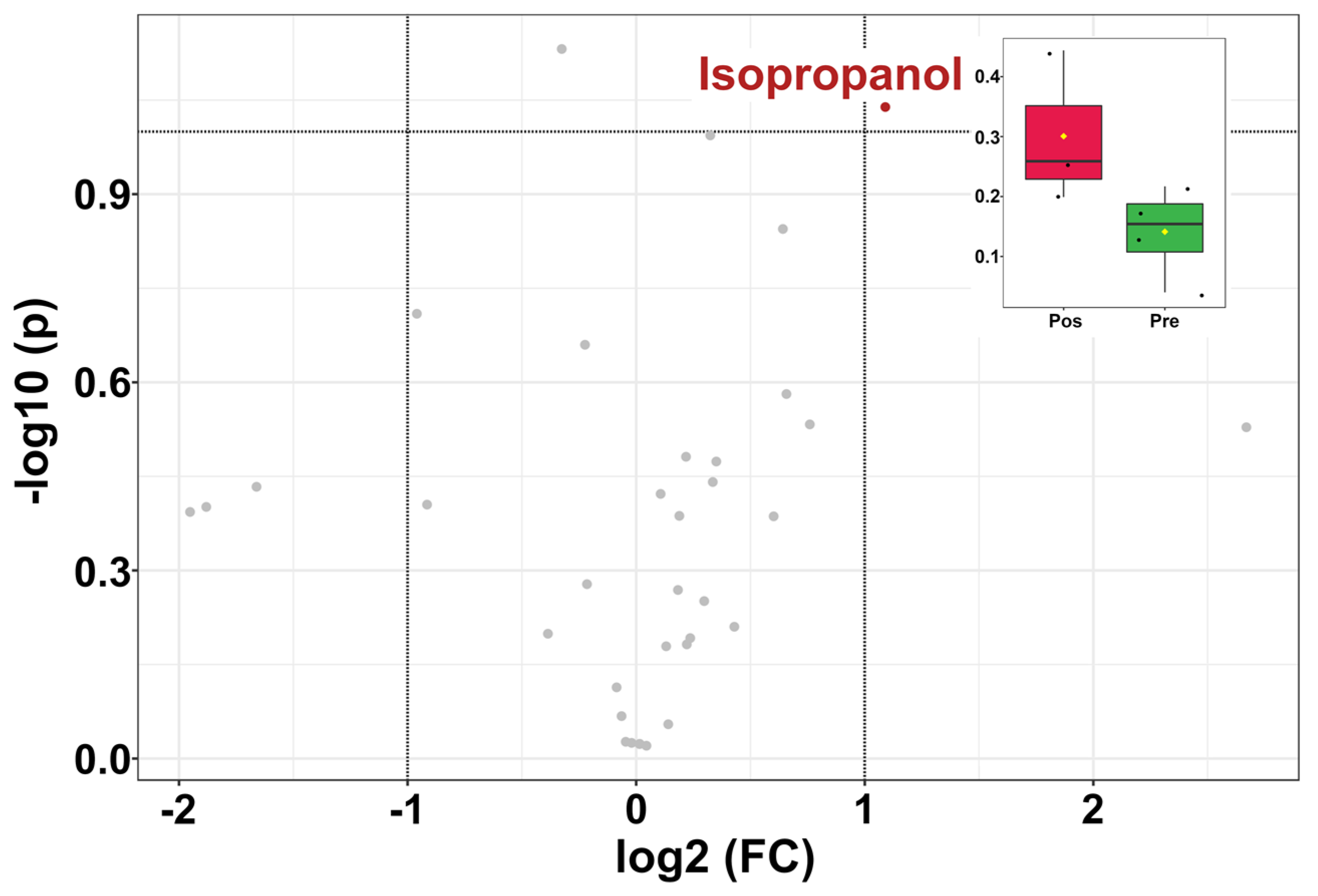
3.5. Giant Panda Seminal Plasma Metabolome Is Affected by Storage Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swaisgood, R.; Wang, D.; Wei, F. The IUCN Red List of Threatened Species. 2016. Available online: https://www.iucnredlist.org/species/712/121745669 (accessed on 10 March 2022).
- Feng, W.H.; Zhao, J.; Fujihara, N. Physiological Characteristics of Electro-Ejaculated Giant Panda (Ailuropoda melanoleuca) Semen. Jpn. J. Zoo Wildl. Med. 1997, 2, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, T.; Hori, T.; Nakashige, T.; Narushima, E.; Hara, T.; Akikawa, T.; Nose, N.; Saito, K.; Shichiri, S.; Hashizaki, F.; et al. Semen Quality in a Giant Panda (Ailuropoda melanoleuca) in Relation to Estrus of a Nearby Resident Female Panda. Theriogenology 2006, 66, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Weixin, L. A Note on the Artificial Insemination of Giant Panda. Chin. J. Anim. Vet. Sci. 1981, 2, 73–76. [Google Scholar]
- Huang, Y.; Wang, P.; Zhang, G.; Zhang, H.; Li, D.; Du, J.; Wei, R.; Tang, C.; Spindler, R.E.; Wildt, D.E. Use of Artificial Insemination to Enhance Propagation of Giant Pandas at the Wolong Breeding Center. In Proceedings of the International Symposium on Assisted Reproductive Technologies (ART) for Conservation and Genetic Management of Wildlife, Omaha, NE, USA, 27–28 April 2002. [Google Scholar]
- Seager, S.W.J. The Breeding of Captive Wild Species by Artificial Methods. Zoo Biol. 1983, 2, 235–239. [Google Scholar] [CrossRef]
- Platz, C.C.; Wildt, D.E.; Howard, J.G.; Bush, M. Electroejaculation and Semen Analysis and Freezing in the Giant Panda (Ailuropoda melanoleuca). J. Reprod. Fertil. 1983, 67, 9–12. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, G.; Mainka, S.A. Semen Evaluation of Giant Pandas (Ailuropoda melanoleuca) at the Wolong Reserve. Zoo Biol. 1994, 13, 83–86. [Google Scholar] [CrossRef]
- Menezes, E.B.; Velho, A.L.C.; Santos, F.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Uncovering Sperm Metabolome to Discover Biomarkers for Bull Fertility. BMC Genom. 2019, 20, 714. [Google Scholar] [CrossRef]
- Cazaux Velho, A.L.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic Markers of Fertility in Bull Seminal Plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jørgensen, N.; Andersson, A.M. Parabens in Urine, Serum and Seminal Plasma from Healthy Danish Men Determined by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, V.; Ghosh, S.; Sengupta, A.; Srivastava, S.; Sonawat, H.M.; Narayan, P.K. Identification of Biochemical Differences between Different Forms of Male Infertility by Nuclear Magnetic Resonance (NMR) Spectroscopy. J. Assist. Reprod. Genet. 2014, 31, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Mumcu, A.; Karaer, A.; Dogan, B.; Tuncay, G. Metabolomics Analysis of Seminal Plasma in Patients with Idiopathic Oligoasthenoteratozoospermia Using High-Resolution NMR Spectroscopy. Andrology 2020, 8, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Du Fossé, N.A.; Lashley, E.E.L.O.; van Beelen, E.; Meuleman, T.; le Cessie, S.; van Lith, J.M.M.; Eikmans, M.; van der Hoorn, M.L.P. Identification of Distinct Seminal Plasma Cytokine Profiles Associated with Male Age and Lifestyle Characteristics in Unexplained Recurrent Pregnancy Loss. J. Reprod. Immunol. 2021, 147, 103349. [Google Scholar] [CrossRef] [PubMed]
- Vince, S.; Žura Žaja, I.; Samardžija, M.; Majić Balić, I.; Vilić, M.; Duričić, D.; Valpotić, H.; Marković, F.; Milinković-Tur, S. Age-Related Differences of Semen Quality, Seminal Plasma, and Spermatozoa Antioxidative and Oxidative Stress Variables in Bulls during Cold and Warm Periods of the Year. Animal 2018, 12, 559–568. [Google Scholar] [CrossRef] [Green Version]
- El Sisy, G.A.; Abo El-Maaty, A.M.; Rawash, Z.M. Comparative Blood and Seminal Plasma Oxidant/Antioxidant Status of Arab Stallions with Different Ages and Their Relation to Semen Quality. Asian Pac. J. Reprod. 2016, 5, 428–433. [Google Scholar] [CrossRef]
- Souza, C.E.A.; Araújo, A.A.; Oliveira, J.T.A.; Lima Souza, A.C.; Neiva, J.N.M.; Moura, A.A. Reproductive Development of Santa Inês Rams during the First Year of Life: Body and Testis Growth, Testosterone Concentrations, Sperm Parameters, Age at Puberty and Seminal Plasma Proteins. Reprod. Domest. Anim. 2010, 45, 644–653. [Google Scholar] [CrossRef]
- Abou-Ahmed, M.M.; El-Belely, M.S.; Ismail, S.T.; El-Baghdady, Y.R.M.; Hemeida, N.A. Influence of Age and Season on Certain Biochemical Constituents of Seminal Plasma of Arabian Horses. Anim. Reprod. Sci. 1993, 32, 237–244. [Google Scholar] [CrossRef]
- Aitken-Palmer, C.; Hou, R.; Burrell, C.; Zhang, Z.; Wang, C.; Spindler, R.; Wildt, D.E.; Ottinger, M.A.; Howard, J.G. Protracted Reproductive Seasonality in the Male Giant Panda (Ailuropoda melanoleuca) Reflected by Patterns in Androgen Profiles, Ejaculate Characteristics, and Selected Behaviors. Biol. Reprod. 2012, 86, 195. [Google Scholar] [CrossRef]
- Kareskoski, M.; Katila, T. Components of Stallion Seminal Plasma and the Effects of Seminal Plasma on Sperm Longevity. Anim. Reprod. Sci. 2008, 107, 249–256. [Google Scholar] [CrossRef]
- Pavaneli, A.P.P.; Recuero, S.; Chaves, B.R.; Garcia-Bonavila, E.; Llavanera, M.; Pinart, E.; Bonet, S.; de Andrade, A.F.C.; Yeste, M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. Int. J. Mol. Sci. 2020, 21, 4520. [Google Scholar] [CrossRef]
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear Magnetic Resonance for Foodomics beyond Food Analysis. TrAC Trends Anal. Chem. 2014, 59, 93–102. [Google Scholar] [CrossRef]
- Zhu, C.; Li, C.; Wang, Y.; Laghi, L. Characterization of Yak Common Biofluids Metabolome by Means of Proton Nuclear Magnetic Resonance Spectroscopy. Metabolites 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Faillace, V.; Laus, F.; Bazzano, M.; Laghi, L. Characterization of Trotter Horses Urine Metabolome by Means of Proton Nuclear Magnetic Resonance Spectroscopy. Metabolomics 2018, 14, 106. [Google Scholar] [CrossRef]
- R Development Core Team. Computational Many-Particle Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 1, ISBN 3900051070. [Google Scholar]
- Kneen, M.A.; Annegarn, H.J. Algorithm for Fitting XRF, SEM and PIXE X-Ray Spectra Backgrounds. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1996, 109–110, 209–213. [Google Scholar] [CrossRef]
- Liland, K.H.; Almøy, T.; Mevik, B.H. Optimal Choice of Baseline Correction for Multivariate Calibration of Spectra. Appl. Spectrosc. 2010, 64, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Hubert, M.; Rousseeuw, P.J.; Vanden Branden, K. ROBPCA: A New Approach to Robust Principal Component Analysis. Technometrics 2005, 47, 64–79. [Google Scholar] [CrossRef]
- Zhu, C.; Laghi, L.; Zhang, Z.; He, Y.; Wu, D.; Zhang, H.; Huang, Y.; Li, C.; Zou, L. First Steps toward the Giant Panda Metabolome Database: Untargeted Metabolomics of Feces, Urine, Serum, and Saliva by 1H NMR. J. Proteome Res. 2020, 19, 1052–1059. [Google Scholar] [CrossRef]
- Wei, F.; Hu, J. Studies on the Reproduction of Giant Panda in Wolong Natural Reserve. Acta Theriol. Sin. 1994, 14, 243–248. [Google Scholar]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR Spectroscopic Studies on Human Seminal Plasma: A Probative Discriminant Function Analysis Classification Model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef]
- Meeker, J.D.; Godfrey-Bailey, L.; Hauser, R. Relationships between Serum Hormone Levels and Semen Quality among Men from an Infertility Clinic. J. Androl. 2007, 28, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal Biomarkers for the Evaluation of Male Infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-H.; Froenicke, L.; Lin, C.Y.; Lyons, L.A.; Miller, M.G.; Pinkerton, K.E.; VandeVoort, C.A. Effects of Environmental Tobacco Smoke In Vivo on Rhesus Monkey Semen Quality, Sperm Function, and Sperm Metabolism. Reprod. Toxicol. 2009, 27, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Diao, R.; Zhu, X.; Li, Z.; Cai, Z. Metabolic Characterization of Asthenozoospermia Using Nontargeted Seminal Plasma Metabolomics. Clin. Chim. Acta 2015, 450, 254–261. [Google Scholar] [CrossRef]
- Chen, Z.; Toth, T.; Godfrey-Bailey, L.; Mercedat, N.; Schiff, I.; Hauser, R. Seasonal Variation and Age-Related Changes in Human Semen Parameters. J. Androl. 2003, 24, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Jeyendran, R.S.; Graham, E.F. An Evaluation of Cryoprotective Compounds on Bovine Spermatozoa. Cryobiology 1980, 17, 458–464. [Google Scholar] [CrossRef]
- Bazzano, M.; Laghi, L.; Zhu, C.; di Giambattista, A.; Tesei, B.; Laus, F. Metabolomic Analysis of Seminal Plasma in Stallions Belonging to Two Different Horse Breeds. J. Equine Vet. Sci. 2020, 89, 103038. [Google Scholar] [CrossRef]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef]
- D’Aniello, G.; Ronsini, S.; Notari, T.; Grieco, N.; Infante, V.; D’Angel, N.; Mascia, F.; di Fiore, M.M.; Fisher, G.; D’Aniello, A. D-Aspartate, a Key Element for the Improvement of Sperm Quality. Adv. Sex. Med. 2012, 2, 45–53. [Google Scholar] [CrossRef] [Green Version]
- D’Aniello, G.; Ronsini, S.; Guida, F.; Spinelli, P.; D’Aniello, A. Occurrence of D-Aspartic Acid in Human Seminal Plasma and Spermatozoa: Possible Role in Reproduction. Fertil. Steril. 2005, 84, 1444–1449. [Google Scholar] [CrossRef]
- D’Aniello, A.; di Cosmo, A.; di Cristo, C.; Annunziato, L.; Petrucelli, L.; Fisher, G. Involvement of D-Aspartic Acid in the Synthesis of Testosterone in Rat Testes. Life Sci. 1996, 59, 97–104. [Google Scholar] [CrossRef]
- Macchia, G.; Topo, E.; Mangano, N.; D’Aniello, E.; Boni, R. Dl-Aspartic Acid Administration Improves Semen Quality in Rabbit Bucks. Anim. Reprod. Sci. 2010, 118, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.; Sannikka, E.; Suominen, J.; Santti, R. Glucose Content as a Parameter of Semen Quality. Arch. Androl. 1980, 5, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assumpção, T.I.; Torres, R.A.A.; Sousa, M.V.; Ricart, C.A.O. Correlation between Fertility and Levels of Protein, Sugar and Free Amino Acids in Seminal Plasma of Nelore Bulls. Arq. Bras. Med. Vet. Zootec. 2005, 57, 55–61. [Google Scholar] [CrossRef]
- Çevik, M.; Tuncer, P.B.; Taşdemir, U.; Özgürtaş, T. Comparison of Spermatological Characteristics and Biochemical Seminal Plasma Parameters of Normozoospermic and Oligoasthenozoospermic Bulls of Two Breeds. Turk. J. Vet. Anim. Sci. 2007, 31, 381–387. [Google Scholar]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic Profiling Reveals Correlations between Spermiogram Parameters and the Metabolites Present in Human Spermatozoa and Seminal Plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [Green Version]
- Barazzoni, R.; Zanetti, M.; Vettore, M.; Tessari, P. Relationships between Phenylalanine Hydroxylation and Plasma Aromatic Amino Acid Concentrations in Humans. Metab. Clin. Exp. 1998, 47, 669–674. [Google Scholar] [CrossRef]
- Edwards, K.D.G.; Whyte, H.M. Plasma Creatinine Level and Creatinine Clearance as Tests of Renal Function. Australas. Ann. Med. 1959, 8, 218–224. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Sharma, R.K.; Agarwal, A. Relationship between Creatine Kinase Activity and Semen Characteristics in Subfertile Men. Int. J. Fertil. Women’s Med. 1998, 43, 192–197. [Google Scholar]
- Hallak, J.; Sharma, R.K.; Pasqualotto, F.F.; Ranganathan, P.; Thomas, A.J.; Agarwal, A. Creatine Kinase as an Indicator of Sperm Quality and Maturity in Men with Oligospermia. Urology 2001, 58, 446–451. [Google Scholar] [CrossRef]
- Ford, G.C.; Harrison, P.M.; Rice, D.W.; Smith, J.M.; Treffry, A.; White, J.L.; Yariv, J. Ferritin: Design and Formation of an Iron-Storage Molecule. Philos. Trans. R. Soc. B Biol. Sci. 1984, 304, 551–565. [Google Scholar] [CrossRef]
- Hamamah, S.; Seguin, F.; Bujan, L.; Barthelemy, C.; Mieusset, R.; Lansac, J. Quantification by Magnetic Resonance Spectroscopy of Metabolites in Seminal Plasma Able to Differentiate Different Forms of Azoospermia. Hum. Reprod. 1998, 13, 132–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-Associated Metabolites in Bull Seminal Plasma and Blood Serum: 1H Nuclear Magnetic Resonance Analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G.; Jockenhövel, F.; Nieschlag, E. Variations in Semen Parameters from Fathers. Hum. Reprod. 1991, 6, 859–866. [Google Scholar] [CrossRef]
- Schreier, C.; Kremer, W.; Huber, F.; Neumann, S.; Pagel, P.; Lienemann, K.; Pestel, S. Reproducibility of NMR Analysis of Urine Samples: Impact of Sample Preparation, Storage Conditions, and Animal Health Status. BioMed Res. Int. 2013, 2013, 878374. [Google Scholar] [CrossRef]
- O’Sullivan, V.; Madrid-Gambin, F.; Alegra, T.; Gibbons, H.; Brennan, L. Impact of Sample Storage on the NMR Fecal Water Metabolome. ACS Omega 2018, 3, 16585–16590. [Google Scholar] [CrossRef] [Green Version]
| Sample ID | Age (Years) | Sample Collection Date |
|---|---|---|
| G1 | 6 | April 2010 |
| G2 | 6 | April 2013 |
| G3 | 6 | May 2019 |
| G4 | 6 | April 2015 |
| G5 | 7 | March 2016 |
| G6 | 8 | April 2013 |
| G7 | 12 | April 2016 |
| G8 | 10 | April 2019 |
| G9 | 9 | March 2019 |
| G10 | 13 | February 2012 |
| G11 | 13 | February 2012 |
| G12 | 18 | April 2010 |
| G13 | 13 | April 2018 |
| G14 | 14 | June 2018 |
| G15 | 14 | June 2018 |
| Amino Acids, Peptides, and Derivatives | Adult Phase I | Adult Phase II | Adult Phase III |
|---|---|---|---|
| Alanine | 8.17 × 10−1 ± 2.07 × 10−1 | 9.41 × 10−1 ± 7.01 × 10−2 | 7.29 × 10−1 ± 1.77 × 10−1 |
| Asparagine | 6.63 × 10−1 ± 1.99 × 10−1 | 5.95 × 10−1 ± 8.45 × 10−2 | 5.65 × 10−1 ± 9.66 × 10−2 |
| Aspartate | 9.67 × 10−1 ± 2.89 × 10−1 | 1.10 ± 3.77 × 10−1 | 1.29 ± 3.77 × 10−1 |
| Carnitine | 13.1 ± 5.82 | 12.9 ± 5.36 | 12.8 ± 5.83 |
| Creatinine | 1.04 × 10−1 ± 2.75 × 10−2 | 3.26 × 10−1 ± 4.27 × 10−1 | 5.16 × 10−1 ± 6.86 × 10−1 |
| Glutamate | 4.37 ± 8.04 × 10−1 | 5.23 ± 2.54 × 10−1 | 4.50 ± 9.85 × 10−1 |
| Isoleucine | 5.34 × 10−1 ± 2.15 × 10−1 | 6.19 × 10−1 ± 2.21 × 10−2 | 5.29 × 10−1 ± 1.64 × 10−1 |
| Leucine | 9.56 × 10−1 ± 4.05 × 10−1 | 1.22 ± 1.13 × 10−1 | 9.71 × 10−1 ± 3.15 × 10−1 |
| Methionine | 4.41 × 10−1 ± 1.17 × 10−1 | 4.35 × 10−1 ± 2.53 × 10−2 | 3.95 × 10−1 ± 9.33 × 10−2 |
| O-Acetylcarnitine | 1.41 ± 7.18 × 10−1 | 9.65 × 10−1 ± 1.55 × 10−1 | 1.14 ± 3.82 × 10−1 |
| Phenylalanine | 7.62 × 10−1 ± 3.12 × 10−1 | 6.08 × 10−1 ± 1.45 × 10−1 | 6.32 × 10−1 ± 4.21 × 10−1 |
| Threonine | 9.37 × 10−1 ± 2.63 × 10−1 | 8.84 × 10−1 ± 2.26 × 10−1 | 7.53 × 10−1 ± 1.81 × 10−1 |
| Tyrosine | 2.17 ± 2.57 | 9.62 × 10−1 ± 1.88 × 10−1 | 2.16 ± 3.35 |
| Valine | 9.09 × 10−1 ± 3.31 × 10−1 | 9.87 × 10−1 ± 9.85 × 10−2 | 7.80 × 10−1 ± 2.22 × 10−1 |
| Organic acids and derivatives | |||
| cis-Aconitate | 3.76 × 10−1 ± 8.03 × 10−2 | 3.52 × 10−1 ± 1.30 × 10−1 | 3.67 × 10−1 ± 9.55 × 10−2 |
| Citrate | 17.0 ± 11.7 | 11.3 ± 3.89 | 16.9 ± 15.1 |
| Formate | 8.74 × 10−1 ± 8.09 × 10−2 | 9.67 × 10−1 ± 1.02 × 10−1 | 8.63 × 10−1 ± 1.50 × 10−1 |
| Galactarate | 4.45 × 10−1 ± 5.01 × 10−2 | 4.28 × 10−1 ± 4.33 × 10−2 | 4.14 × 10−1 ± 9.55 × 10−2 |
| Lactate | 24.8 ± 6.00 | 25.8 ± 5.45 | 23.7 ± 7.77 |
| Malonate | 7.43 × 10−1 ± 2.79 × 10−1 | 6.93 × 10−1 ± 2.46 × 10−1 | 8.11 × 10−1 ± 3.13 × 10−1 |
| Carbohydrates | |||
| Glucose | 6.95 ± 9.30 | 2.39 ± 2.90 × 10−1 | 5.47 ± 10.5 |
| Nucleosides, nucleotides, and analogs | |||
| Hypoxanthine | 3.53 × 10−1 ± 7.11 × 10−2 | 3.42 × 10−1 ± 1.23 × 10−1 | 4.15 × 10−1 ± 1.08 × 10−1 |
| Xanthine | 1.33 × 10−1 ± 1.75 × 10−2 | 1.47 × 10−1 ± 4.38 × 10−2 | 1.26 × 10−1 ± 1.67 × 10−2 |
| Miscellaneous | |||
| 1,3-Dihydroxyacetone | 8.21 × 10−1 ± 4.47 × 10−1 | 6.49 × 10−1 ± 2.55 × 10−1 | 8.08 × 10−1 ± 2.97 × 10−1 |
| 2,3-Butanediol | 2.14 × 10−2 ± 1.08 × 10−2 | 4.10 × 10−2 ± 3.42 × 10−2 | 6.22 × 10−2 ± 3.39 × 10−2 |
| Betaine | 3.69 × 10−1 ± 1.66 × 10−1 | 2.49 × 10−1 ± 6.09 × 10−2 | 2.91 × 10−1 ± 2.28 × 10−1 |
| Cadaverine | 5.10 × 10−1 ± 1.51 × 10−1 | 5.23 × 10−1 ± 4.58 × 10−2 | 4.21 × 10−1 ± 7.30 × 10−2 |
| Choline | 5.13 × 10−1 ± 1.61 × 10−1 | 5.78 × 10−1 ± 1.42 × 10−1 | 6.16 × 10−1 ± 2.36 × 10−1 |
| Ethanol | 2.92 × 10−1 ± 5.09 × 10−2 | 2.85 × 10−1 ± 5.15 × 10−2 | 5.56 × 10−1 ± 7.00 × 10−1 |
| Ethanolamine | 1.56 × 10−1 ± 5.11 × 10−2 | 2.89 × 10−1 ± 7.71 × 10−2 | 2.29 × 10−1 ± 1.10 × 10−1 |
| Isopropanol | 1.88 × 10−1 ± 1.53 × 10−1 | 2.11 × 10−1 ± 6.18 × 10−2 | 1.70 × 10−1 ± 7.19 × 10−2 |
| Methanol | 9.94 × 10−1 ± 8.35 × 10−1 | 1.23 ± 1.14 | 5.28 × 10−1 ± 2.15 × 10−1 |
| myo-Inositol | 1.68 ± 1.18 | 2.34 ± 8.57 × 10−1 | 2.18 ± 1.24 |
| sn-Glycero-3-phosphocholine | 28.2 ± 11.8 | 25.3 ± 6.70 | 29.3 ± 10.4 |
| TMAO | 7.27 × 10−2 ± 4.52 × 10−2 | 1.46 × 10−1 ± 1.22 × 10−2 | 1.03 × 10−1 ± 5.23 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Jin, L.; Luo, B.; Zhou, Q.; Dong, L.; Li, X.; Zhang, H.; Huang, Y.; Li, C.; Zou, L.; et al. Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy. Animals 2022, 12, 1536. https://doi.org/10.3390/ani12121536
Zhu C, Jin L, Luo B, Zhou Q, Dong L, Li X, Zhang H, Huang Y, Li C, Zou L, et al. Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy. Animals. 2022; 12(12):1536. https://doi.org/10.3390/ani12121536
Chicago/Turabian StyleZhu, Chenglin, Lei Jin, Bo Luo, Qiang Zhou, Li Dong, Xiaoyan Li, Hemin Zhang, Yan Huang, Caiwu Li, Likou Zou, and et al. 2022. "Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy" Animals 12, no. 12: 1536. https://doi.org/10.3390/ani12121536
APA StyleZhu, C., Jin, L., Luo, B., Zhou, Q., Dong, L., Li, X., Zhang, H., Huang, Y., Li, C., Zou, L., & Laghi, L. (2022). Dominant Components of the Giant Panda Seminal Plasma Metabolome, Characterized by 1H-NMR Spectroscopy. Animals, 12(12), 1536. https://doi.org/10.3390/ani12121536






