Concentrations of Boar Taint Compounds Are Weakly Associated with Sexual Behavior of Young Boars
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Quantification of Boar Taint Compounds
2.3. Assessment of Sexual Behavior Traits
2.4. Statistical Analysis
2.4.1. Sexual Behavior Traits
2.4.2. Androstenone, Skatole, and Indole Levels as Predictors of Boar Performance
2.4.3. Duration of the Training Session
3. Results and Discussion
3.1. Androstenone, Skatole, and Indole Concentrations
3.2. Correlations between Variables
3.3. Distribution of Scores for Sexual Behavior Traits and Duration of the Training Session
3.4. Effect of Farm, Body Weight, and Age on Sexual Behaviour Traits
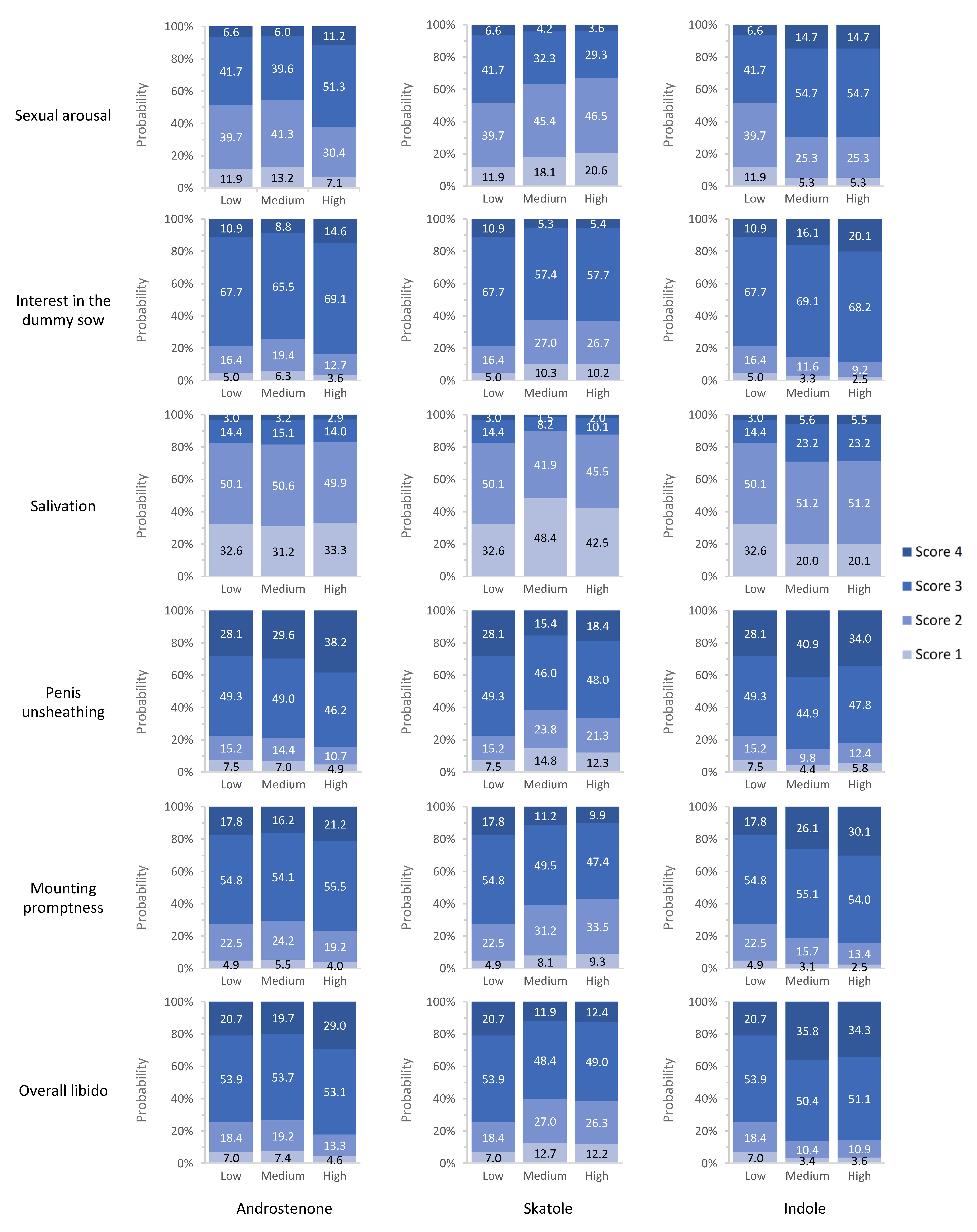
3.5. Association between Boar Taint Compound Levels and Sexual Behavior Traits
3.6. Androstenone, Skatole, and Indole Levels as Predictors of Boar Performance
3.7. Association between Boar Taint Compound Levels and Duration of the Session
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamaratskaia, G.; Squires, E.J. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 3, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Bone, C.; Cameron, J. Pork production with entire males: Directions for control of boar taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Babol, J.; Andersson, H.; Lundström, K. Plasma skatole and androstenone levels in entire male pigs and relationship between boar taint compounds, sex steroids and thyroxine at various ages. Livest. Prod. Sci. 2004, 87, 91–98. [Google Scholar] [CrossRef]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Claus, R.; Raab, S. Influences on skatole formation from tryptophan in the pig colon. Adv. Exp. Med. Biol. 1999, 467, 679–684. [Google Scholar] [CrossRef]
- Han, X.; Zhou, M.; Cao, X.; Du, X.; Meng, F.; Bu, G.; Kong, F.; Huang, A.; Zeng, X. Mechanistic insight into the role of immunocastration on eliminating skatole in boars. Theriogenology 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Prosciutto di Parma (Parma Ham) Protected Designation of Origin (Specifications and Dossier Pursuant to Article 4 of Council Regulation EEC no. 2081/92 Dated 14 July 1992). Available online: https://www.prosciuttodiparma.com/wp-content/uploads/2019/07/Parma_Ham_Specifications_Disciplinare_Consolidato_Nov_13.pdf (accessed on 6 January 2022).
- Consorzio del Prosciutto di San Daniele. Production Specification for the Protected Designation of Origin “Prosciutto di San Daniele”. 2007. Available online: https://www.prosciuttosandaniele.it/content/uploads/2018/05/Production-Specifications-SAN-DANIELE.pdf (accessed on 6 January 2022).
- Robinson, J.A.B.; Buhr, M.M. Impact of genetic selection on management of boar replacement. Theriogenology 2005, 63, 668–678. [Google Scholar] [CrossRef]
- Savić, R.; Petrovic, M.; Radojkovic, D.; Radovic, C.; Parunovic, N. Variability of libido and properties of boar ejaculate. Indian J. Anim. Res. 2014, 48, 422. [Google Scholar] [CrossRef]
- Willeke, H.; Claus, R.; Müller, E.; Pirchner, F.; Karg, H. Selection for high and low level of 5α-androst-16-en-3-one in boars. I. direct and correlated response of endocrinological traits. J. Anim. Breed. Genet. 1987, 104, 64–73. [Google Scholar] [CrossRef]
- Baes, C.; Mattei, S.; Luther, H.; Ampuero, S.; Sidler, X.; Bee, G.; Spring, P.; Hofer, A. A performance test for boar taint compounds in live boars. Animal 2013, 7, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Rostellato, R.; Bonfatti, V.; Larzul, C.; Bidanel, J.P.; Carnier, P. Estimates of genetic parameters for content of boar taint compounds in adipose tissue of intact males at 160 and 220 days of age1. J. Anim. Sci. 2015, 93, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 16 December 2021).
- Christensen, R.H.B. Ordinal-Regression Models for Ordinal Data. 2019. R Package Version 2019.12–10. Available online: https://cran.r-project.org/package=ordinal (accessed on 2 August 2021).
- Christensen, R.H.B. Cumulative Link Models for Ordinal Regression with the R Package Ordinal; Technical University of Denmark & Christensen Statistics: Lyngby, Denmark, 2018. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Hand, D.J.; Till, R.J. A Simple Generalisation of the Area Under the ROC Curve for Multiple Class Classification Problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Dugué, C. Relations entre le Risque d’Odeur de Verrat et les Caractères d’Intérêt chez le Verrat et le Porc Charcutier. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2020. Available online: https://oatao.univ-toulouse.fr/28152/1/Dugue.pdf (accessed on 7 October 2021).
- Wesoly, R.; Jungbluth, I.; Stefanski, V.; Weiler, U. Pre-slaughter conditions influence skatole and androstenone in adipose tissue of boars. Meat Sci. 2015, 99, 60–67. [Google Scholar] [CrossRef]
- Babol, J.; Zamaratskaia, G.; Juneja, R.K.; Lundström, K. The effect of age on distribution of skatole and indole levels in entire male pigs in four breeds: Yorkshire, Landrace, Hampshire and Duroc. Meat Sci. 2004, 67, 351–358. [Google Scholar] [CrossRef]
- Bonneau, M. Factors affecting the level of androstenone. Acta Vet. Scand. 2006, 48, S7. [Google Scholar] [CrossRef]
- Walstra, P.; Claudi-Magnussen, C.; Chevillon, P.; von Seth, G.; Diestre, A.; Matthews, K.R.; Homer, D.B.; Bonneau, M. An international study on the importance of androstenone and skatole for boar taint: Levels of androstenone and skatole by country and season. Livest. Prod. Sci. 1999, 62, 15–28. [Google Scholar] [CrossRef]
- Bonneau, M.; Carrie-Lemoine, J.; Mesure-Morat. Genital tract development and histomorphometrical traits of the testis in the young boar: Relationships with fat 5α-androstenone levels. Anim. Reprod. Sci. 1987, 15, 259–263. [Google Scholar] [CrossRef]
- Babol, J.; Squires, E.J.; Lundström, K. Relationship between metabolism of skatole and androstenone in intact male pigs. J. Anim. Sci. 1999, 77, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Parois, S.; Larzul, C.; Prunier, A. Associations between the dominance status and sexual development, skin lesions or feeding behaviour of intact male pigs. Appl. Anim. Behav. Sci. 2017, 187, 15–22. [Google Scholar] [CrossRef]
- Chutia, T.; Ahmed, F.A.; Lalrintluanga, K.; Kalita, G.; Gali, J.M.; Roychoudhury, P.; Mayengbam, P.; Chaudary, J.K. Training and libido assessment in boar during semen collection. Haryana Vet. 2019, 58, 50–52. [Google Scholar]
- Steybe, L.; Kress, K.; Schmucker, S.; Stefanski, V. Impact of housing condition on welfare and behavior of immunocastrated fattening pigs (Sus scrofa domestica). Animals 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Weiler, U.; Claus, R.; Schnoebelen-Combes, S.; Louveau, I. Influence of age and genotype on endocrine parameters and growth performance: A comparative study in wild boars, Meishan and Large White boars. Livest. Prod. Sci. 1998, 54, 21–31. [Google Scholar] [CrossRef]
- Westendorf, P.; Richter, L. Nutrition of the boar. Ubersicht Furtierernahrung 1977, 5, 161–184. [Google Scholar]
- Zamaratskaia, G.; Babol, J.; Madej, A.; Squires, E.; Lundstrom, K. Age-related variation of plasma concentrations of skatole, androstenone, testosterone, oestradiol-17beta, oestrone sulphate, dehydroepiandrosterone sulphate, triiodothyronine and igf-1 in six entire male pigs. Reprod. Domest. Anim. 2004, 39, 168–172. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Serum androgen level is determined by autosomal dominant inheritance and regulates sex-related CYP genes in pigs. Biochem. Biophys. Res. Commun. 2013, 430, 833–838. [Google Scholar] [CrossRef]
- Claus, R.; Weiler, U. Endocrine regulation of growth and metabolism in the pig: A review. Livest. Prod. Sci. 1994, 37, 245–260. [Google Scholar] [CrossRef]
- Øverland, M.; Berg, J.; Matre, T. The effect of feed and feeding regime on skatole and androstenone levels and on sensory attributes of entire male and female pigs. In Proceedings of the EAAP Working Group Production and Utilisation of Meat from Entire Male Pigs, Milton Keynes, UK, 27–29 September 1995. [Google Scholar]
- Bonneau, M.; Chevillon, P. Acceptability of entire male pork with various levels of androstenone and skatole by consumers according to their sensitivity to androstenone. Meat Sci. 2012, 90, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Mörlein, D.; Trautmann, J.; Gertheiss, J.; Meier-Dinkel, L.; Fischer, J.; Eynck, H.-J.; Heres, L.; Looft, C.; Tholen, E. Interaction of skatole and androstenone in the olfactory perception of boar taint. J. Agric. Food Chem. 2016, 64, 4556–4565. [Google Scholar] [CrossRef] [PubMed]
- Hodel, C.; Nathues, H.; Grahofer, A. Effect of housing conditions, management procedures and traits of the external male reproductive tract on the sexual behaviour of natural mating boars. Theriogenology 2021, 167, 44–50. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Tilbrook, A.J. Sexual behavior of male pigs. Horm. Behav. 2007, 52, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Petrovic, M. Effect of photoperiod on sexual activity of boar. Rev. Bras. Zootec. 2015, 44, 276–282. [Google Scholar] [CrossRef][Green Version]
- Savić, R.; Ausejo Marcos, R.; Petrović, M.; Radojković, D.; Radović, Č.; Gogić, M. Fertility of boars—What is important to know. Biotechnol. Anim. Husb. 2017, 33, 135–149. [Google Scholar] [CrossRef]





| Boar Taint Compound | Class of Concentration | ||
|---|---|---|---|
| Low | Intermediate | High | |
| Androstenone (µg/g) | 0.42 (0.08–0.62) | 0.85 (0.64–1.17) | 2.11 (1.17–8.28) |
| Skatole (ng/g) | 8.65 (0.35–14.66) | 25.57 (14.76–42.40) | 116.83 (43.00–605.04) |
| Indole (ng/g) | 4.76 (1.34–7.62) | 12.69 (7.65–19.32) | 40.20 (19.40–191.20) |
| Trait | Body Weight | Log (AND) | Log (SKA) | Log (IND) |
|---|---|---|---|---|
| Age | 0.252 *** | 0.068 | 0.097 | 0.055 |
| Body weight | 0.126 ** | 0.017 | −0.043 | |
| log(AND) | 0.309 *** | 0.389 *** | ||
| log(SKA) | 0.695 *** |
| Effect | DF | MS | F | p |
|---|---|---|---|---|
| Farm | 1 | 1.39 | 10.27 | 0.001 |
| Androstenone concentration class | 2 | 0.20 | 1.45 | 0.236 |
| Skatole concentration class | 2 | 0.15 | 1.09 | 0.338 |
| Indole concentration class | 2 | 0.12 | 0.88 | 0.415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschi, E.; Faggion, S.; Mondin, C.; Carnier, P.; Bonfatti, V. Concentrations of Boar Taint Compounds Are Weakly Associated with Sexual Behavior of Young Boars. Animals 2022, 12, 1499. https://doi.org/10.3390/ani12121499
Boschi E, Faggion S, Mondin C, Carnier P, Bonfatti V. Concentrations of Boar Taint Compounds Are Weakly Associated with Sexual Behavior of Young Boars. Animals. 2022; 12(12):1499. https://doi.org/10.3390/ani12121499
Chicago/Turabian StyleBoschi, Elena, Sara Faggion, Chiara Mondin, Paolo Carnier, and Valentina Bonfatti. 2022. "Concentrations of Boar Taint Compounds Are Weakly Associated with Sexual Behavior of Young Boars" Animals 12, no. 12: 1499. https://doi.org/10.3390/ani12121499
APA StyleBoschi, E., Faggion, S., Mondin, C., Carnier, P., & Bonfatti, V. (2022). Concentrations of Boar Taint Compounds Are Weakly Associated with Sexual Behavior of Young Boars. Animals, 12(12), 1499. https://doi.org/10.3390/ani12121499







