Study of the Mandibular Bone Microstructure and Blood Minerals Bioavailability in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) from Freshwater
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens Sampling
2.2. Sample Processing
2.3. Quantification of Serum Metabolites
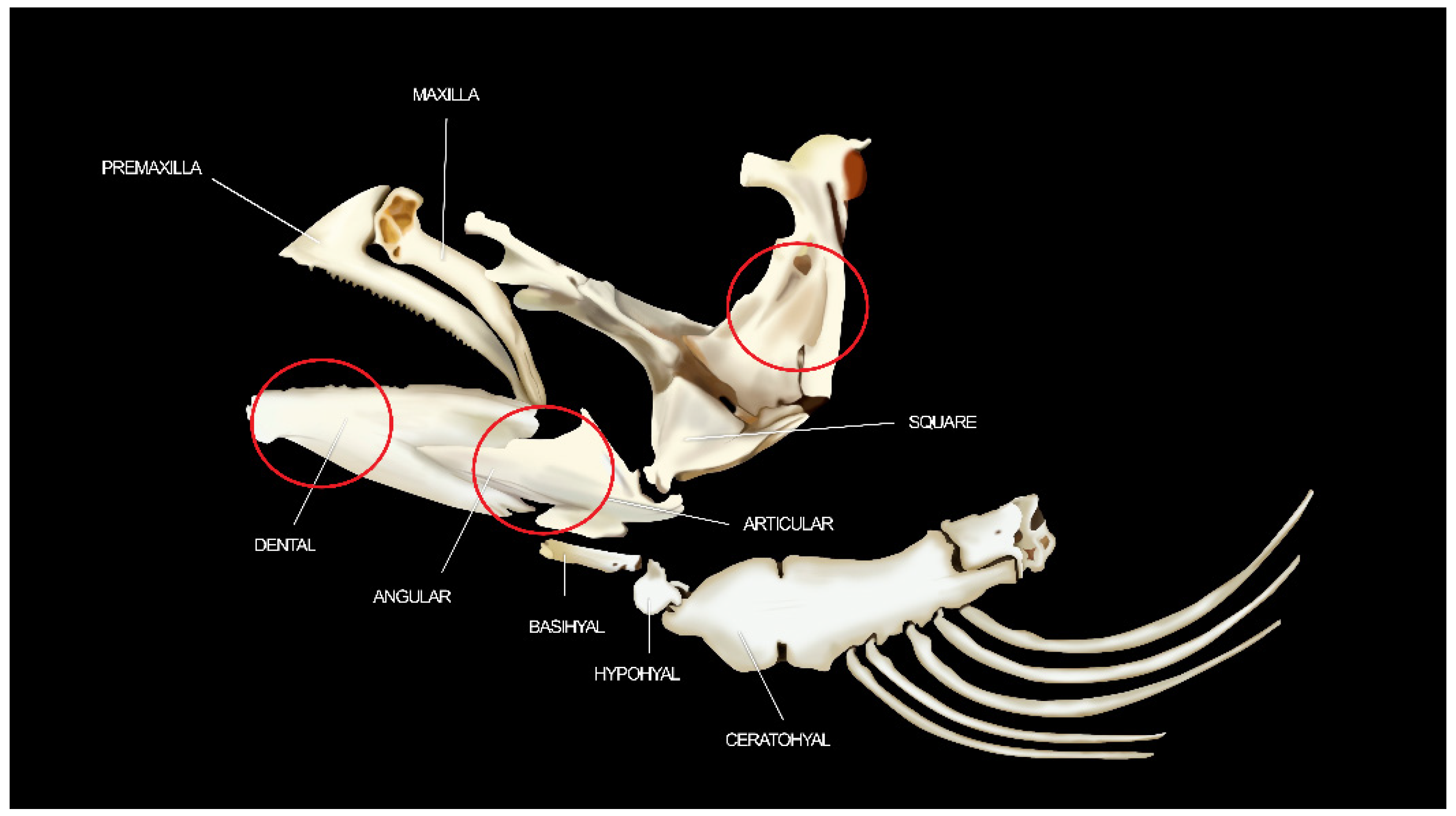
2.4. Scanning Electron Microscopy (VP-SEM) Microstructure and Semi-Quantitative Elemental Microanalysis in Mandibular Bone
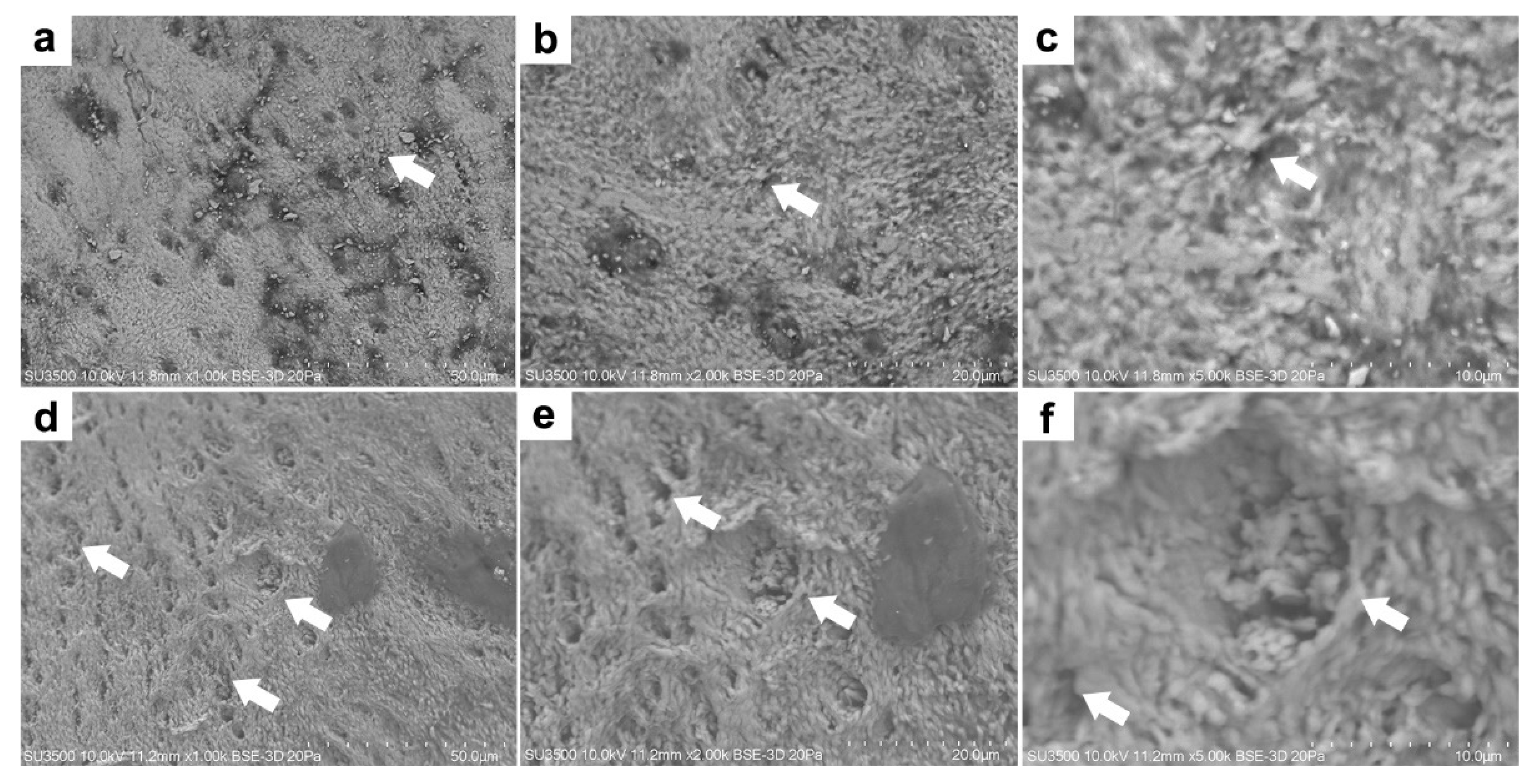
2.4.1. Microstructural Analysis
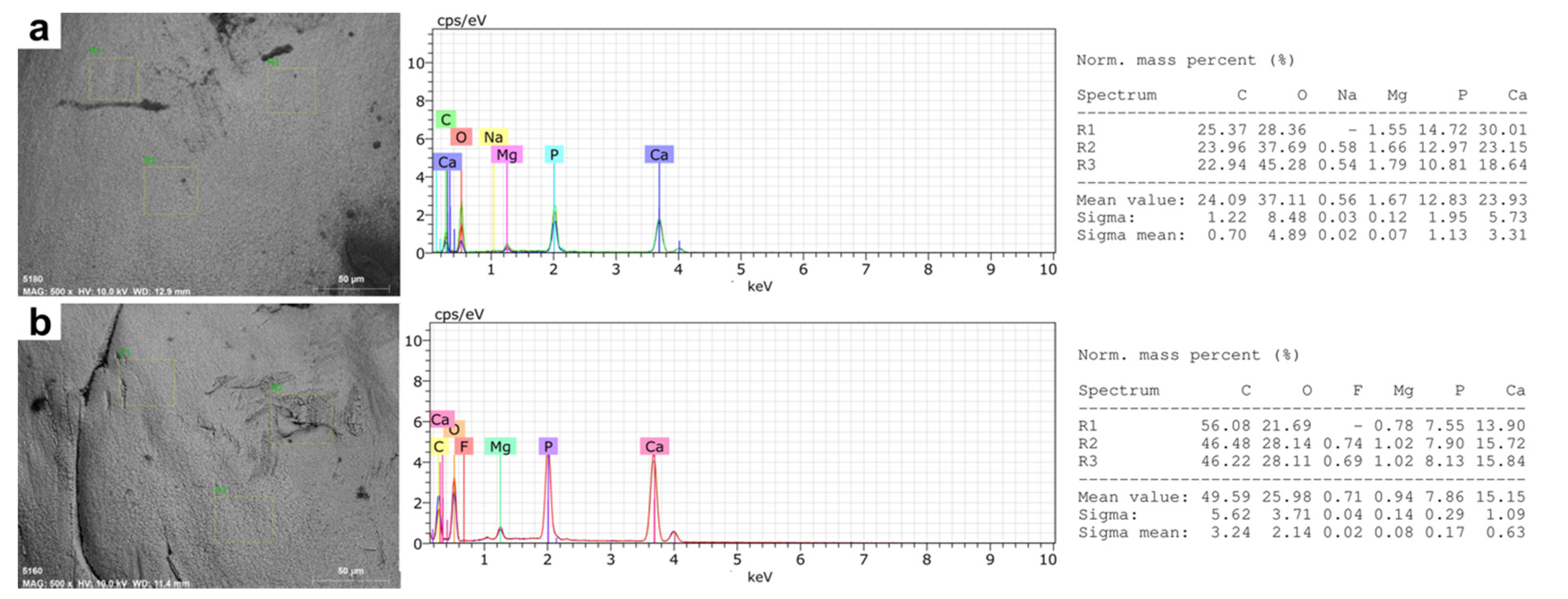
2.4.2. Semi-Quantitative Elemental Microanalysis
2.5. Thermogravimetric Analysis in Mandibular Bone (TGA-DSC)
2.6. Statistical Analysis
3. Results
3.1. Bioavailability of Metabolites and Microelements
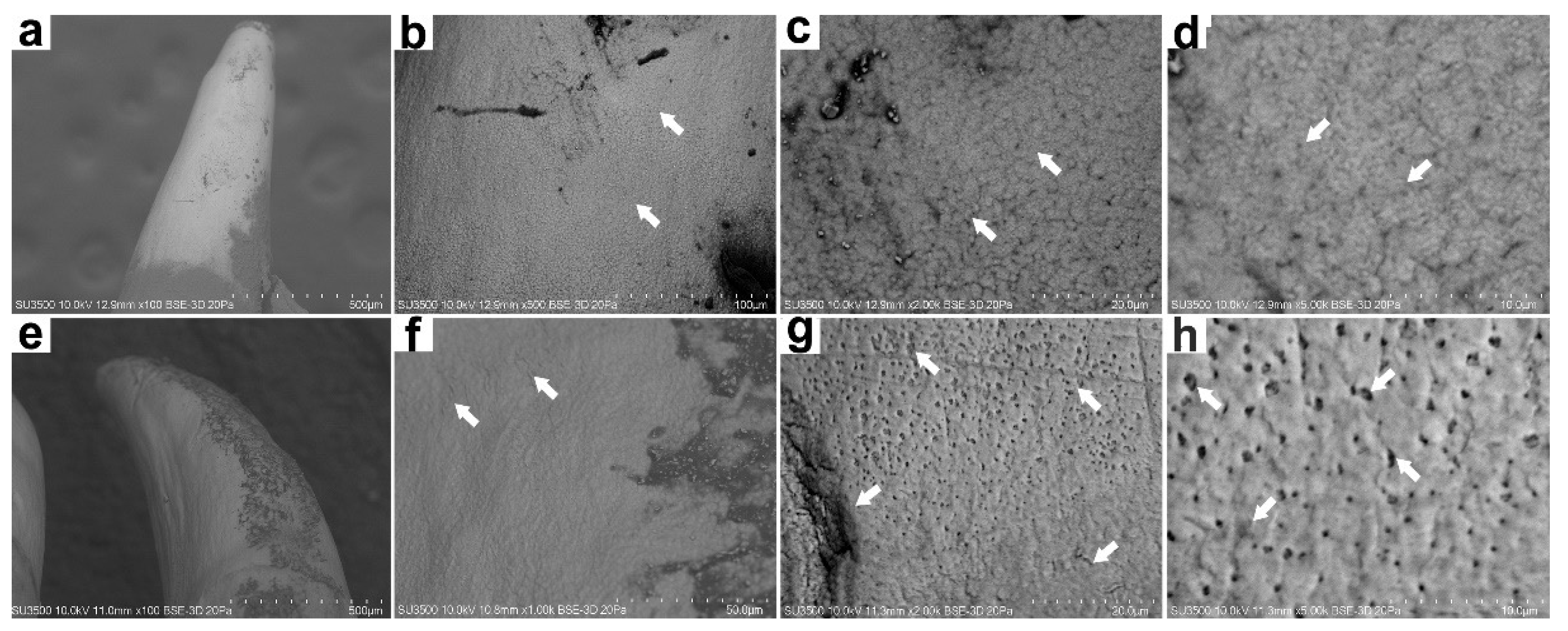
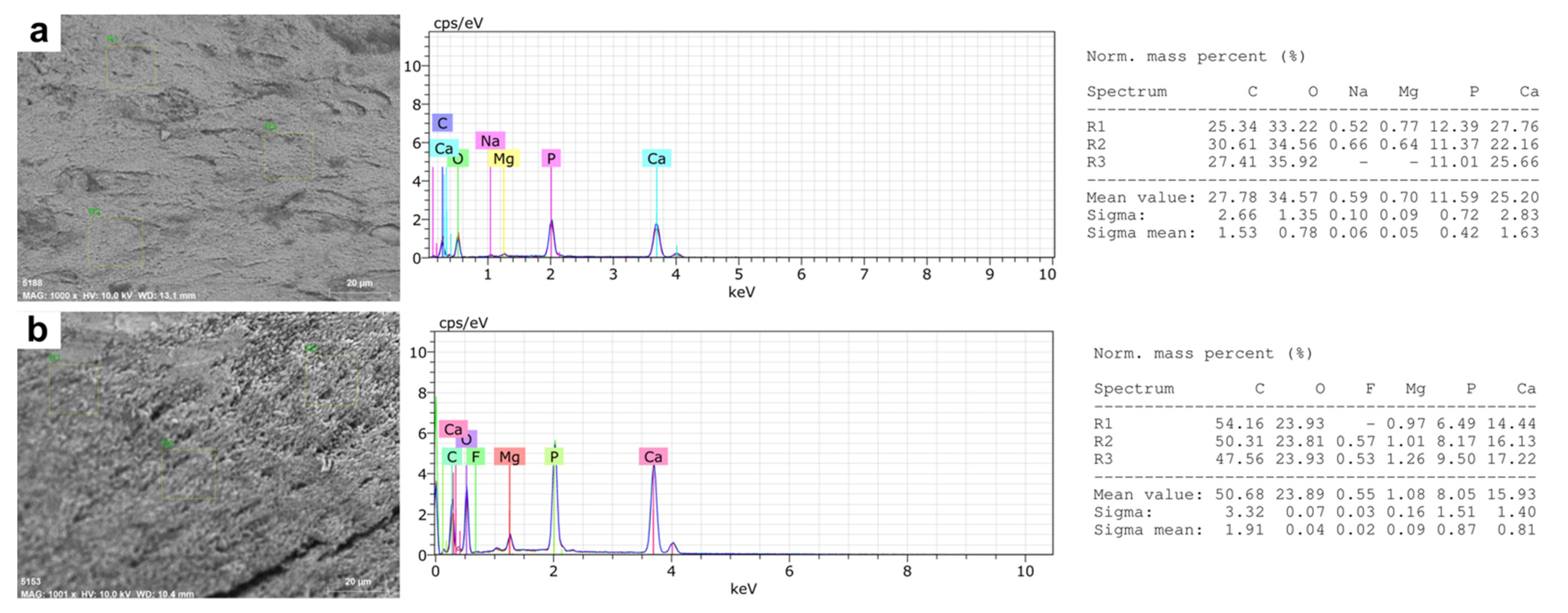
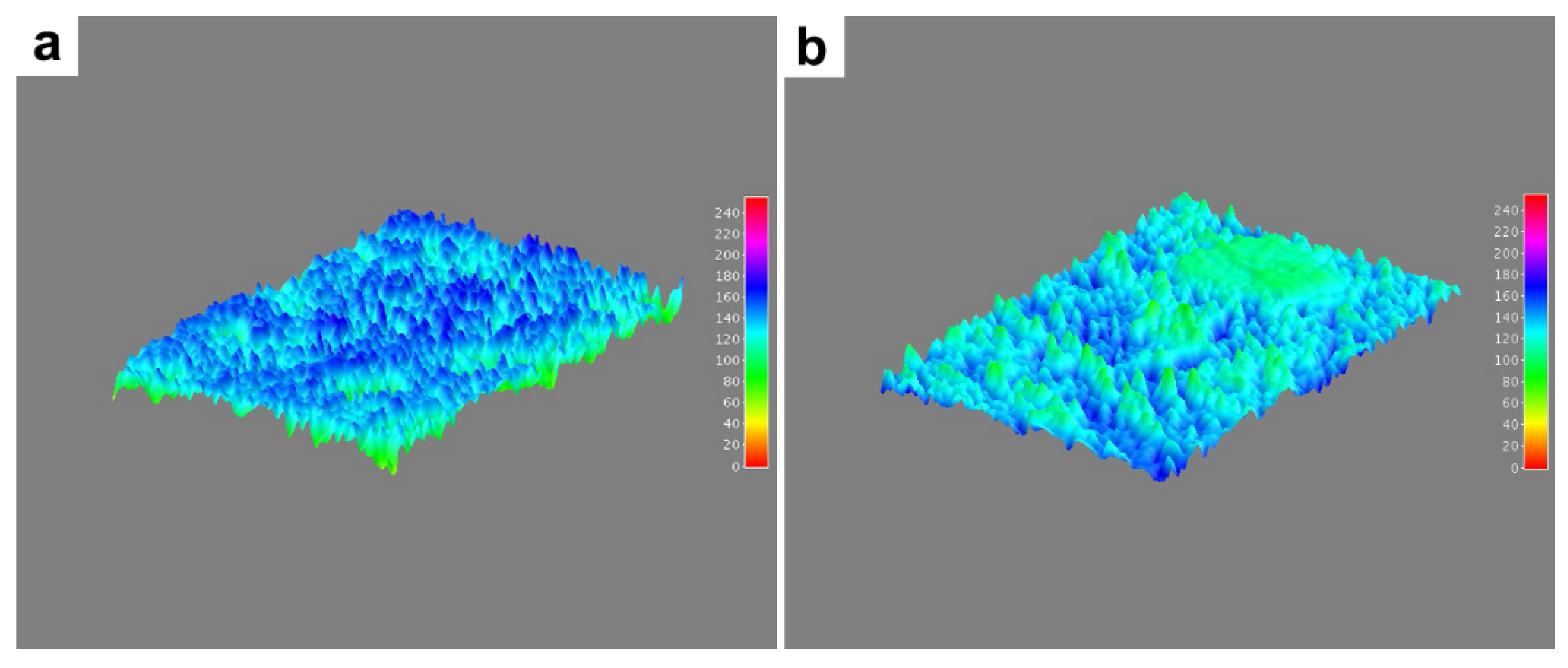
3.2. Microstructural and Microelemental Analysis in Mandibular Bone
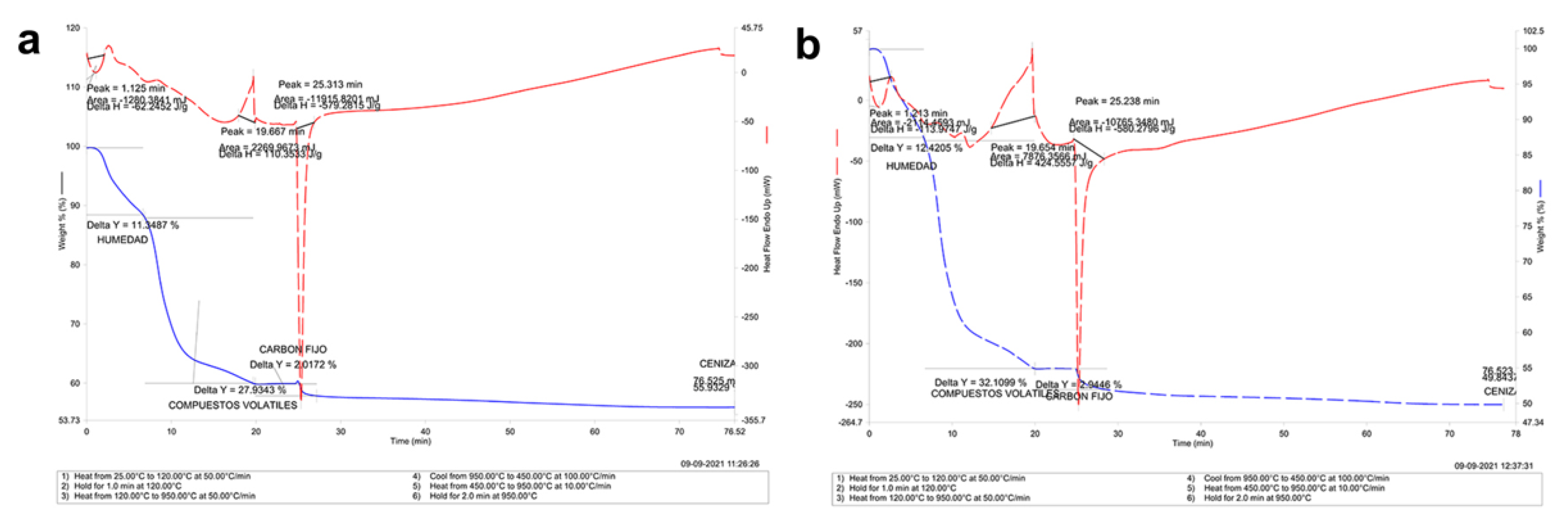
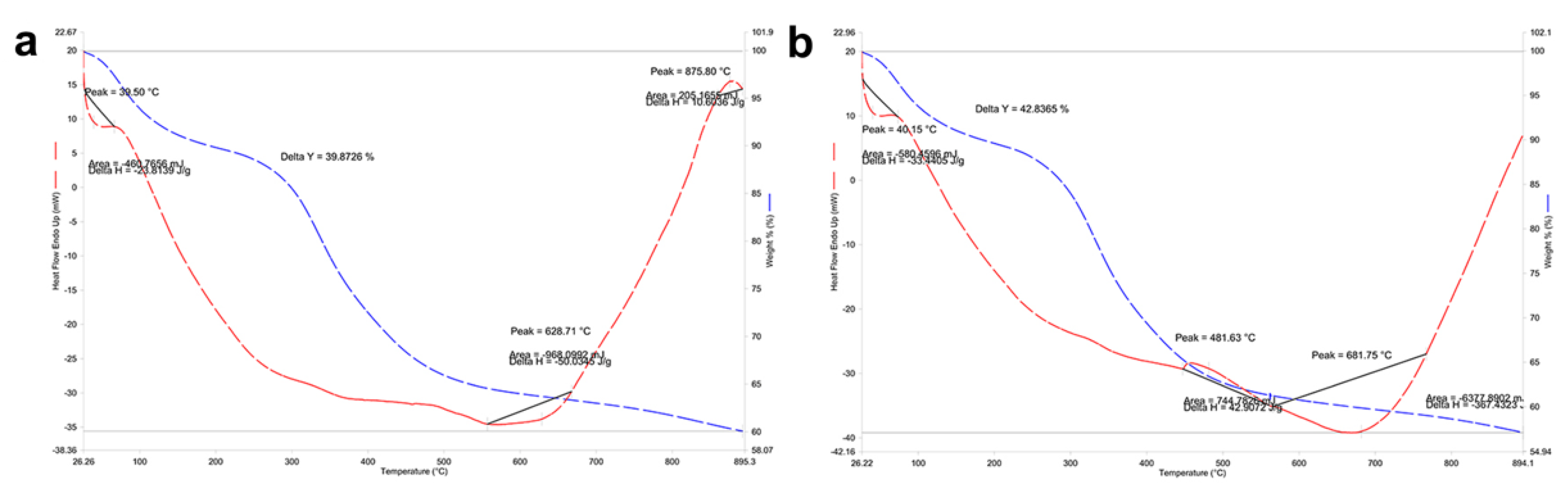
3.3. Thermogravimetric Analysis in Mandibular Bone (TGA-DSC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Luyer, J.; Deschamps, M.H.; Proulx, E.; Poirier Stewart, N.; Droit, A.; Sire, J.Y. RNA-Seq transcriptome analysis of pronounced biconcave vertebrae: A common abnormality in rainbow trout (Oncorhynchus mykiss, Walbaum) fed a low-phosphorus diet. J. Next. Gen. Seq. Appl. 2015, 2, 1000112. [Google Scholar]
- Barahona-Fernándes, M.J. Body deformation in hatchery reared European seabass Dicentrarchus labrax (L). Types, prevalence and effect on fish survival. J. Fish Biol. 1982, 21, 239–249. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Angelopoulou, A.; Kaspiris, P.; Divanach, P.; Koumoundouros, G. Temperature effects on cranial deformities in European sea bass, Dicentrarchus labrax (L.). J. Appl. Ichthyol. 2007, 23, 99–103. [Google Scholar] [CrossRef]
- Argüello-Guevara, W.; Bohórquez-Cruz, M.; Silva, A. Malformaciones craneales en larvas y juveniles de peces cultiva-dos. Lat. Am. J. Aquat. Res. 2017, 42, 950–962. [Google Scholar] [CrossRef]
- Valheim, M.; Brun, E. A Visit to Intesal and the Jaw Deformity Problem in the Chilean Salmon Industry; Instituto Tecnológico del Salmón: Puerto Montt, Chile, 1999. [Google Scholar]
- Rojas, H.; Maretto, D.; Cassigoli, J.; Uribe, C. Epidemiología y Control de la Deformación Mandibular en Salmones; Instituto Tecnológico del Salmón: Puerto Montt, Chile, 2000. [Google Scholar]
- Lall, S.; Lewis, L. Role of nutrients in skeletal metabolism and pathology in fish. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Haga, Y.; Du, S.-J.; Satoh, S.; Kotani, T.; Fushimi, H.; Takeuchi, T. Analysis of the mechanism of skeletal deformity in fish larvae using a vitamin A-induced bone deformity model. Aquaculture 2011, 315, 26–33. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Prabhu, A.; Fjelldal, P.; Albrektsen, S.; Hatlen, B.; Denstadli, V.; Elisabeth Ytteborg, E.; Takle, H.; Lock, E.; Berntssen, M.; et al. Mineral nutrition and bone health in salmonids. Rev. Aquaculture 2019, 11, 740–765. [Google Scholar] [CrossRef]
- Andrades, J.A.; Becerra, J.; Fernández-Llebrez, P. Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.). Aquaculture 1996, 141, 1–11. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino, J.; Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Wargelius, A.; Fjelldal, P.; Nordgarden, U.; Hansen, T. Continuous light affects mineralization and delays osteoid in-corporation in vertebral bone of Atlantic salmon (Salmo salar L.). J. Exp. Biol. 2009, 212, 656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Mori Moren, M.; Fontagn, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2, main typologies, occurrences and causative factors. Rev. Aquaculture 2013, 5, 121–167. [Google Scholar] [CrossRef]
- Afonso, J.M.; Roo, F.J. Anomalías Morfológicas en Peces Cultivados: Heredabilidad y Selección. In Genética y Genómica en Acuicultura; Martínez, P., Figueras, A., Eds.; Publicaciones Científicas y Tecnológicas de la Fundación Observatorio Español de Acuicultura: Madrid, Spain, 2009. [Google Scholar]
- Krossoy, C.; Robin Ornsrud, R.; Wargelius, A. Differential gene expression of bgp and mgp in trabecular and compact bone of Atlantic salmon (Salmo salar L.) vertebrae. J. Anat. 2009, 215, 663–672. [Google Scholar] [CrossRef]
- Battaglene, S.C.; Talbot, R.B. Effects of Salinity and Aeration on Survival of and Initial Swim Bladder Inflation in Larval Australian Bass. Progress. Fish Cult. 1993, 55, 35–39. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Koumoundorus, G.; Divanach, P.; Kentorui, M. Osteological development of the vertebral column and of the fins in Pagellus erythrinus (L. 1758). Temperature effect on the developmental plasticity and mor-pho-anatomical abnormalities. Aquaculture 2004, 232, 407–424. [Google Scholar] [CrossRef]
- Ytteborg, E.; Baeverfjord, G.; Torgersen, J.; Hjelde, K.; Harald Takle, H. Molecular pathology of vertebral deformities in hyperthermic Atlantic salmon (Salmo salar). BMC Physiol. 2010, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Bregua, P.; Guerreiro, P.; Rotllant, J. Stress, Glucocorticoids and Bone: A Review from Mammals and Fish. Front. Endocrinol. 2018, 9, 516. [Google Scholar] [CrossRef]
- Halver, J. The vitamins required for cultivated salmonids. Comp. Biochem. Physiol. 1982, 73, 43–50. [Google Scholar] [CrossRef]
- Lonchmann, R.; Phillips, H. Dietary protein requirement of juvenile golden shiners (Notemigonus crysoleucas) and goldfish (Carassius auratus) in aquaria. Aquaculture 1994, 128, 277–285. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Cahu, C.L.; Zambonino-Infante, J.L. Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture 2011, 315, 49–60. [Google Scholar] [CrossRef]
- Snellgrove, D.; Alexander, L. Whole-body amino acid composition of adult fancy ranchu goldfish (Carassius auratus). Br. J. Nutr. 2011, 106, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, S.H.; Rafiee, G.H.; Farhangi, M.; Mojazi, B. Effect of Different Levels of Dietary Calcium and Potassium on Growth Indices, Biochemical Composition and Some Whole Body Minerals in Rainbow Trout (Oncorhynchus mykiss) Fingerlings. J. Aquac. Res. Dev. 2013, 4, 2–8. [Google Scholar]
- Rojas, M.; Ramírez, E.; del Sol, M. Morphological Study and Mineral Analysis of the Lower Mandible of Adult Atlantic Salmon (Salmo salar) from Scotland with Mandibular Deformation. Int. J. Morphol. 2016, 34, 1097–1104. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, S.; Cai, Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS ONE 2012, 7, e46286. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V.; et al. Toxic Effects of Zinc Chloride on the Bone Development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tresguerres, I.; Hernández-Gil, M.; Alobera, G.; del Canto Pingarrón, M.; Blanco Jerez, L. Physiological bases of bone regeneration I. Histology and physiology of bone tissue. Med. Oral Patol. Oral Cir. Bucal 2006, 11, 47–51. [Google Scholar]
- Boskey, A.; Gehron Robey, P. The Regulatory Role of Matrix Proteins in Mineralization of Bone. Osteoporosis 2013, 4, 235–255. [Google Scholar]
- Uyanab, O.; Koshiob, S.; Ishikawab, M. Effects of dietary phosphorus and phospholipid level on growth, and phospho-rus deficiency signs in juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2007, 267, 44–54. [Google Scholar]
- Lee, K.J.; Poweli, M.; Barrows, F.; Smiley, S.; Bechtel, P.; Hardy, R. Evaluation of supplemental fish bone meal made from Alaska seafood processing byproducts and dicalcium phosphate in plant protein based diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 2010, 302, 248–255. [Google Scholar] [CrossRef]
- Saris, N.; Mervaala, E.; Karppanen, H.; Khawaja, J.; Lewenstam, A. Magnesium An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Ciosek, Z.; Kot, K.; Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. 2-Oxoglutaratedependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: Potential role in the regulation of aging process. Cell. Mol. Life Sci. 2015, 72, 3897–3914. [Google Scholar] [CrossRef] [PubMed]
- Bishop, T.; Ratcliffe, P.J. Signaling hypoxia by hypoxiainducible factor protein hydroxylases: A historical overview and future perspectives. Hypoxia 2014, 2, 197–213. [Google Scholar] [PubMed]
- Medeiros, D.M.; Stoecker, B.; Plattner, A.; Jennings, D.; Haub, M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J. Nutr. 2004, 134, 3061–3067. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Plattner, A.; Jennings, D.; Stoecker, B. Bone morphology, strength and density are compromised in iron deficient rats and exacerbated by calcium restriction. J. Nutr. 2002, 132, 3135–3141. [Google Scholar] [CrossRef]
- Parelman, M.; Stoecker, B.; Baker, A.; Medeiros, D. Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp. Biol. Med. 2006, 231, 378–386. [Google Scholar] [CrossRef]
- Herrera, E. Perfil Metabólico de Salmón Atlántico Salmo Salar y Trucha Arcoiris Oncorhynchus mykiss de Tres Pisciculturas en Fase de Agua Dulce en el sur de Chile; Universidad Austral de Chile: Valdivia, Chile, 2004. [Google Scholar]
- Stockham, S.L.; Scott, M.A. Fundamentals of Veterinary Clinical Pathology; Iowa State University Press: Ames, IA, USA, 2008. [Google Scholar]
- Thrall, M.A.; Baker, D.C.; Campbell, T.W.; DeNicolla, D.; Fettman, M.J.; Lassen, E.D.; Rebar, A.; Weiser, G. Veterinary Hematology and Clinical Chemistry; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Wittwer, F. Manual de Patología Clínica Veterinaria, 2nd ed.; Imprenta America: Valdivia, Chile, 2012. [Google Scholar]
- Müller, A.; Bittencourt, P.; Rozas, M.; Walker, R. Clinical Pathology Manual of Salmonid Fish. In Aquaculture Sanitary Management Program of the National Service for Fisheries and Aquaculture; Servicio Nacional de Pesca y Acuicultura: Santiago, Chile, 2018. [Google Scholar]
- Sadovy, Y.J.; Vincent, A.C.J. Ecological Issues and the Trades in Live Reef Fishes. Coral Reef Fishes. Dynamics and Diversity in a Complex Ecosystem; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Godoy, K.; Sandoval, C.; Manterola-Barroso, C.; Salinas, P.; Salazar, L.A.; Rojas, M. Evaluation of mineralization in jaw and teeth of rainbow trout (Oncorhynchus mykiss) using variable pressure scanning electron microscopy coupled to energy-dispersive X-ray spectroscopy detector. Int. J. Morphol. 2022, 40, 530–539. [Google Scholar] [CrossRef]
- Li, Z.H.; Velisek, J.; Zlabek, V.; Grabic, R.; Machova, J.; Kolarova, J.; Randak, T. Hepatic antioxidant status and hemato-logical parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem. Biol. Interact. 2010, 183, 98–104. [Google Scholar] [CrossRef]
- Kaneko, J. Clinical Biochemistry of Domestic Animals, 5th ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Bellier, S. Interprétation etvaleur susuelles des paramètres sanguins en Biochimie clinique vétérinaire. Rev. Francoph. Lab. 2010, 2010, 43–56. [Google Scholar]
- Cáceres, G. Caracterización del Perfil Bioquímico del Salmón del Atlántico “Salmo Salar” Sanos, y Serovariación Ante Desafío con Virus ISA y Piscirickettsia salmonis; Universidad de Chile: Santiago, Chile, 2018. [Google Scholar]
- Polakof, S.; Mommsen, T.; Soengas, J. Glucosensing and glucose homeostasis: From fish to mammals. Comp. Biochem. Physiol. B 2011, 160, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2022, 42, 517–525. [Google Scholar]
- Servicio Nacional de Pesca y Acuicultura (SERNAPESCA). Informe Sanitario de Salmonicultura; Departamento de Salud Animal, Subdirección de Acuicultura, Ministerio de Economía, Fomento y Turismo: Santiago, Chile, 2021. [Google Scholar]
- Organización de la Naciones Unidas para la Alimentación y la Agricultura (FAO). Manual Práctico Para el Cultivo de Trucha Arcoiris; Organización de la Naciones Unidas para la Alimentación y la Agricultura: Guatemala, Guatemala, 2014. [Google Scholar]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.; Cancela, M.L. Development of two bone-derived cell lines from the marine teleost Sparus aurata, evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.H.; McDaniel, N.K.; Ferraris, R.P. In vivo fractional P (i) absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. Am. J. Phys. Regul. Integr. Comp. Phys. 2003, 285, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R.J.; Zerler, B.; Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef]
- Liang, H.; Mi, H.; Ji, K.; Ge, X.; Re, M.; Xie, J. Effects of Dietary Calcium Levels on Growth Performance, Blood Biochemistry and Whole Body Composition in Juvenile Bighead Carp (Aristichthys nobilis). Turkish J. Fish. Aquat. Sci. 2018, 18, 623–631. [Google Scholar]
- Penido, M.; Alon, U. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Bonanno, M.; Rey, M.; Seijo, M.; Zeni, S. Rol de la Osteocalcina más allá del hueso. Osteolgia 2019, 15, 78–93. [Google Scholar]
- Londoño, M.; Echavarría, A.; De la Calle, F. Características cristaloquímicas de la Hidroxiapatita sintética tratada a diferentes temperaturas. Rev. EIA. Esc. Ing. Antioq. 2006, 5, 109–118. [Google Scholar]
- Pareja, N.; Escobar, D.; Ossa, C.; Echavarría, A. Synthesis and characterization of microporous hydroxyapatite, comparison with a commercial product. Rev. Fac. Ing. Univ. Antioq. 2008, 43, 67–76. [Google Scholar]
- Hernández, G. Biocerámicos Porosos Compuestos de Hidroxiapatita y Dióxido de Titanio; Universidad Nacional de Mar del Plata: Mar del Plata, Argentina, 2020. [Google Scholar]


| Medium (Minimum–Maximum) | p-Value | ||
|---|---|---|---|
| Blood Metabolites | Farmed Fish | Wild Fish | |
| Albumin (g/L) | 30.0 (24.7–41.8) | 18.1 (15.5–20.4) | <0.001 |
| Acid Phosphatase (U/L) | 5.88 (3.73–7.56) | 13.3 (8.51–21.6) | <0.001 |
| Alkaline Phosphatase (U/L) | 229 (131–394) | 594 (340–860) | <0.001 |
| Creatine kinase (U/L) | 7021 (513–15,920) | 2192 (1045–3249) | 0.024 |
| Globulin (g/L) | 37.6 (15.0–67.2) | 28.9 (18.1–37.7) | 0.053 |
| Glucose (mmol/L) | 4.49 (2.75–6.64) | 3.43 (3.26–3.63) | <0.001 |
| Total Protein (g/L) | 67.6 (41.1–95.6) | 47.0 (34.6–56.4) | <0.001 |
| Medium (Minimum–Maximum) | p-Value | ||
|---|---|---|---|
| Blood Microelements | Farmed Fish | Wild Fish | |
| Calcium (mmol/L) | 4.16 (2.70–5.90) | 4.07 (3.60–4.50) | 0.516 |
| Phosphorus (mmol/L) | 6.63 (4.60–8.60) | 8.54 (6.80–11.40) | <0.001 |
| Ca/P ratio | 0.63 (0.49–0.81) | 0.48 (0.39–0.60) | 0.106 |
| Iron (µmol/L) | 15.19 (12.80–18.20) | 13.28 (9.70–19.80) | 0.001 |
| Magnesium (mmol/L) | 2.14 (0.89–4.32) | 2.95 (2.68–3.28) | <0.001 |
| Medium (Minimum–Maximum) | p-Value | ||
|---|---|---|---|
| Mass Per Cent (%W) | Farmed Fish | Wild Fish | |
| Carbon | 22.72 (18.31–35.31) | 46.87 (37.38–56.08) | <0.001 |
| Oxygen | 39.44 (28.36–45.28) | 25.70 (15.75–33.71) | <0.001 |
| Calcium | 23.78 (18.45–30.15) | 17.63 (13.90–20.74) | 0.001 |
| Phosphorus | 11.70 (7.90–14.72) | 8.32 (5.89–10.57) | 0.001 |
| Ca/P ratio | 2.06 (1.72–2.99) | 2.17 (1.82–3.45) | 0.637 |
| Magnesium | 1.40 (0.55–2.18) | 0.89 (0.75–1.02) | 0.094 |
| Fluoride | 0.59 (0.00–1.70) | 0.81 (0.69–0.87) | 0.73 |
| Medium (Minimum–Maximum) | p-Value | ||
|---|---|---|---|
| Mass Per Cent (% W) | Farmed Fish | Wild Fish | |
| Carbon | 31.31 (15.30–48.84) | 44.61 (31.11–57.68) | 0.009 |
| Oxygen | 34.47 (29.95–42.61) | 29.91 (22.26–36.03) | 0.049 |
| Calcium | 21.90 (12.60–29.26) | 14.07 (9.20–17.98) | 0.006 |
| Phosphorus | 10.11 (5.78–14.11) | 6.83 (4.81–8.67) | 0.012 |
| Ca/P ratio | 2.18 (1.81–2.50) | 2.06 (1.73–2.32) | 0.993 |
| Magnesium | 0.78 (0.00–1.11) | 0.62 (0.52–0.78) | 0.018 |
| Fluoride | 0.10 (0.00–0.92) | 0.16 (0.00–0.64) | 0.702 |
| Medium (Minimum–Maximum) | p-Value | ||
|---|---|---|---|
| Parameters | Farmed Fish | Wild Fish | |
| Pore Number | 7940.83 (6281.92–9598.84) | 11,339.60 (7071.97–15,607.23) | 0.048 |
| Total Area (µm2) | 34.97 (22.69–47.25) | 55.18 (32.19–78.17) | 0.14 |
| Pore Size (nm) | 4.45 (3.42–5.48) | 4.89 (3.19–6.57) | 0.667 |
| % of Total Area | 3.46 (2.67–4.25) | 5.81 (4.67–6.95) | 0.261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, K.; Sandoval, C.; Manterola-Barroso, C.; Vásquez, C.; Sepúlveda, N.; Rojas, M.; Salazar, L.A. Study of the Mandibular Bone Microstructure and Blood Minerals Bioavailability in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) from Freshwater. Animals 2022, 12, 1476. https://doi.org/10.3390/ani12121476
Godoy K, Sandoval C, Manterola-Barroso C, Vásquez C, Sepúlveda N, Rojas M, Salazar LA. Study of the Mandibular Bone Microstructure and Blood Minerals Bioavailability in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) from Freshwater. Animals. 2022; 12(12):1476. https://doi.org/10.3390/ani12121476
Chicago/Turabian StyleGodoy, Karina, Cristian Sandoval, Carlos Manterola-Barroso, Claudio Vásquez, Noelia Sepúlveda, Mariana Rojas, and Luis A. Salazar. 2022. "Study of the Mandibular Bone Microstructure and Blood Minerals Bioavailability in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) from Freshwater" Animals 12, no. 12: 1476. https://doi.org/10.3390/ani12121476
APA StyleGodoy, K., Sandoval, C., Manterola-Barroso, C., Vásquez, C., Sepúlveda, N., Rojas, M., & Salazar, L. A. (2022). Study of the Mandibular Bone Microstructure and Blood Minerals Bioavailability in Rainbow Trout (Oncorhynchus mykiss, Walbaum 1792) from Freshwater. Animals, 12(12), 1476. https://doi.org/10.3390/ani12121476









