Laparoscopic versus Open Ovariectomy in Bitches: Changes in Cardiorespiratory Values, Blood Parameters, and Sevoflurane Requirements Associated with the Surgical Technique
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Equipment
2.3. Anesthesia and Analgesia
2.4. Surgical Procedure
2.4.1. Laparoscopic Ovariectomy Group
2.4.2. Laparotomy Ovariectomy Group
2.5. Blood Sampling and Recorded Data
2.6. Statistical Analysis
3. Results
3.1. Age, Weight and Body Condition
3.2. Cardiorespiratory and End-Tidal Sevoflurane Concentration Values
3.3. Blood Gas and Hematochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devitt, C.M.; Cox, R.E.; Hailey, J.J. Duration, Complications, Stress, and Pain of Open Ovariohysterectomy versus a Simple Method of Laparoscopic-Assisted Ovariohysterectomy in Dogs. J. Am. Vet. Med. Assoc. 2005, 227, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Dupré, G.; Fiorbianco, V.; Skalicky, M.; Gültiken, N.; Ay, S.S.; Findik, M. Laparoscopic Ovariectomy in Dogs: Comparison between Single Portal and Two-Portal Access. Vet. Surg. 2009, 38, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.B.; Moll, H.D.; Payton, M.E. Comparison of Laparoscopic Ovariohysterectomy and Ovariohysterectomy in Dogs. Vet. Surg. 2004, 33, 62–69. [Google Scholar] [CrossRef]
- Nylund, A.M.; Drury, A.; Weir, H.; Monnet, E. Rates of Intraoperative Complications and Conversion to Laparotomy during Laparoscopic Ovariectomy Performed by Veterinary Students: 161 Cases (2010–2014). J. Am. Vet. Med. Assoc. 2017, 251, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, J.; Khalaj, A.R.; Aminlou, E.; Niasari-Naslaji, A. Comparative Evaluation of Conventional and Transvaginal Laparoscopic Ovariohysterectomy in Dogs. Vet. Surg. 2012, 41, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, K.M.; Giuffrida, M.A.; Mayhew, P.D.; Runge, J.J. Outcome of Laparoscopic Ovariectomy and Laparoscopic-Assisted Ovariohysterectomy in Dogs: 278 Cases (2003–2013). J. Am. Vet. Med. Assoc. 2017, 251, 443–450. [Google Scholar] [CrossRef]
- Cicirelli, V.; Lacalandra, G.M.; Aiudi, G.G. The Effect of Splash Block on the Need for Analgesia in Dogs Subjected to Video-assisted Ovariectomy. Vet. Med. Sci. 2022, 8, 104. [Google Scholar] [CrossRef]
- Radford, A.C.; Bonaventura, N.C.; Ganjei, J.B. Combined Laparoscopic Ovariectomy and Laparoscopic-Assisted Gastropexy Utilizing a 2-Port Technique in 10 Dogs. Can. Vet. J. 2021, 62, 1111. [Google Scholar]
- Culp, W.T.N.; Mayhew, P.D.; Brown, D.C. The Effect of Laparoscopic versus Open Ovariectomy on Postsurgical Activity in Small Dogs. Vet. Surg. 2009, 38, 811–817. [Google Scholar] [CrossRef]
- Shariati, E.; Bakhtiari, J.; Khalaj, A.; Niasari-Naslaji, A. Comparison between Two Portal Laparoscopy and Open Surgery for Ovariectomy in Dogs. Vet. Res. Forum. Int. Q. J. 2014, 5, 219–223. [Google Scholar]
- Hancock, R.B.; Lanz, O.I.; Waldron, D.R.; Duncan, R.B.; Broadstone, R.V.; Hendrix, P.K. Comparison of Postoperative Pain after Ovariohysterectomy by Harmonic Scalpel-Assisted Laparoscopy Compared with Median Celiotomy and Ligation in Dogs. Vet. Surg. 2005, 34, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.D.; Freeman, L.; Kwan, T.; Brown, D.C. Comparison of Surgical Site Infection Rates in Clean and Clean-Contaminated Wounds in Dogs and Cats after Minimally Invasive versus Open Surgery: 179 Cases (2007–2008). J. Am. Vet. Med. Assoc. 2012, 240, 193–198. [Google Scholar] [CrossRef]
- Mayhew, P.D. Complications of Minimally Invasive Surgery in Companion Animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Collivignarelli, F.; Vignoli, M.; Scaletta, L.; Cuomo, A.; Falerno, I.; Paolini, A.; Tamburro, R.; Phillips, C. A Comparison of Times Taken for the Placement of the First Portal and Complication Rates between the Veress Needle Technique and the Modified Hasson Technique in Canine Ovariectomy Laparoscopic Surgery. Animals 2021, 11, 2936. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.P.; Mullins, R.A.; Singh, A.; Mayhew, P.D. A Systematic Review of Complications Related to Laparoscopic and Laparoscopic-Assisted Procedures in Dogs. Vet. Surg. 2020, 49, O5–O14. [Google Scholar] [CrossRef] [PubMed]
- Safran, D.B.; Orlando, R. Physiologic Effects of Pneumoperitoneum. Am. J. Surg. 1994, 167, 281–286. [Google Scholar] [CrossRef]
- Di Bella, C.; Lacitignola, L.; Grasso, S.; Centonze, P.; Greco, A.; Ostuni, R.; Crovace, A.; Staffieri, F. An Alveolar Recruitment Maneuver Followed by Positive End-Expiratory Pressure Improves Lung Function in Healthy Dogs Undergoing Laparoscopy. Vet. Anaesth. Analg. 2018, 45, 618–629. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.C. Comparison of Oxidative Stress Status in Dogs Undergoing Laparoscopic and Open Ovariectomy. J. Vet. Med. Sci. 2013, 76, 273–276. [Google Scholar] [CrossRef]
- Rauh, R.; Hemmerling, T.M.; Rist, M.; Jacobi, K.E. Influence of Pneumoperitoneum and Patient Positioning on Respiratory System Compliance. J. Clin. Anesth. 2001, 13, 361–365. [Google Scholar] [CrossRef]
- Baki, E.D.; Kokulu, S.; Bal, A.; Ela, Y.; Sivaci, R.G.; Yoldas, M.; Çelik, F.; Ozturk, N.K. Evaluation of Low Tidal Volume with Positive End-Expiratory Pressure Application Effects on Arterial Blood Gases during Laparoscopic Surgery. J. Chin. Med. Assoc. 2014, 77, 374–378. [Google Scholar] [CrossRef][Green Version]
- Fukushima, F.B.; Malm, C.; Andrade, M.E.J.; Oliveira, H.P.; Melo, E.G.; Caldeira, F.M.C.; Gheller, V.A.; Palhares, M.S.; Macedo, S.P.; Figueiredo, M.S.; et al. Cardiorespiratory and Blood Gas Alterations during Laparoscopic Surgery for Intra-Uterine Artificial Insemination in Dogs. Can. Vet. J. 2011, 52, 77–79. [Google Scholar] [PubMed]
- Scott, J.; Singh, A.; Valverde, A. Pneumoperitoneum in Veterinary Laparoscopy: A Review. Vet. Sci. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Umano, G.R.; Delehaye, G.; Noviello, C.; Papparella, A. The “Dark Side” of Pneumoperitoneum and Laparoscopy. Minim. Invasive Surg. 2021, 2021, 5564745. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.V.; Ganem, E.M.; Carraretto, A.R.; Vianna, P.T. Hemodynamic Changes during Pneumoperitoneum in Volume and Pressure Controlled Ventilated Dogs. Rev. Bras. Anestesiol. 2003, 53, 756–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duke, T.; Steinacher, S.L.; Remedios, A.M. Cardiopulmonary Effects of Using Carbon Dioxide for Laparoscopic Surgery in Dogs. Vet. Surg. 1996, 25, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.D.; Pascoe, P.J.; Kass, P.H.; Shilo-Benjamini, Y. Effects of Pneumoperitoneum Induced at Various Pressures on Cardiorespiratory Function and Working Space during Laparoscopy in Cats. Am. J. Vet. Res. 2013, 74, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.C.; Case, J.B.; Coisman, J.G.; Isaza, N.M.; Amora-Junior, D.; Maisenbacher, H.W. Cardiopulmonary Effects of Laparoscopic Ovariectomy of Variable Duration in Cats. Vet. Surg. 2015, 44, 2–6. [Google Scholar] [CrossRef]
- Dorn, M.; Becher-Deichsel, A.; Bockstahler, B.; Peham, C.; Dupré, G. Pressure–Volume Curve during Capnoperitoneum in Cats. Animals 2020, 10, 1408. [Google Scholar] [CrossRef]
- Sümpelmann, R.; Schuerholz, T.; Marx, G.; Härtel, D.; Hecker, H.; Ure, B.M.; Jesch, N.K. Haemodynamic, Acid–Base and Blood Volume Changes during Prolonged Low Pressure Pneumoperitoneum in Rabbits. Br. J. Anaesth. 2006, 96, 563–568. [Google Scholar] [CrossRef][Green Version]
- Russo, A.; Di Stasio, E.; Scagliusi, A.; Bevilacqua, F.; Isgrò, M.A.; Marana, R.; Marana, E. Positive End-Expiratory Pressure during Laparoscopy: Cardiac and Respiratory Effects. J. Clin. Anesth. 2013, 25, 314–320. [Google Scholar] [CrossRef]
- Hirvonen, E.A.; Nuutinen, L.S.; Kauko, M. Ventilatory Effects, Blood Gas Changes, and Oxygen Consumption During Laparoscopic Hysterectomy. Anesth. Analg. 1995, 80, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.M.; Giraud, G.D.; Togioka, B.M.; Jones, D.B.; Cigarroa, J.E. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017, 135, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Lacitignola, L.; Fracassi, L.; Skouropoulou, D.; Crovace, A.; Staffieri, F. Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study. Vet. Sci. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Beazley, S.G.; Cosford, K.; Duke-Novakovski, T. Cardiopulmonary Effects of Using Carbon Dioxide for Laparoscopic Surgery in Cats. Can. Vet. J. 2011, 52, 973. [Google Scholar] [PubMed]
- Ivankovich, A.D.; Miletich, D.J.; Albrecht, R.F.; Heyman, H.J.; Bonnet, R.F. Cardiovascular Effects of Intraperitoneal Insufflation with Carbon Dioxide and Nitrous Oxide in the Dog. Anesthesiology 1975, 42, 281–287. [Google Scholar] [CrossRef]
- Kabakchiev, C.; Valverde, A.; Singh, A.; Beaufrère, H. Cardiovascular and Respiratory Effects of Carbon Dioxide Pneumoperitoneum in the Domestic Rabbit (Oryctolagus Cuniculus). Can. J. Vet. Res. 2020, 84, 108. [Google Scholar]
- Duerr, F.M.; Twedt, D.C.; Monnet, E. Changes in PH of Peritoneal Fluid Associated with Carbon Dioxide Insufflation during Laparoscopic Surgery in Dogs. Am. J. Vet. Res. 2008, 69, 298–301. [Google Scholar] [CrossRef]
- Freeman, L.J.; Rahmani, E.Y.; Al-Haddad, M.; Sherman, S.; Chiorean, M.V.; Selzer, D.J.; Snyder, P.W.; Constable, P.D. Comparison of Pain and Postoperative Stress in Dogs Undergoing Natural Orifice Transluminal Endoscopic Surgery, Laparoscopic, and Open Oophorectomy. Gastrointest. Endosc. 2010, 72, 373–380. [Google Scholar] [CrossRef]
- Haraguchi, T.; Kimura, S.; Itoh, H.; Nishikawa, S.; Hiyama, M.; Tani, K.; Iseri, T.; Itoh, Y.; Nakaichi, M.; Taura, Y.; et al. Comparison of Postoperative Pain and Inflammation Reaction in Dogs Undergoing Preventive Laparoscopic-Assisted and Incisional Gastropexy. J. Vet. Med. Sci. 2017, 79, 1524–1531. [Google Scholar] [CrossRef]
- Del Romero, A.; Cuervo, B.; Peláez, P.; Miguel, L.; Torres, M.; Yeste, M.; Rivera Del Alamo, M.M.; Rubio, C.P.; Rubio, M. Changes in Acute Phase Proteins in Bitches after Laparoscopic, Midline, and Flank Ovariectomy Using the Same Method for Hemostasis. Animals 2020, 10, 2223. [Google Scholar] [CrossRef]
- Höglund, O.V.; Olsson, K.; Hagman, R.; Öhlund, M.; Olsson, U.; Lagerstedt, A.S. Comparison of Haemodynamic Changes during Two Surgical Methods for Neutering Female Dogs. Res. Vet. Sci. 2011, 91, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Guha, S.K.; Tiwary, R.; Kanti Guha, S.; Ansari, M. Haemato-Biochemical Indices in Female Dogs Undergoing Laparoscopic and Open Elective Ovariectomy. Pharma Innov. J. 2018, 7, 168–176. [Google Scholar]
- Impellizeri, J.A.; Tetrick, M.A.; Muir, P. Effect of Weight Reduction on Clinical Signs of Lameness in Dogs with Hip Osteoarthritis. J. Am. Vet. Med. Assoc. 2000, 216, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Cassata, G.; Palumbo, V.D.; Cicero, L.; Damiano, G.; Maenza, A.; Migliazzo, A.; Di Paola, G.; Vicari, D.; Fazzotta, S.; Lo Monte, A.I. Laparotomic vs Laparoscopic Ovariectomy: Comparing the Two Methods. The Ovariectomy in the Bitch in Laparoscopic Era. Acta Biomed. 2016, 87, 271–274. [Google Scholar]
- Bendinelli, C.; Properzi, R.; Boschi, P.; Bresciani, C.; Rocca, E.; Sabbioni, A.; Leonardi, F. Meloxicam vs Robenacoxib for Postoperative Pain Management in Dogs Undergoing Combined Laparoscopic Ovariectomy and Laparoscopic-Assisted Gastropexy. Vet. Surg. 2019, 48, 578–583. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Ferreira, D.A.; Brás, S.; Gonzalo-Orden, J.M.; Antunes, L.M. Correlation between Clinical Signs of Depth of Anaesthesia and Cerebral State Index Responses in Dogs with Different Target-Controlled Infusions of Propofol. Vet. Anaesth. Analg. 2012, 39, 21–28. [Google Scholar] [CrossRef]
- Wagner, M.C.; Hecker, K.G.; Pang, D.S.J. Sedation Levels in Dogs: A Validation Study. BMC Vet. Res. 2017, 13, 110. [Google Scholar] [CrossRef]
- Hernández-Avalos, I.; Valverde, A.; Ibancovichi-Camarillo, J.A.; Sánchez-Aparicio, P.; Recillas-Morales, S.; Osorio-Avalos, J.; Rodríguez-Velázquez, D.; Miranda-Cortés, A.E. Clinical Evaluation of Postoperative Analgesia, Cardiorespiratory Parameters and Changes in Liver and Renal Function Tests of Paracetamol Compared to Meloxicam and Carprofen in Dogs Undergoing Ovariohysterectomy. PLoS ONE 2020, 15, e0223697. [Google Scholar] [CrossRef]
- Cicirelli, V.; Debidda, P.; Maggio, N.; Caira, M.; Lacalandra, G.M.; Aiudi, G.G. Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs. Animals 2021, 11, 1275. [Google Scholar] [CrossRef]
- Goethem, B.E.B.J.; Rosenveldt, K.W.; Kirpensteijn, J. Monopolar versus Bipolar Electrocoagulation in Canine Laparoscopic Ovariectomy: A Nonrandomized, Prospective, Clinical Trial. Vet. Surg. 2003, 32, 464–470. [Google Scholar] [CrossRef]
- Hasson, H.M. Open Laparoscopy as a Method of Access in Laparoscopic Surgery. Gynaecol. Endosc. 1999, 8, 353–362. [Google Scholar] [CrossRef]
- Wauters, J.; Claus, P.; Brosens, N.; McLaughlin, M.; Hermans, G.; Malbrain, M.; Wilmer, A. Relationship between Abdominal Pressure, Pulmonary Compliance, and Cardiac Preload in a Porcine Model. Crit. Care Res. Pract. 2012, 2012, 763181. [Google Scholar] [CrossRef] [PubMed]
- Aranake, A.; Mashour, G.A.; Avidan, M.S. Minimum Alveolar Concentration: Ongoing Relevance and Clinical Utility. Anaesthesia 2013, 68, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Boscan, P.; Monnet, E.; Mama, K.; Twedt, D.C.; Congdon, J.; Eickhoff, J.C.; Steffey, E.P. A Dog Model to Study Ovary, Ovarian Ligament and Visceral Pain. Vet. Anaesth. Analg. 2011, 38, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Case, J.B.; Marvel, S.J.; Boscan, P.; Monnet, E.L. Surgical Time and Severity of Postoperative Pain with One, Two, or Three Instrument Cannulas. JAVMA J. Am. Vet. Med. Assoc. 2011, 239, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Katic, N.; Aurich, J.E.; Dupré, G. Postoperative Complications and Owner Assessment of Single Portal Laparoscopic Ovariectomy in Dogs. Vet. Rec. 2018, 183, 745. [Google Scholar] [CrossRef] [PubMed]
- Granados, J.R.; Usón-Casaus, J.; Martínez, J.M.; Sánchez-Margallo, F.; Pérez-Merino, E. Canine Laparoscopic Ovariectomy Using Two 3- And 5-Mm Portal Sites: A Prospective Randomized Clinical Trial. Can. Vet. J. 2017, 58, 565–570. [Google Scholar]
- Smith, J.D.; Allen, S.W.; Quandt, J.E.; Tackett, R.L. Indicators of Postoperative Pain in Cats and Correlation with Clinical Criteria. Am. J. Vet. Res. 1996, 57, 1674–1678. [Google Scholar]
- Moldal, E.R.; Kjelgaard-Hansen, M.J.; Peeters, M.E.; Nødtvedt, A.; Kirpensteijn, J. C-Reactive Protein, Glucose and Iron Concentrations Are Significantly Altered in Dogs Undergoing Open Ovariohysterectomy or Ovariectomy. Acta Vet. Scand. 2018, 60, 32. [Google Scholar] [CrossRef]
- Rubio, M.; Satué, K.; Carrillo, J.M.; Guerra, Á.H.; Cuervo, B.; Chicharro, D.; Damiá, E.; Romero, A.D.; Sopena, J. Changes in Hematological and Biochemical Profiles in Ovariohysterectomized Bitches Using an Alfaxalone–Midazolam–Morphine–Sevoflurane Protocol. Animals 2022, 12, 914. [Google Scholar] [CrossRef]
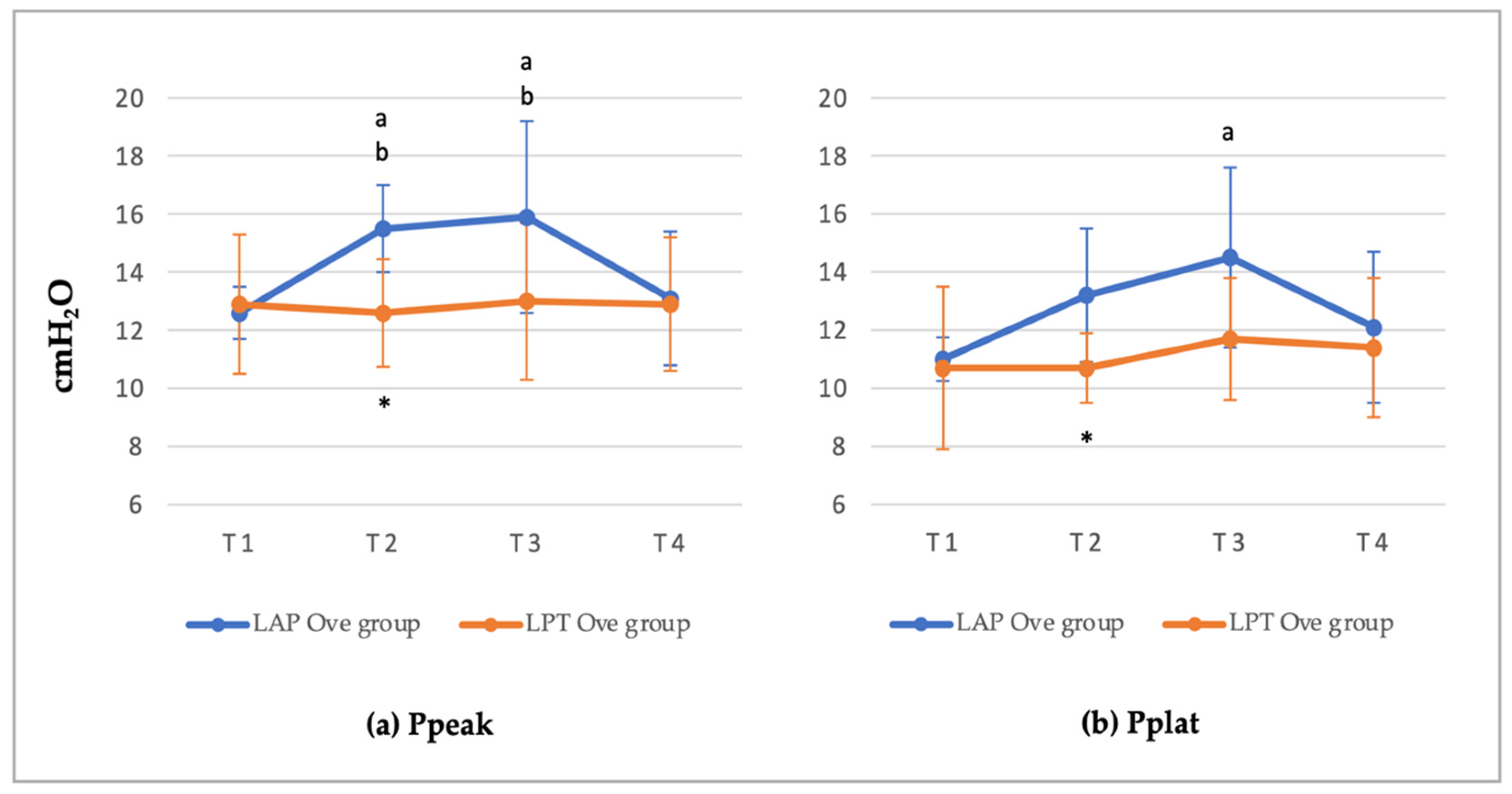
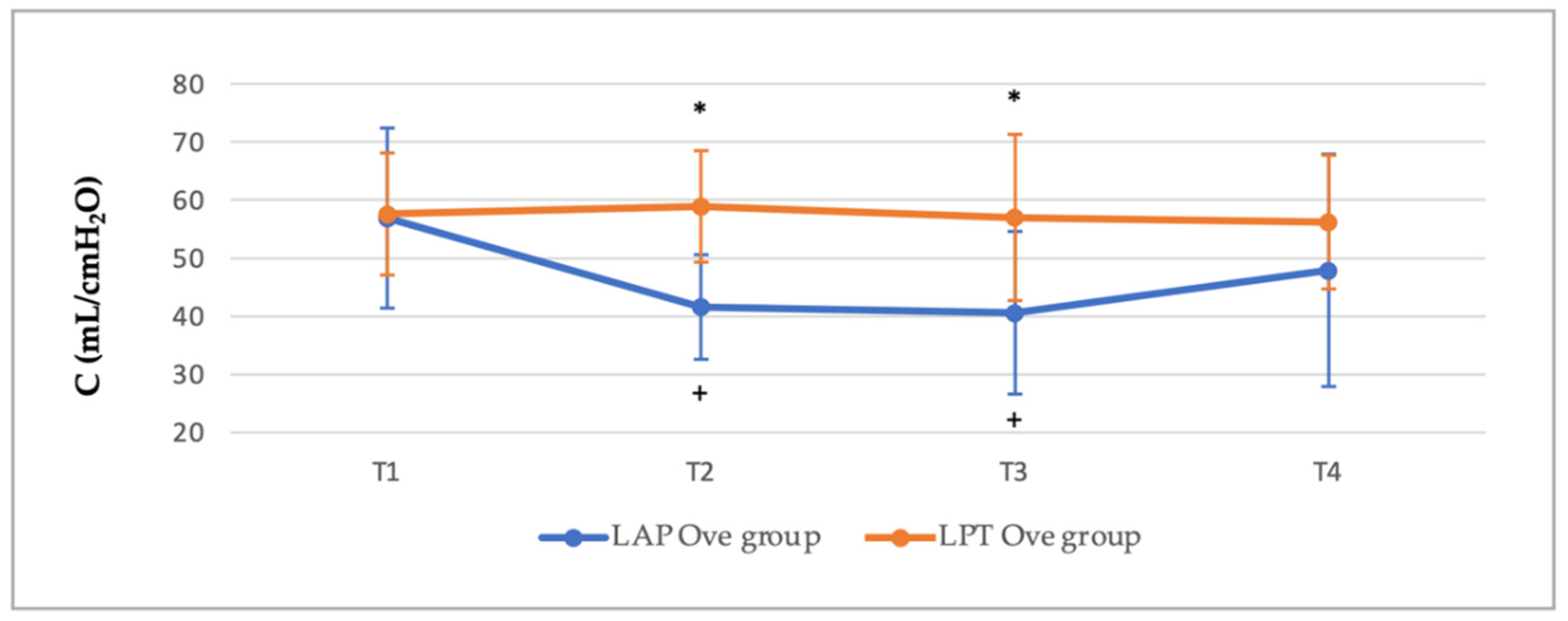
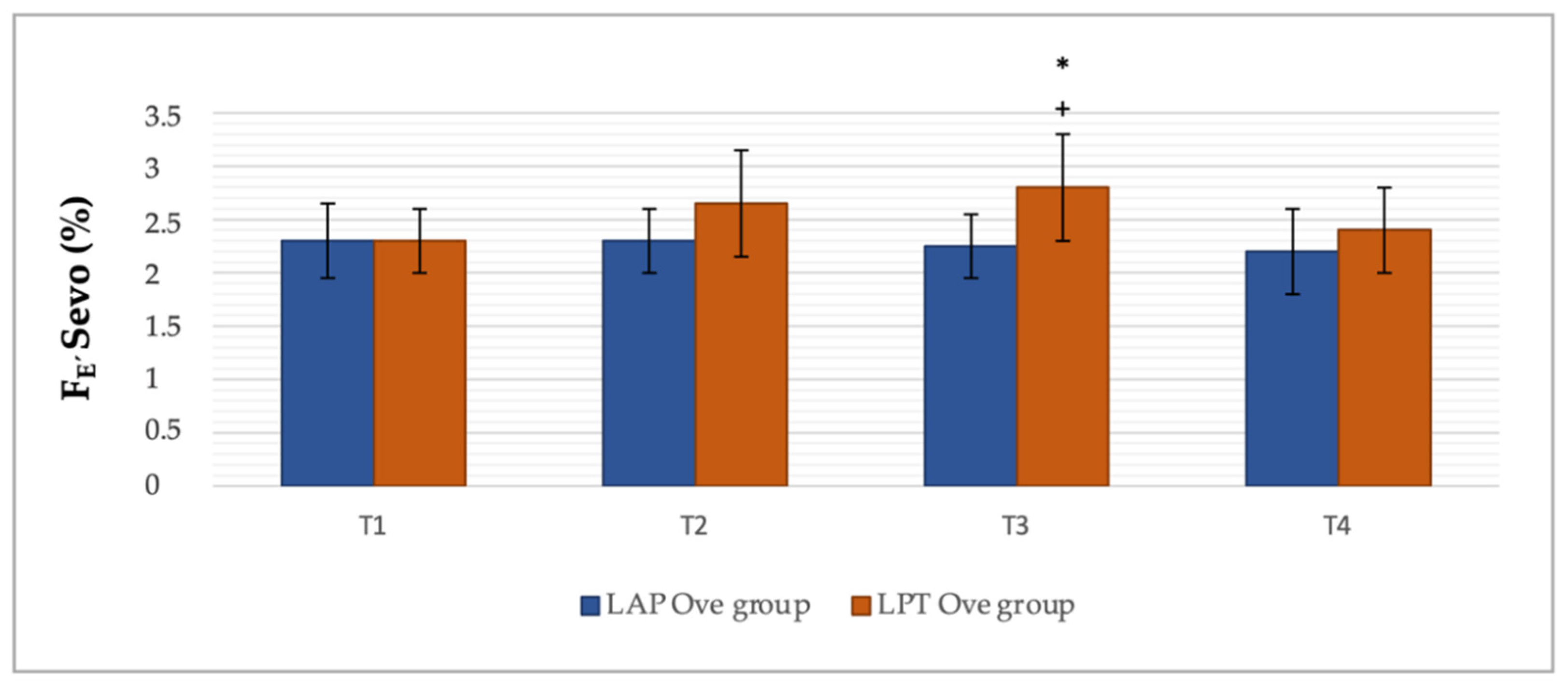
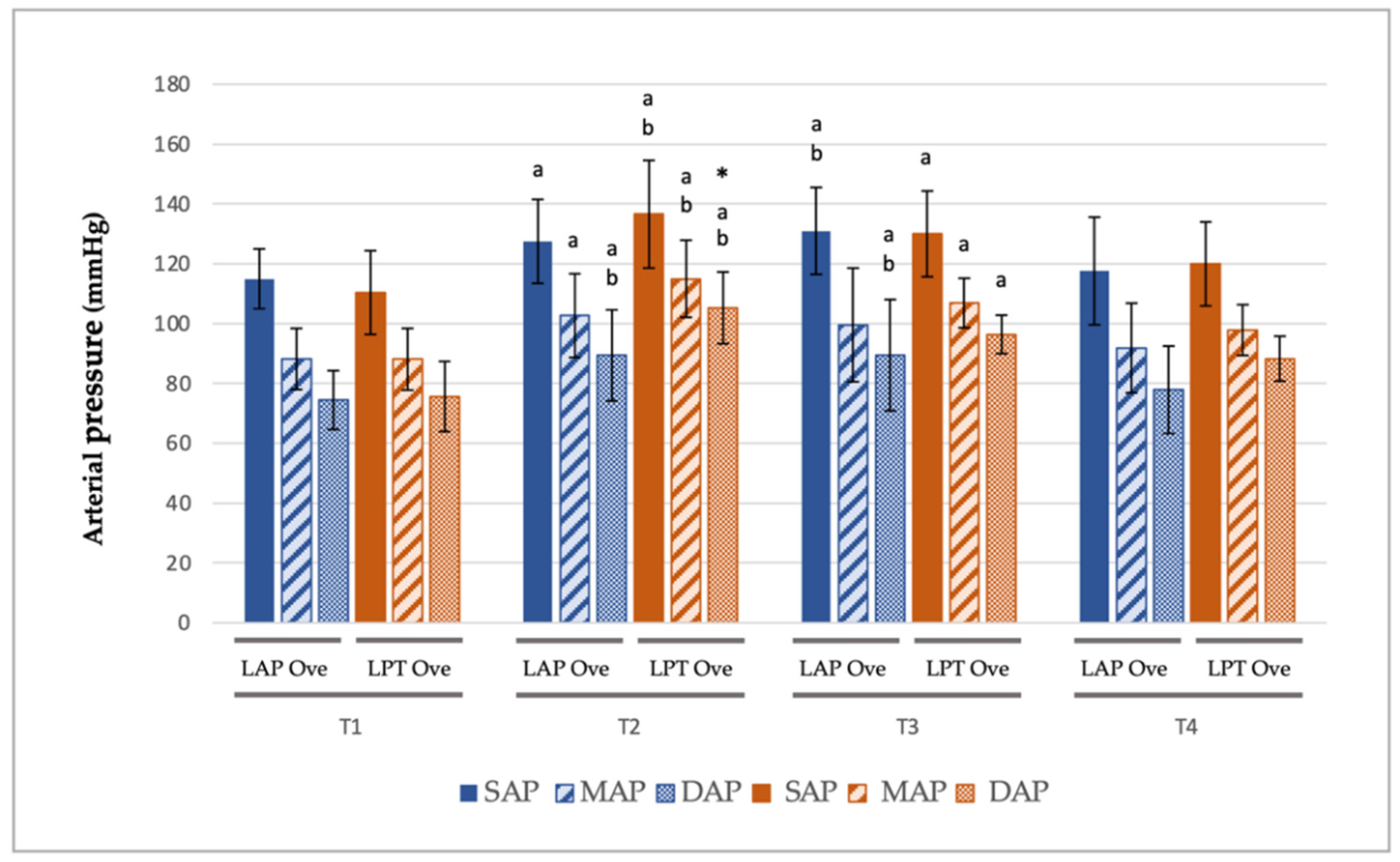
| LAP Ove Group | LPT Ove Group | |||
|---|---|---|---|---|
| Variables | PRE | POST | PRE | POST |
| pH | 7.38 ± 0.05 | 7.39 ± 0.05 | 7.40 ± 0.04 | 7.41 ± 0.04 |
| PaCO2 (mmHg) | 41.25 ± 3 | 40.12 ± 4.3 | 41.9 ± 2.35 | 39.12 ± 4 |
| HCO3 (mEq/L) | 24.28 ± 1.7 | 23.1 ± 1.29 | 25.4 ± 1.34 | 24.75 ± 1.24 |
| BE (mmol/L) | −0.38 ± 1.8 | −1.6 ± 1.4 | 1.07 ± 1.44 | 0.46 ± 1.3 |
| PaO2 (mmHg) | 418 ± 58 | 434 ± 32 | 434 ± 40 | 429 ± 45 |
| Na++ (mmol/L) | 154.25 ± 2.8 | 151.6 ± 3.1 + | 150.75 ± 3.3 * | 150.5 ± 1.8 |
| K+ (mmol/L) | 3.62 ± 0.4 | 3.99 ± 0.6 | 3.71 ± 0.2 | 4 ± 0.16 + |
| Cl− (mmol/L) | 119.25 ± 1.99 | 119 ± 1.5 | 117.62 ± 1.5 | 118 ± 1 |
| LAP Ove Group | LPT Ove Group | |||
|---|---|---|---|---|
| Variables | PRE | POST | PRE | POST |
| GLU (mg/dL) | 102.9 ± 18.5 | 151.2 ± 34.8 + | 107.87 ± 17.2 | 153.8 ± 29.3 + |
| CREA (mg/dL) | 0.85 ± 0.14 | 0.85 ± 0.14 | 0.7 ± 0.15 | 0.71 ± 0.13 |
| TPP (g/dL) | 6.7 ± 0.6 | 6.1 ± 0.8 + | 6.5 ± 0.4 | 6 ± 0.5 + |
| ALB (g/dL) | 3.2 ± 0.16 | 2.8 ± 0.2 + | 2.9 ± 0.2 | 2.7 ± 0.2 + |
| GLOB (g/dL) | 3.57 ± 0.6 | 3.26 ± 0.6 + | 3.59 ± 0.35 | 3.3 ± 0.3 + |
| ALT (UI/L) | 39.37 ± 18.4 | 37.37 ± 22.2 | 34.6 ± 12.4 | 30.87 ± 13.5 |
| ALP (UI/L) | 49.87 ± 21.16 | 44.75 ± 20.1 | 45 ± 23.5 | 37 ± 19.6 |
| PCV (%) | 43.3 ± 7.3 | 39.7 ± 6.6 + | 43.3 ± 3.9 | 38.1 ± 3.8 + |
| HB (g/dL) | 15.82 ± 2.6 | 14.56 ± 2.5 + | 15.9 ± 1.16 | 14 ± 1.34 + |
| WBC (K/μL) | 11.34 ± 3.2 | 7.35 ± 1.8 + | 13.9 ± 2.38 | 10.45 ± 2.4 *, + |
| PLT (K/μL) | 327.5 ± 127 | 293.4 ± 98.8 + | 324.5 ± 29.7 | 283.5 ± 33.2 + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Martín, S.; Valiño-Cultelli, V.; González-Cantalapiedra, A. Laparoscopic versus Open Ovariectomy in Bitches: Changes in Cardiorespiratory Values, Blood Parameters, and Sevoflurane Requirements Associated with the Surgical Technique. Animals 2022, 12, 1438. https://doi.org/10.3390/ani12111438
Fernández-Martín S, Valiño-Cultelli V, González-Cantalapiedra A. Laparoscopic versus Open Ovariectomy in Bitches: Changes in Cardiorespiratory Values, Blood Parameters, and Sevoflurane Requirements Associated with the Surgical Technique. Animals. 2022; 12(11):1438. https://doi.org/10.3390/ani12111438
Chicago/Turabian StyleFernández-Martín, Silvia, Victoria Valiño-Cultelli, and Antonio González-Cantalapiedra. 2022. "Laparoscopic versus Open Ovariectomy in Bitches: Changes in Cardiorespiratory Values, Blood Parameters, and Sevoflurane Requirements Associated with the Surgical Technique" Animals 12, no. 11: 1438. https://doi.org/10.3390/ani12111438
APA StyleFernández-Martín, S., Valiño-Cultelli, V., & González-Cantalapiedra, A. (2022). Laparoscopic versus Open Ovariectomy in Bitches: Changes in Cardiorespiratory Values, Blood Parameters, and Sevoflurane Requirements Associated with the Surgical Technique. Animals, 12(11), 1438. https://doi.org/10.3390/ani12111438






