Direct Rub Inoculation of Fungal Flora Changes Fatty Acid Composition and Volatile Flavors in Dry-Aged Beef: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Dry-Aged Beef
2.2. Observation of the Appearance of Dry-Aged Beef during the Aging Process
2.3. Proximate Composition
2.4. Water-Holding Capacity and Shear Force Measurements
2.5. Fatty Acid Analysis
2.6. Volatile Aromatic Compound Analysis
2.7. Statistical Analysis
3. Results and Discussion
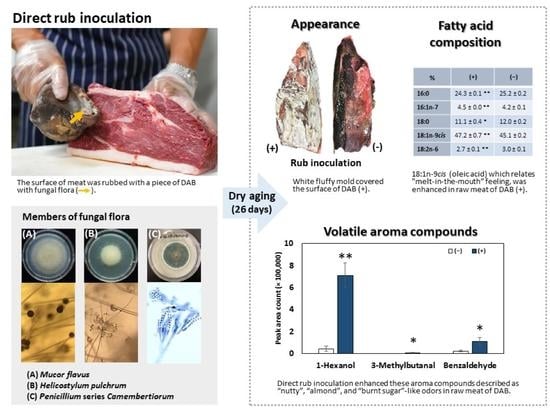
3.1. Appearance and Yield of DAB
3.2. Proximate Composition
3.3. Water-Holding Capacity and Shear Force
3.4. Fatty Acid Composition
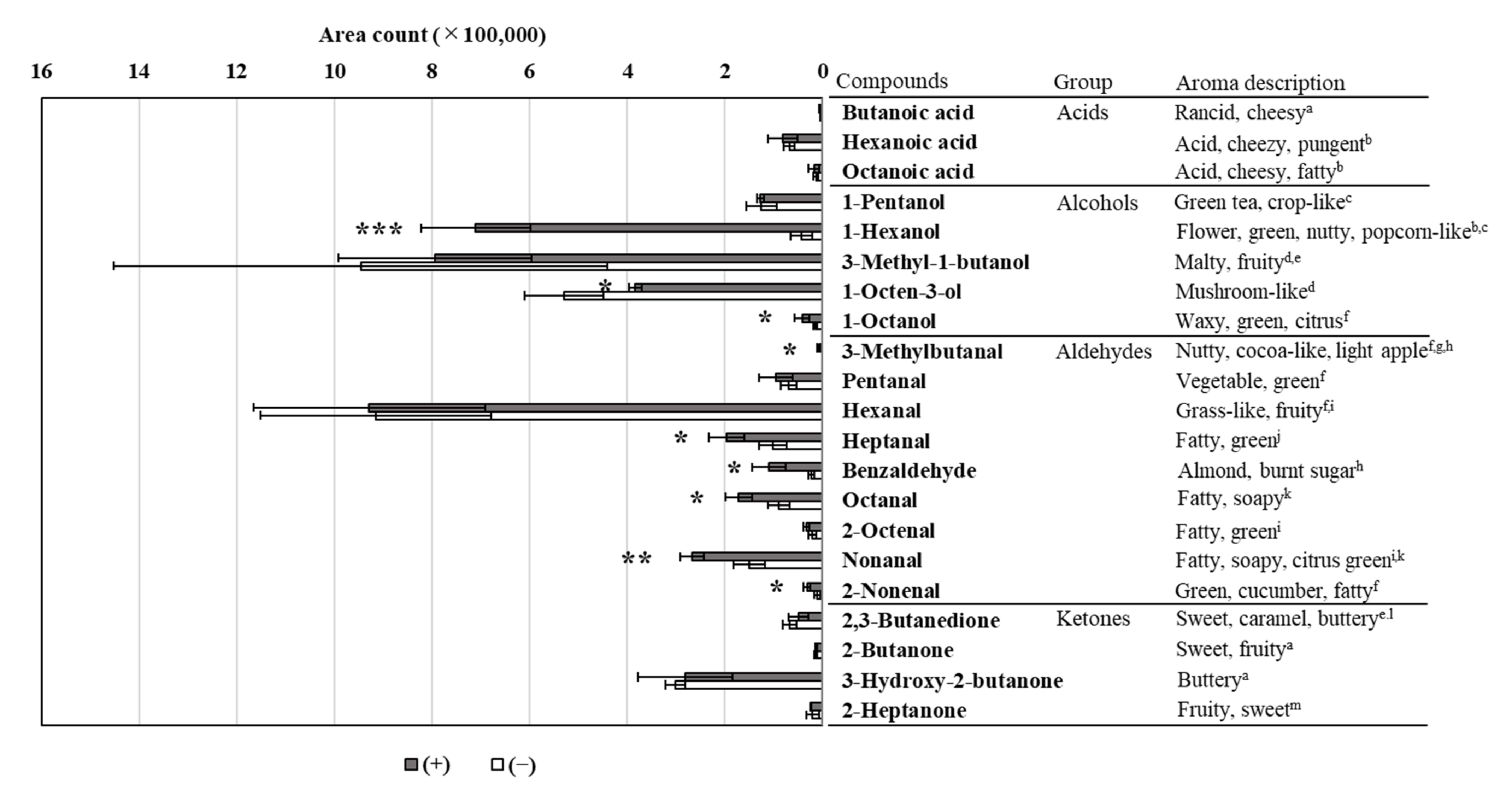
3.5. Volatile Aromatic Compound Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Further Reading
References
- Ryu, S.; Park, M.R.; Maburutse, B.E.; Lee, W.J.; Park, D.-J.; Cho, S.; Hwang, I.; Oh, S.; Kim, Y. Diversity and characteristics of the meat microbiological community on dry aged beef. J. Microbiol. Biotechnol. 2018, 28, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Yoon, J.W.; Kim, M.; Oh, H.; Yoon, Y.; Jo, C. Changes in microbial composition on the crust by different air flow velocities and their effect on sensory properties of dry-aged beef. Meat Sci. 2019, 153, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Koizumi, R.; Nakazawa, Y.; Shiwa, Y.; Maeno, S.; Kido, Y.; Irisawa, T.; Muramatsu, Y.; Tada, K.; Yamazaki, M.; et al. Characterization of the microbiota and chemical properties of pork loins during dry aging. Microbiologyopen 2021, 10, e1157. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Shin, M.; Cho, S.; Hwang, I.; Kim, Y.; Oh, S. Molecular Characterization of microbial and fungal communities on dryaged beef of Hanwoo using metagenomic analysis. Foods 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Hanagasaki, T.; Asato, N. Changes in free amino acid content and hardness of beef while dry-aging with Mucor flavus: Changes in the quality of beef while dry-aging with Mucor flavus. J. Anim. Sci. Technol. 2018, 60, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, H.; Lee, H.J.; Lee, J.; Jo, C.; Yoon, Y. Identification of microorganisms associated with the quality improvement of dry-aged beef through microbiome analysis and DNA sequencing, and evaluation of their effects on beef quality. J. Food Sci. 2019, 84, 2944–2954. [Google Scholar] [CrossRef]
- Dashdorj, D.; Tripathi, V.K.; Cho, S.; Kim, Y.; Hwang, I. Erratum to: Dry aging of beef; Review. J. Anim. Sci. Technol. 2016, 58, 28. [Google Scholar] [CrossRef] [Green Version]
- Mikami, N.; Toyotome, T.; Yamashiro, Y.; Sugo, K.; Yoshitomi, K.; Takaya, M.; Han, K.-H.; Fukushima, M.; Shimada, K. Dry-aged beef manufactured in Japan: Microbiota identification and their effects on product characteristics. Food Res. Int. 2021, 140, 110020. [Google Scholar] [CrossRef]
- Hamm, R. Functional properties of the myofibrillar system and their measurements. In Muscle as Food, 1st ed.; Bechtel, P.J., Ed.; Academic Press: London, UK, 1986; pp. 135–199. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Prevot, A.F.; Mordret, F.X. Use of glass capillary columns for the analysis of fatty substances by gas chromatography. Rev. Fr. Corps Gras. 1976, 23, 409–423. (In French) [Google Scholar]
- Van Long, N.N.; Rigalma, K.; Coroller, L.; Dadure, R.; Debaets, S.; Mounier, J.; Vasseur, V. Modelling the effect of water activity reduction by sodium chloride or glycerol on conidial germination and radial growth of filamentous fungi encountered in dairy foods. Food Microbiol. 2017, 68, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Maniaci, G.; Alabiso, M.; Francesca, N.; Giosue, C.; Di Grigoli, A.; Corona, O.; Cardamone, C.; Graci, G.; Portolano, B.; Bonanno, A. Bresaola made from Cinisara cattle: Effect of muscle type and animal category on physicochemical and sensory traits. CyTA J. Food 2020, 18, 383–391. [Google Scholar] [CrossRef]
- Irie, M.; Izumo, A.; Mohri, S. Rapid method for determining water-holding capacity in meat using video image analysis and simple formulae. Meat Sci. 1996, 42, 95–102. [Google Scholar] [CrossRef]
- Silva, D.R.G.; de Moura, A.P.R.; Ramos, A.L.S.; Ramos, E.M. Comparison of Warner-Bratzler shear force values between round and square cross-section cores for assessment of beef Longissimus tenderness. Meat Sci. 2017, 125, 102–105. [Google Scholar] [CrossRef]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between beef consumer tenderness perception and Warner-Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Silva, J.A.; Patarata, L.; Martins, C. Influence of ultimate pH on bovine meat tenderness during ageing. Meat Sci. 1999, 52, 453–459. [Google Scholar] [CrossRef]
- Nakahashi, Y.; Maruyama, S.; Seki, S.; Hidaka, S.; Kuchida, K. Relationships between monounsaturated fatty acids of marbling flecks and image analysis traits in longissimus muscle for Japanese Black steers. J. Anim. Sci. 2008, 86, 3551–3556. [Google Scholar] [CrossRef] [Green Version]
- Westerling, D.B.; Hedrick, H.B. Fatty acid composition of bovine lipids as influenced by diet, sex and anatomical location and relationship to sensory characteristics. J. Anim. Sci. 1979, 48, 1343–1348. [Google Scholar] [CrossRef]
- Alabiso, M.; Maniaci, G.; Giosuè, C.; Gaglioa, G.; Francesca, N.; Di Grigoli, A.; Portolano, B.; Bonanno, A. Effect of muscle type and animal category on fatty acid composition of bresaola made from meat of Cinisara cattle: Preliminary investigation. CyTA J. Food 2020, 18, 734–741. [Google Scholar] [CrossRef]
- Maw, S.J.; Fowler, V.R.; Hamilton, M.; Petchey, A.M. Physical characteristics of pig fat and their relation to fatty acid composition. Meat Sci. 2003, 63, 185–190. [Google Scholar] [CrossRef]
- Stukey, J.E.; McDonough, V.M.; Martin, C.E. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J. Biol. Chem. 1989, 264, 16537–16544. [Google Scholar] [CrossRef]
- Shanklin, J.; Somerville, C. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc. Natl Acad. Sci. USA 1991, 88, 2510–2514. [Google Scholar] [CrossRef] [Green Version]
- Mu, S.; Liu, L.; Liu, H.; Shen, Q.; Luo, J. Characterization of the relationship between olfactory perception and the release of aroma compounds before and after simulated oral processing. J. Dairy Sci. 2021, 104, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Iiduka, T.; Tsukamoto, K.; Sugiura, S.; Tanaka, G.; Sakamaki, G.; Yamamoto, Y.; Takeshige, Y.; Miyazawa, T.; Kojima, A.; et al. Retort beef aroma that gives preferable properties to canned beef products and its aroma components. Anim. Sci. J. 2017, 88, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, C.; Zhao, L.; Zhang, M.; Wang, F.; Sun, E.; Ren, F. Keto acid decarboxylase and keto acid dehydrogenase activity detected during the biosynthesis of flavor compound 3-methylbutanal by the nondairy adjunct culture Lactococcus lactis ssp. lactis F9. J. Dairy Sci. 2018, 101, 9725–9735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef]
- Feng, T.; Shui, M.; Song, S.; Zhuang, H.; Sun, M.; Yao, L. Characterization of the key aroma compounds in three truffle varieties from China by flavoromics approach. Molecules 2019, 24, 3305. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Xu, X.; Luo, D.; Lao, F.; Pang, X.; Shen, Q.; Hu, X.; Wu, J. Characterization of key aroma compounds in raw and roasted peas (Pisum sativum L.) by application of instrumental and sensory techniques. J. Agric. Food Chem. 2020, 68, 2718–2727. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of key aroma compounds in Beijing roasted duck by gas chromatography–olfactometry–mass spectrometry, odor-activity values, and aroma-recombination experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by polypropylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.T.; Kim, Y.J.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, S.K. Comparison of meat quality, fatty acid composition and aroma volatiles of dry-aged beef from Hanwoo cows slaughtered at 60 or 80 months old. Asian-Australas. J. Anim. Sci. 2020, 33, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.M.; Hierro, E.M.; de la Hoz, L.; Mottram, D.S.; Fernández, M.; Ordóñez, J.A. Changes in selected biochemical and sensory parameters as affected by the superficial inoculation of Penicillium camemberti on dry fermented sausages. Int. J. Food Microbiol. 2003, 85, 111–125. [Google Scholar] [CrossRef]
- Flores, M.; Corral, S.; Cano-García, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, R.; Sun, F.; Li, X.-A.; Chen, Q.; Kong, B. The potential correlations between the fungal communities and volatile compounds of traditional dry sausages from Northeast China. Food Microbiol. 2021, 98, 103787. [Google Scholar] [CrossRef]
- Liang, J.; Xie, J.; Hou, L.; Zhao, M.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.-G. Aroma constituents in Shanxi aged vinegar before and after aging. J. Agric. Food Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef]
- Frauendorfer, F.; Schieberle, P. Changes in key aroma compounds of Criollo cocoa beans during roasting. J. Agric. Food Chem. 2008, 56, 10244–10251. [Google Scholar] [CrossRef]
- Majcher, M.A.; Olszak-Ossowska, D.; Szudera-Kończal, K.; Jeleń, H.H. Formation of key aroma compounds during preparation of pumpernickel bread. J. Agric. Food Chem. 2020, 68, 10352–10360. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yu, H.; Zhu, J. Characterization of odor-active compounds of various cherry wines by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2287–2293. [Google Scholar] [CrossRef]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.-S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef]

| Characteristics | + | − |
|---|---|---|
| Moisture (%) | 58.9 ± 1.5 ** | 63.9 ± 0.5 |
| Crude protein (%) | 20.8 ± 0.2 | 21.5 ± 0.7 |
| Crude fat (%) | 20.1 ± 1.0 ** | 13.4 ± 0.1 |
| Ash (%) | 1.03 ± 0.02 ** | 1.14 ± 0.04 |
| Expressible drip loss (%) | 31.2 ± 2.3 | 27.3 ± 0.9 |
| Cooking loss (%) | 26.0 ± 0.3 ** | 16.6 ± 2.2 |
| WBSF (kg/cm2) | 2.64 ± 0.32 * | 1.68 ± 0.17 |
| FAME (%) | With (+) | Without (−) |
|---|---|---|
| 8:0 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 12:0 | 0.04 ± 0.00 * | 0.04 ± 0.00 |
| 13:0 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 14:0 | 2.44 ± 0.01 * | 2.63 ± 0.08 |
| 14:1n-5 | 0.70 ± 0.02 * | 0.64 ± 0.02 |
| 15:0 | 0.44 ± 0.01 * | 0.47 ± 0.01 |
| 15:1n-5 | 0.11 ± 0.00 | 0.12 ± 0.00 |
| 16:0 | 24.25 ± 0.09 ** | 25.20 ± 0.24 |
| 16:1n-7 | 4.51 ± 0.04 ** | 4.21 ± 0.08 |
| 17:0 | 1.32 ± 0.02 ** | 1.40 ± 0.01 |
| 17:1n-7 | 1.25 ± 0.02 ** | 1.16 ± 0.02 |
| 18:0 | 11.09 ± 0.40 * | 11.97± 0.15 |
| 18:1n-9(cis) | 47.21 ± 0.66 ** | 45.14 ± 0.24 |
| 18:2n-9 | 0.44 ± 0.00 ** | 0.47 ± 0.00 |
| 18:2n-6 | 2.65 ± 0.09 ** | 2.99 ± 0.06 |
| 18:3n-3 | 0.11 ± 0.00 | 0.11± 0.00 |
| 20:0 | 0.35 ± 0.00 | 0.34 ± 0.00 |
| 20:4n-6 | 0.29 ± 0.03 ** | 0.40 ± 0.03 |
| SFA | 39.96 ± 0.49 ** | 42.09 ± 0.34 |
| MUFA | 53.79 ± 0.64 ** | 51.27 ± 0.16 |
| PUFA | 3.49 ± 0.12 ** | 3.98 ± 0.09 |
| 16:1/16:0 ratio | 0.19 ± 0.00 *** | 0.17 ± 0.00 |
| 18:1/18:0 ratio | 4.26 ± 0.21 * | 3.77 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikami, N.; Toyotome, T.; Takaya, M.; Tamura, K. Direct Rub Inoculation of Fungal Flora Changes Fatty Acid Composition and Volatile Flavors in Dry-Aged Beef: A Preliminary Study. Animals 2022, 12, 1391. https://doi.org/10.3390/ani12111391
Mikami N, Toyotome T, Takaya M, Tamura K. Direct Rub Inoculation of Fungal Flora Changes Fatty Acid Composition and Volatile Flavors in Dry-Aged Beef: A Preliminary Study. Animals. 2022; 12(11):1391. https://doi.org/10.3390/ani12111391
Chicago/Turabian StyleMikami, Nana, Takahito Toyotome, Masahiro Takaya, and Kenichi Tamura. 2022. "Direct Rub Inoculation of Fungal Flora Changes Fatty Acid Composition and Volatile Flavors in Dry-Aged Beef: A Preliminary Study" Animals 12, no. 11: 1391. https://doi.org/10.3390/ani12111391
APA StyleMikami, N., Toyotome, T., Takaya, M., & Tamura, K. (2022). Direct Rub Inoculation of Fungal Flora Changes Fatty Acid Composition and Volatile Flavors in Dry-Aged Beef: A Preliminary Study. Animals, 12(11), 1391. https://doi.org/10.3390/ani12111391