Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares
Abstract
Simple Summary
Abstract
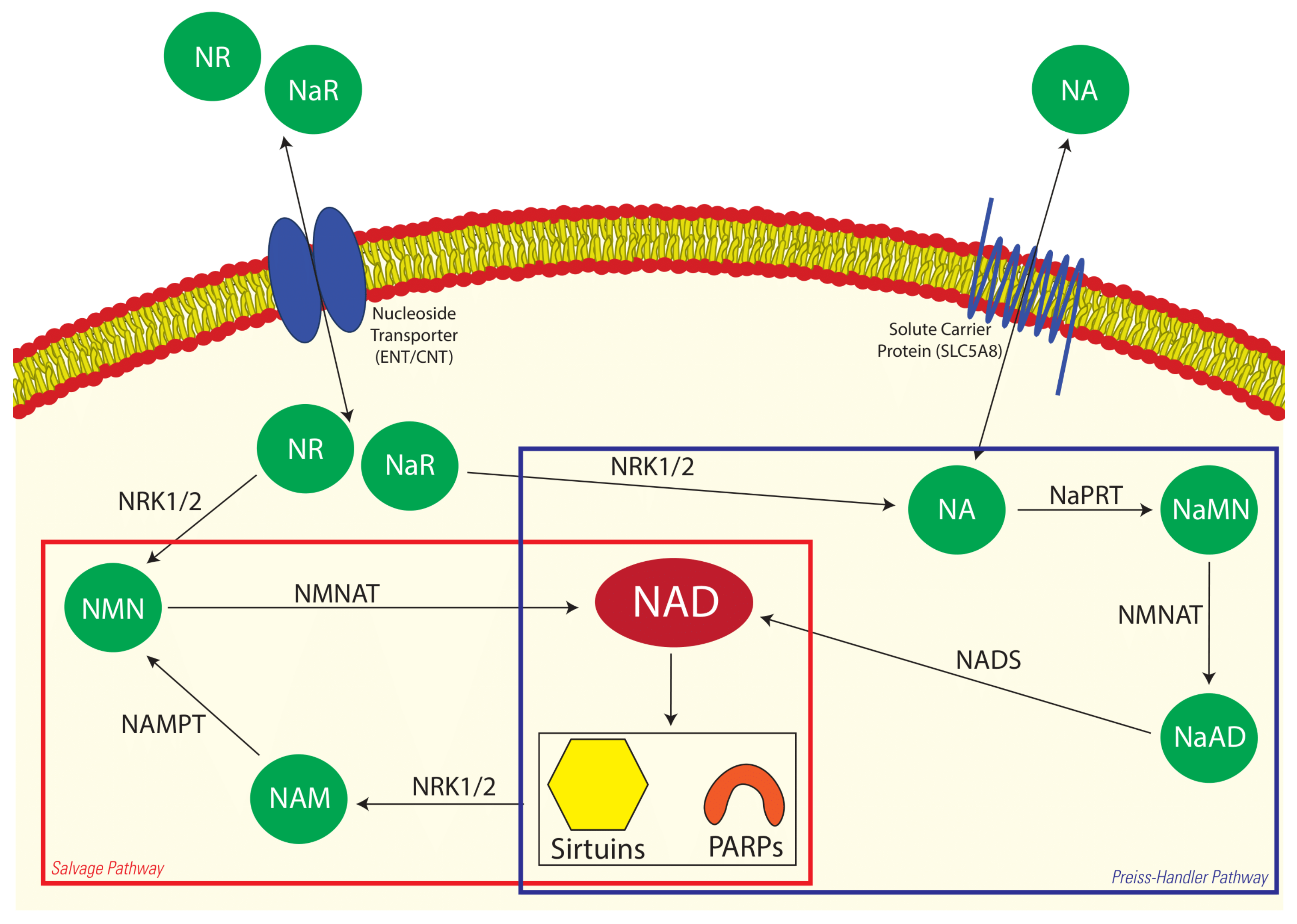
1. Introduction
2. Materials and Methods
2.1. Animals
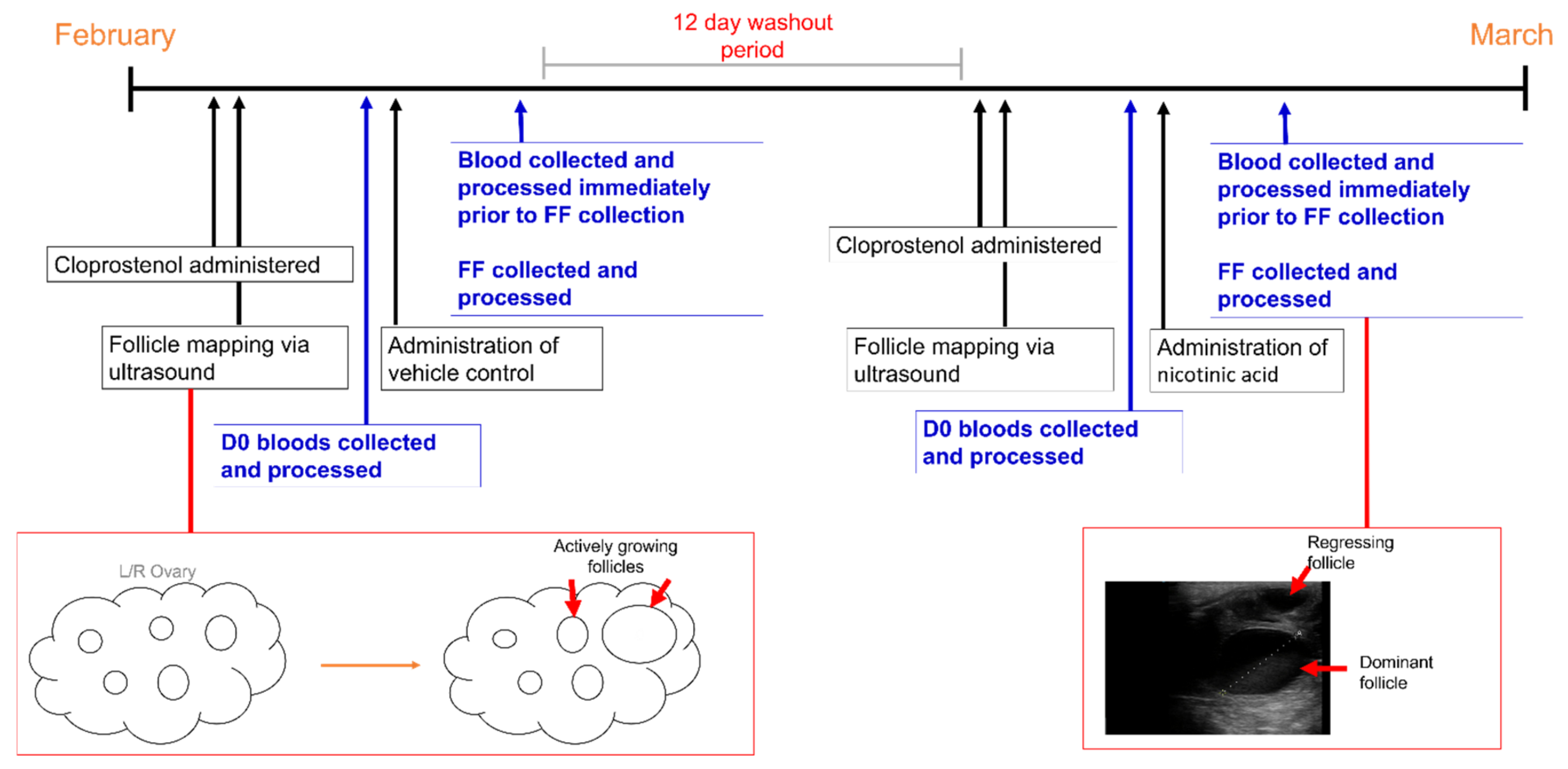
2.2. Study Design
2.3. Supplemental Feeding and Blood Collections
2.4. Follicular Fluid Aspiration and Mare Husbandry
2.5. Chemicals and Reagents
2.6. Solid-Phase Extraction
2.7. Mass Spectrometry Instrumentation and Conditions
2.8. Statistical Analysis
3. Results
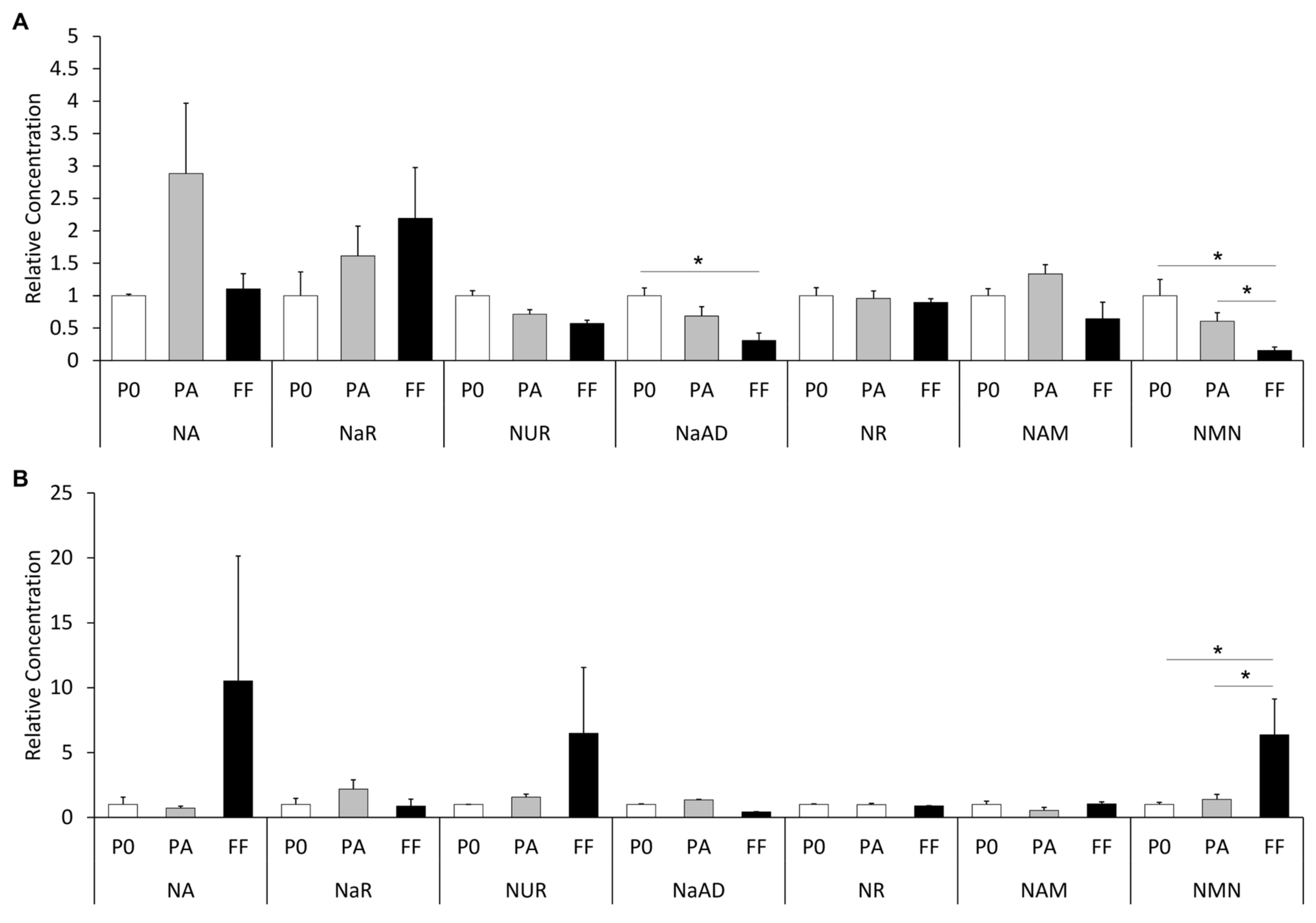
3.1. Metabolite Concentrations in Plasma
3.2. Metabolite Concentrations in the Follicular Fluid of Dominant Follicles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nath, L.C.; Anderson, G.A.; McKinnon, A.O. Reproductive efficiency of Thoroughbred and Standardbred horses in north-east Victoria. Aust. Vet. J. 2010, 88, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.A.; Bijnen, M.L.J.; Osborne, M.; More, S.J.; Henderson, I.S.F.; Duffy, P.; Crowe, M.A. Key factors affecting reproductive success of Thoroughbred mares and stallions on a commercial stud farm. Reprod. Domest. Anim. 2016, 51, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.H.; Allen, W.R. Reproductive efficiency of intensively managed Thoroughbred mares in Newmarket. Equine Vet. J. 2002, 34, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bosh, K.A.; Powell, D.; Shelton, B.; Zent, W. Reproductive performance measures among Thoroughbred mares in central Kentucky, during the 2004 mating season. Equine Vet. J. 2009, 41, 883–888. [Google Scholar] [CrossRef]
- Stout, T.A.E. How to manage early embryonic death. How to manage the subfertile mare. AAEP Proc. 2012, 58, 331–333. [Google Scholar]
- Vanderwall, D.K. Early embryonic loss in the mare. J. Equine Vet. Sci. 2008, 28, 691–702. [Google Scholar] [CrossRef]
- Van Niekerk, F.E.; van Niekerk, C.H. The effect of dietary protein on reproduction in the mare. VII. Embryonic development, early embryonic death, foetal losses and their relationship with serum progestagen. J. S. Afr. Vet. Assoc. 1998, 69, 150–155. [Google Scholar] [CrossRef][Green Version]
- Johnson, S.; Imai, S.I. NAD+ biosynthesis, aging, and disease. F1000Research 2018, 7, 132. [Google Scholar] [CrossRef]
- Li, J.; Bonkowski, M.S.; Moniot, S.; Zhang, D.; Hubbard, B.P.; Ling, A.J.; Rajman, L.A.; Qin, B.; Lou, Z.; Gorbunova, V.; et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science 2017, 355, 1312–1317. [Google Scholar] [CrossRef]
- Ghosh, S.; George, S.; Roy, U.; Ramachandran, D.; Kolthur-Seetharam, U. NAD: A master regulator of transcription. Biochim. Biophys. Acta 2010, 1799, 681–693. [Google Scholar] [CrossRef]
- Bender, D.A.; Olufunwa, R. Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br. J. Nutr. 1988, 59, 279–287. [Google Scholar] [CrossRef]
- Shi, H.; Enriquez, A.; Rapadas, M.; Martin, E.; Wang, R.; Moreau, J.; Lim, C.K.; Szot, J.O.; Ip, E.; Hughes, J.N.; et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N. Engl. J. Med. 2017, 377, 544–552. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef]
- INRA. L’alimentation des Chevaux; Martin-Rosset, W., Ed.; INRA Publications: Versailles, France, 1990; Volume 232. [Google Scholar]
- Frape, D.L. Equine Nutrition and Feeding, 3rd ed.; Blackwell Publishing Limited: Oxford, UK, 2004. [Google Scholar]
- National Research Council. Nutrient Requirements of Horses, 6th ed.; The National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Parker, A.L.; Lawrence, L.M.; Rokuroda, S.; Warren, L.K. The effects of niacin supplementation on niacin status and exercise metabolism in horses. In Proceedings of the 15th Symposium of the Equine Nutrition and Physiology Society, Fort Worth, TX, USA, 4–7 June 1997; p. 19. [Google Scholar]
- Pearson, P.B.; Sheybani, M.K.; Schmidt, H. The B vitamin requirements of the horse. J. Anim. Sci. 1944, 3, 166–174. [Google Scholar] [CrossRef]
- Bustamante, S.; Jayasena, T.; Richani, D.; Gilchrist, R.B.; Wu, L.E.; Sinclair, D.A.; Sachdev, P.S.; Braidy, N. Quantifying the cellular NAD+ metabolome using a tandem liquid chromatography mass spectrometry approach. Metabolomics 2017, 14, 15. [Google Scholar] [CrossRef]
- Rechsteiner, M.; Hillyard, D.; Olivera, B.M. Turnover of nicotinamide adenine dinucleotide in cultures of human cells. J. Cell Physiol. 1976, 88, 207–217. [Google Scholar] [CrossRef]
- Pfuhl, P.; Karcher, U.; Haring, N.; Baumeister, A.; Tawab, M.A.; Schubert-Zsilavecz, M. Simultaneous determination of niacin, niacinamide and nicotinuric acid in human plasma. J. Pharm. Biomed. Anal. 2005, 36, 1045–1052. [Google Scholar] [CrossRef]
- Evered, D.F.; Sadoogh-Abasian, F.; Patel, P.D. Absorption of nicotinic acid and nicotinamide across human buccal mucosa in vivo. Life Sci. 1980, 27, 1649–1651. [Google Scholar] [CrossRef]
- Carroll, F.D.; Goss, H.; Howell, C.E. The synthesis of B vitamins in the horse. J. Anim. Sci. 1949, 8, 290–299. [Google Scholar] [CrossRef]
- Pollard, C.L.; Gibb, Z.; Swegen, A.; Lawson, E.F.; Grupen, C.G. Nicotinic acid supplementation at a supraphysiological dose increases the bioavailability of NAD+ precursors in mares. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, G.A.; Sarett, H.P.; Register, U.D.; Gibbens, J. Studies on niacin requirement in man. 1. Experimental pellagra in subjects on corn diets low in niacin and tryptophan. J. Clin. Investig. 1952, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, G.A.; Rosenthal, H.L.; Gibbens, J.; Unglaub, W.G. Studies on niacin requirement in man. 2. Requirement on wheat and corn diets low in tryptophan. J. Nutr. 1955, 56, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Horwitt, M.K.; Harvey, C.C.; Rothwell, W.S.; Cutler, J.L.; Haffron, D. Tryptophanniacin relationships in man: Studies with diets deficient in riboflavin and niacin, together with observations on the excretion of nitrogen and niacin metabolites. J. Nutr. 1956, 60, 1–43. [Google Scholar] [CrossRef]
- Jacob, R.A.; Swendseid, M.E.; McKee, R.W.; Fu, C.S.; Clemens, R.A. Biochemical markers for assessment of niacin status in young men: Urinary and blood levels of niacin metabolites. J. Nutr. 1989, 119, 591–598. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine/National Academy of Sciences-National Research Council. Dietary Reference Intake: Folate, Other B Vitamins, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Catandi, G.D.; Fresa, K. Equine Aging and the Oocyte: A Potential Model for Reproductive Aging in Women. J. Equine Vet. Sci. 2020, 89, 103022. [Google Scholar] [CrossRef]
- Franciosi, F.; Goudet, G.; Tessaro, I.; Papillier, P.; Dalbies-Tran, R.; Reigner, F.; Deleuze, S.; Douet, C.; Miclea, I.; Lodde, V.; et al. In vitro maturation affects chromosome segregation, spindle morphology and acetylation of lysine 16 on histone H4 in horse oocytes. Reprod. Fertil. Dev. 2017, 29, 721–730. [Google Scholar] [CrossRef]
- Rizzo, M.; Ducheyne, K.D.; Deelen, C.; Beitsma, M.; Cristarella, S.; Quartuccio, M.; Stout, T.A.E.; de Ruijter-Villani, M. Advanced mare age impairs the ability of in vitro-matured oocytes to correctly align chromosomes on the metaphase plate. Equine Vet. J. 2019, 51, 252–257. [Google Scholar] [CrossRef]
- Kafi, M.; Ashrafi, M.; Azari, M.; Jandarroodi, B.; Abouhamzeh, B.; Asl, A.R. Niacin improves maturation and cryo-tolerance of bovine in vitro matured oocytes: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 621–628. [Google Scholar] [CrossRef]
- Pollard, C.L.; Gibb, Z.; Hawdon, A.; Swegen, A.; Grupen, C.G. Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes. J. Reprod. Dev. 2021, 67, 319–326. [Google Scholar] [CrossRef]
- Pollard, C.L.; Younan, A.; Swegen, A.; Gibb, Z.; Grupen, C.G. Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes. J. Reprod. Dev. 2022. ahead of print. [Google Scholar] [CrossRef]
- Panda, S.; Panda, N.; Panigraphy, K.K.; Gupta, S.K.; Mishra, S.P.; Laishram, M. Role of niacin supplementation in dairy cattle: A review. Asian J. Dairy Food Sci. 2017, 36, 93–99. [Google Scholar] [CrossRef]
- Dolle, C.; Rack, J.G.; Ziegler, M. NAD and ADP-ribose metabolism in mitochondria. Fed. Eur. Biochem. Soc. J. 2013, 280, 3530–3541. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Ballet, N.; Robert, J.C.; Williams, P.E.V. Vitamins in forages. In Forage Evaluation in Ruminant Nutrition; Givens, D.I., Owen, E., Axford, R.F.E., Omed, H.M., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 399–431. [Google Scholar]
- Lopez-Bucio, J.; Nieto-Jacobo, M.F.; Ramirez-Rodriguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
| Metabolite | Vehicle Treatment | NA Treatment | ||||
|---|---|---|---|---|---|---|
| Plasma Prior to First Feed * | Plasma at FF Aspiration † | FF | Plasma Prior to First Feed * | Plasma at FF Aspiration † | FF | |
| NA | 19.9 ± 0.5 | 57.3 ± 21.5 | 22.0 ± 4.6 a | 99.2 ± 55.7 | 70.8 ± 15.4 | 1043.9 ± 953.5 a |
| NAM | 149.7 ± 16.1 a | 200.3 ± 20.9 | 96.5 ± 38.4 b | 1581.3 ± 416.9 a | 875.6 ± 361.1 | 1641.7 ± 256.1 b |
| NaAD | 6.6 ± 0.8 a | 4.6 ± 1.0 b | 2.0 ± 0.8 | 0.7 ± 0.3 a | 0.9 ± 0.3 b | 0.3 ± 0.2 |
| NMN | 9.5 ± 2.4 a | 5.7 ± 1.3 | 1.5 ± 0.5 b | 2.4 ± 0.4 a | 3.3 ± 0.9 | 15.0 ± 6.5 b |
| NUR | 3.6 ± 0.3 a | 2.6 ± 0.2 | 2.1 ± 0.2 | 0.0 ± 0.0 a | 0.6 ± 0.2 | 5.5 ± 5.1 |
| NaR | 2082.1 ± 761.4 | 3363.4 ± 952.9 | 4564.4 ± 1635.2 | 3843.8 ± 1823.3 | 8408.5 ± 2700.4 | 3363.3 ± 2094.3 |
| NR | 12161.5 ± 1490.8 a | 11653.5 ± 1417.0 b | 10871.6 ± 721.6 c | 2029.6 ± 97.9 a | 2073.5 ± 195.3 b | 1858.0 ± 51.9 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, C.-L.; Gibb, Z.; Clulow, J.; Ruiz, A.; Sheridan, A.; Bahrami, M.; Swegen, A.; Grupen, C.G. Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares. Animals 2022, 12, 1383. https://doi.org/10.3390/ani12111383
Pollard C-L, Gibb Z, Clulow J, Ruiz A, Sheridan A, Bahrami M, Swegen A, Grupen CG. Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares. Animals. 2022; 12(11):1383. https://doi.org/10.3390/ani12111383
Chicago/Turabian StylePollard, Charley-Lea, Zamira Gibb, Jennifer Clulow, Agustin Ruiz, Alecia Sheridan, Mohammad Bahrami, Aleona Swegen, and Christopher G. Grupen. 2022. "Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares" Animals 12, no. 11: 1383. https://doi.org/10.3390/ani12111383
APA StylePollard, C.-L., Gibb, Z., Clulow, J., Ruiz, A., Sheridan, A., Bahrami, M., Swegen, A., & Grupen, C. G. (2022). Supplemental Nicotinic Acid Elevates NAD+ Precursors in the Follicular Fluid of Mares. Animals, 12(11), 1383. https://doi.org/10.3390/ani12111383









