Pathological Findings in African Pygmy Hedgehogs Admitted into a Portuguese Rehabilitation Center
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Histopathology and Histochemistry
2.3. Immunohistochemistry
3. Results
3.1. Skin and Subcutaneous Tissue
3.2. Respiratory Tract
3.3. Digestive tract
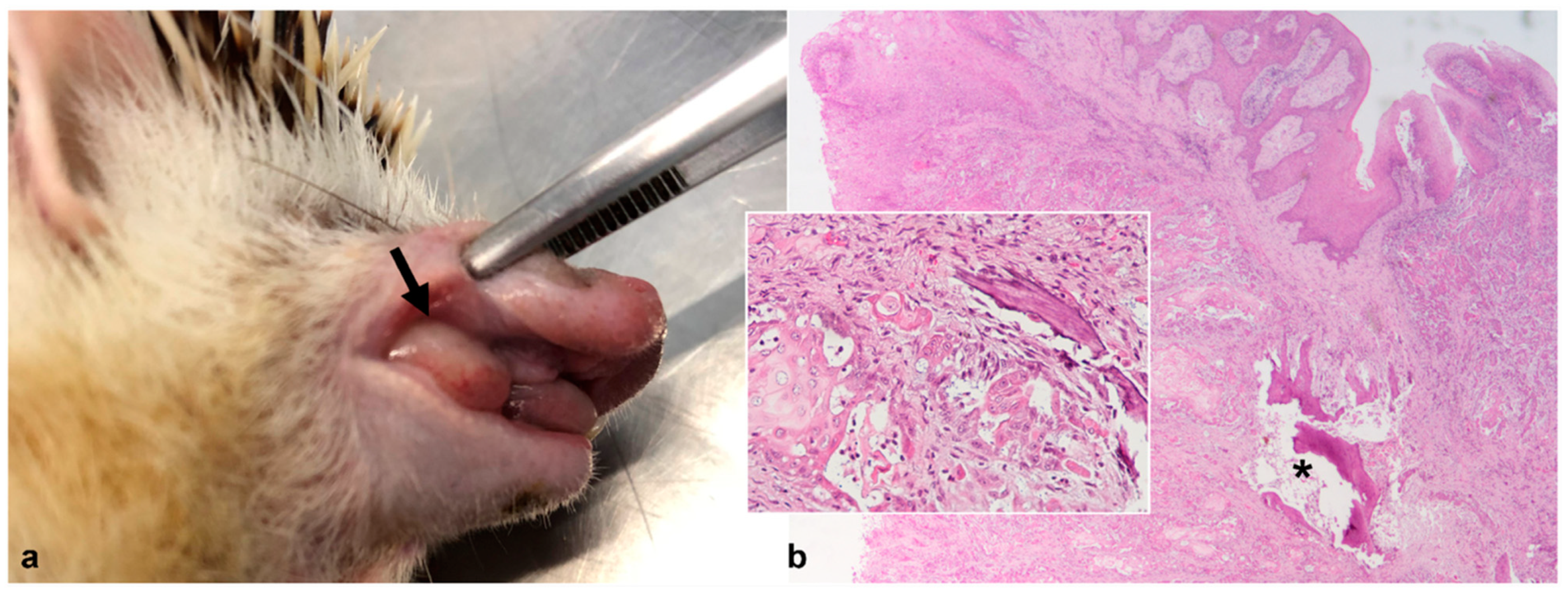
3.3.1. Oral Cavity
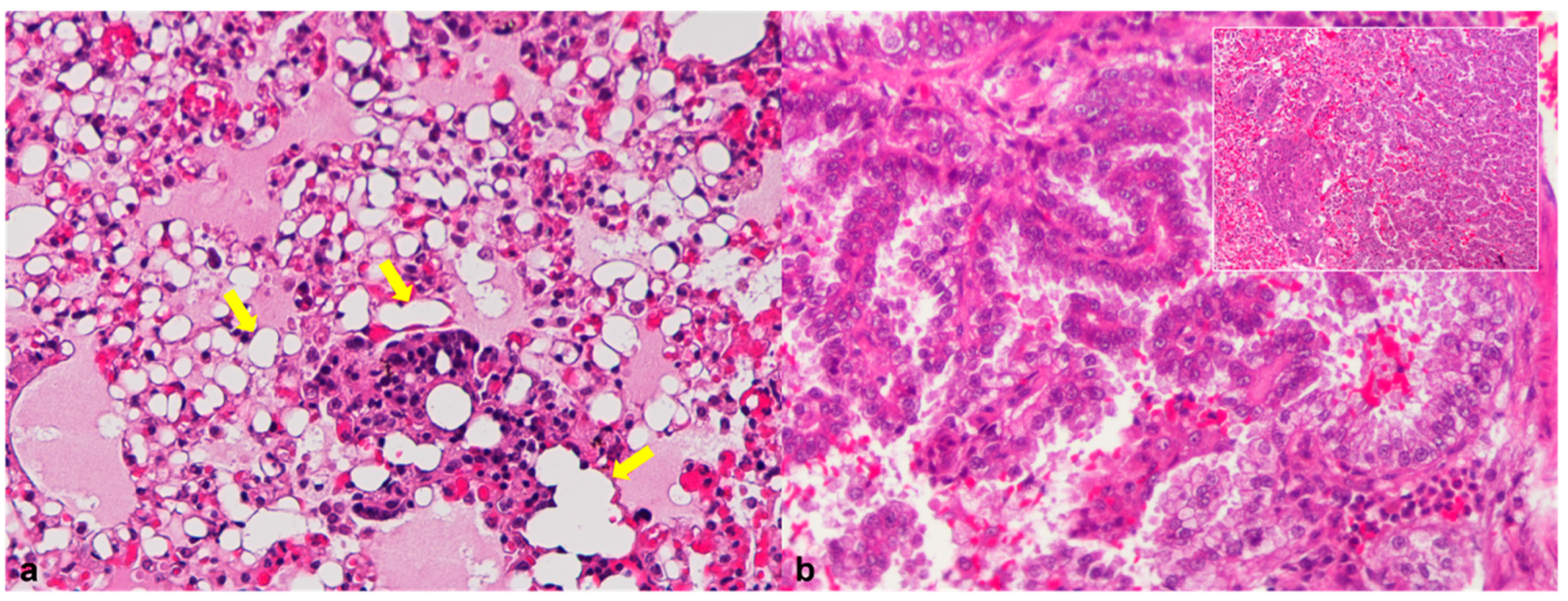
3.3.2. Liver
3.4. Urinary System
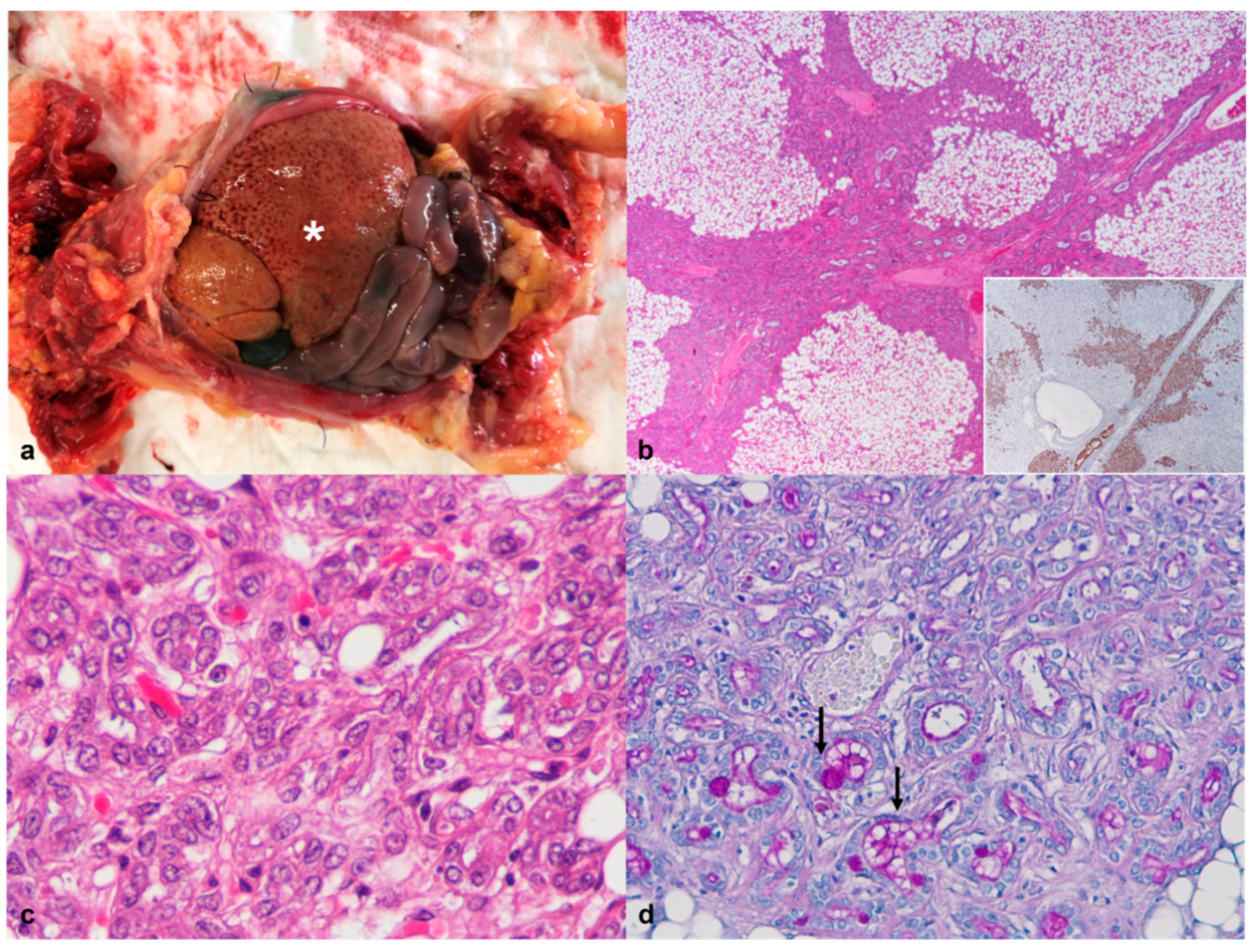
3.5. Endocrine System
3.6. Lymphatic System
Spleen
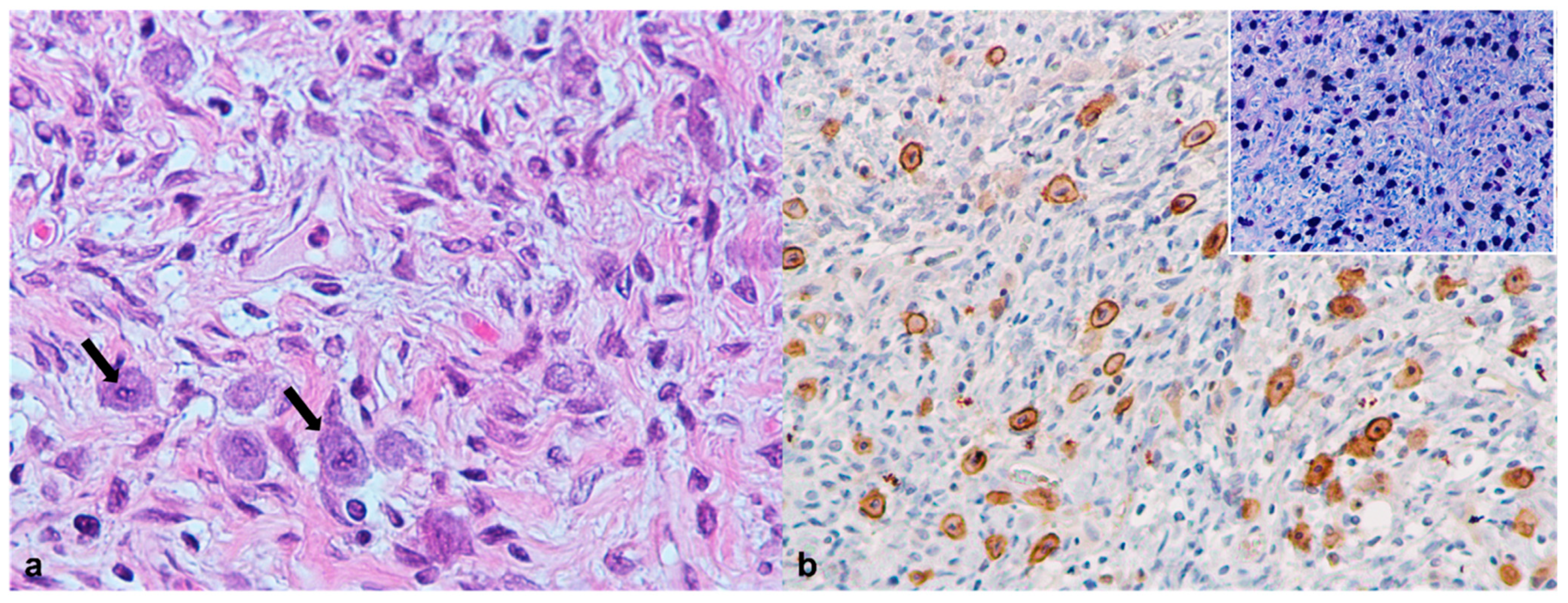
3.7. Central Nervous System
3.8. Reproductive Tract
4. Discussion
4.1. Case 1
4.2. Case 2
4.3. Case 4
4.4. Case 6
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amori, G. Erinaceus europaeus. The IUCN Red List of Threatened Species 2016. E.T29650A2791303. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T29650A2791303.en (accessed on 28 February 2022).
- Riley, P.Y.; Chomel, B.B. Hedgehog zoonoses. Emerg. Infect. Dis. 2005, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowski, J.J.; Hetman, M.; Turlewicz-Podbielska, H.; Pomorska-Mól, M. Hedgehogs as a potential source of zoonotic pathogens—A review and an update of knowledge. Animals 2021, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Cassola, F. Atelerix albiventris. The IUCN Red List of Threatened Species 2016. E.T40602A115174097. Available online: https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T40602A22324217.en (accessed on 28 February 2022).
- Heatley, J.J. Hedgehogs. In Manual-of-Exotic-Pet-Practice; Saunders Elsevier: St. Louis, MO, USA, 2009; pp. 433–455. [Google Scholar] [CrossRef]
- Gardhouse, S.; Eshar, D. Retrospective study of disease occurrence in captive African Pygmy hedgehogs (Atelerix albiventris). Isr. J. Vet. Med. 2015, 70, 32–36. [Google Scholar]
- Keeble, E.; Koterwas, B. Selected Emerging Diseases of Pet Hedgehogs. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Kondo, H.; Sumi, A.; Kagawa, Y. A retrospective study of disease incidence in African pygmy hedgehogs (Atelerix albiventris). J. Vet. Med. Sci. 2018, 80, 1504–1510. [Google Scholar] [CrossRef] [Green Version]
- Doss, G.A.; Carpenter, J.W. Diseases of Hedgehogs. 2021. Available online: https://www.msdvetmanual.com/exotic-and-laboratory-animals/hedgehogs/diseases-of-hedgehogs (accessed on 24 November 2021).
- Ivey, E.; Carpenter, J.W. African Hedgehogs. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery; Elsevier: St. Louis, MO, USA, 2012. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Whitley, D.B.; Storts, R.W.; Heatley, J.J.; Hoppes, S.; Porter, B.F. The Pathology of Wobbly Hedgehog Syndrome. Vet. Pathol. 2018, 55, 711–718. [Google Scholar] [CrossRef]
- Johnson, D.H. Geriatric Hedgehogs. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 615–637. [Google Scholar] [CrossRef]
- Del Aguila, G.; Torres, C.G.; Carvallo, F.R.; Gonzalez, C.M.; Cifuentes, F.F. Oral masses in African pygmy hedgehogs. J. Vet. Diagn. Investig. 2019, 31, 864–867. [Google Scholar] [CrossRef]
- Heatley, J.; Mauldin, G.; Cho, D. A Review of Neoplasia in the Captive African Hedgehog. Semin. Avian Exot. Pet Med. 2005, 14, 182–192. [Google Scholar] [CrossRef]
- Raymond, J.T.; Garner, M.M. Spontaneous tumours in captive African hedgehogs (Atelerix albiventris): A retrospective study. J. Comp. Pathol. 2001, 124, 128–133. [Google Scholar] [CrossRef]
- Blume, G.R.; Eloi, R.S.A.; Oliveira, L.B.; Moraes, E.L.S.C.; Seeger, M.G.; Cargnelutti, J.F.; Sant’Ana, F.J.F. Non-tuberculous Mycobacterial Granulomatous Dermatitis in an African Pygmy Hedgehog (Atelerix albiventris). J. Comp. Pathol. 2021, 182, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Juan-Sallés, C.; Garner, M.M. Cytologic Diagnosis of Diseases of Hedgehogs. Vet. Clin. N. Am. Exot. Anim. Pract. 2007, 10, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lugton, I.W.; Morris, R.S.; Johnstone, A.C. Mycobacterium bovis infection in New Zealand hedgehogs (Erinaceus europaeus). N. Z. Vet. J. 1995, 43, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Nugent, G.; Whitford, E.J.; Hunnam, J.C.; Wilson, P.R.; Cross, M.L.; de Lisle, G.W. Mycobacterium avium subsp. paratuberculosis infection in wildlife on three deer farms with a history of Johne’s disease. N. Z. Vet. J. 2011, 59, 293–298. [Google Scholar] [CrossRef]
- Matos, A.C.; Figueira, L.; Martins, M.H.; Pinto, M.L.; Matos, M.; Coelho, A.C. New Insights into Mycobacterium bovis Prevalence in Wild Mammals in Portugal. Transbound. Emerg. Dis. 2016, 63, e313–e322. [Google Scholar] [CrossRef]
- Nakamura, S.I.; Yasuda, M.; Ozaki, K.; Tsukahara, T. Eosinophilic Leukaemia and Systemic Mycobacterium marinum Infection in an African Pygmy Hedgehog (Atelerix albiventris). J. Comp. Pathol. 2020, 181, 33–37. [Google Scholar] [CrossRef]
- Tappe, J.P.; Weitzman, I.; Liu, S.; Dolensek, E.P.; Karp, D. Systemic Mycobacterium marinum infection in a European hedgehog. J. Am. Vet. Med. Assoc. 1983, 183, 1280–1281. [Google Scholar]
- Graesser, D.; Spraker, T.R.; Dressen, P.; Garner, M.M.; Raymond, J.T.; Terwilliger, G.; Kim, J.; Madri, J.A. Wobbly Hedgehog Syndrome in African Pygmy Hedgehogs (Atelerix spp.). J. Exot. Pet Med. 2006, 15, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Barber, L.G. Thyroid Tumors in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 755–773. [Google Scholar] [CrossRef]
- LaRue, M.K.; Flesner, B.K.; Higbie, C.T.; Dehghanpir, S.; Crossland, N.; Nevarez, J.G.; Tully, T.N.; Grasperge, B.J.; Langohr, I.M.; Shiomitsu, K. Treatment of a Thyroid Tumor in an African Pygmy Hedgehog (Atelerix albiventris). J. Exot. Pet Med. 2016, 25, 226–230. [Google Scholar] [CrossRef]
- Miller, D.L.; Styer, E.L.; Stobaeus, J.K.; Norton, T.M. Thyroid C-cell carcinoma in an African pygmy hedgehog (Atelerix albiventris). J. Zoo Wildl. Med. 2002, 33, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Scott-Moncrieff, J.C. Canine Thyroid Tumors and Hyperthyroidism. In Canine and Feline Endocrinology, 4th ed.; Saunders Elsevier Inc.: Missouri, MO, USA, 2015; pp. 196–212. [Google Scholar] [CrossRef]
- Carver, J.R.; Kapatkin, A.; Patnaik, A.K. A Comparison of Medullary Thyroid Carcinoma and Thyroid Adenocarcinoma in Dogs: A Retrospective Study of 38 Cases. Vet. Surg. 1995, 24, 315–319. [Google Scholar] [CrossRef]
- Bicho, R.C.; Amaral, M.J.; Faustino, A.M.R.; Power, D.M.; Rêma, A.; Carretero, M.A.; Soares, A.M.V.M.; Mann, R.M. Thyroid disruption in the lizard Podarcis bocagei exposed to a mixture of herbicides: A field study. Ecotoxicology 2012, 22, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Grífols, J.; Perpiñán, D. Endogenous lipid pneumonia in an african grey parrot (Psittacus erithacus erithacus). J. Comp. Pathol. 2013, 149, 381–384. [Google Scholar] [CrossRef]
- Pérez-Accino, J.; Liuti, T.; Pecceu, E.; Cazzini, P. Endogenous lipoid pneumonia associated with pulmonary neoplasia in three dogs. J. Small Anim. Pract. 2021, 62, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, T.M.; Haeckel, T.; Wagner, T.; von Scheidt, W.; Schwaiblmair, M.G. Endogenous lipoid pneumonia associated with primary sclerosing cholangitis. Lancet 2007, 369, 1140. [Google Scholar] [CrossRef]
- Himsworth, C.G.; Malek, S.; Saville, K.; Allen, A.L. Endogenous lipid pneumonia and what lies beneath. Can. Veter. J. = La Rev. Veter. Can. 2008, 49, 813–815. [Google Scholar]
- Cullen, J.M.; Brown, D.L. Hepatobiliary system and exocrine pancreas. In Pathologic Basis of Veterinary Disease, 5th ed.; Elsevier: Mosby, MO, USA, 2012; pp. 415–418. [Google Scholar]
- Kandefer-Gola, M.; Ciaputa, R.; Sulima, K.; Miszczak, M.; Lachowska, S.; Nowak, M. Metastatic mast cell tumour in African hedgehog: A case report. Vet. Med. (Praha) 2020, 65, 371–376. [Google Scholar] [CrossRef]
- Raymond, J.T.; White, M.R.; Janovitz, E.B. Malignant mast cell tumor in an African hedgehog (Atelerix albiventris). J. Wildl. Dis. 1997, 33, 140–142. [Google Scholar] [CrossRef]
- Toma, C.; Balteanu, V.A.; Tripon, S.; Trifa, A.; Rema, A.; Amorim, I.; Pop, R.M.; Popa, R.; Catoi, C.; Taulescu, M. Exogenous Jaagsiekte Sheep Retrovirus type 2 (exJSRV2) related to ovine pulmonary adenocarcinoma (OPA) in Romania: Prevalence, anatomical forms, pathological description, immunophenotyping and virus identification. BMC Vet. Res. 2020, 16, 296. [Google Scholar] [CrossRef]
- Dungworth, D.L.; Hauser, B.; Hahn, F.F.; Wilson, D.W.; Haenichen, T.; Harkema, J.R. WHO: Histological Classification of Tumors of the Respiratory System of Domestic Animals; 2nd Series; Washington, DC, USA,, 1999; pp. 16–23. [Google Scholar]
- Sato, T.; Ito, J.; Shibuya, H.; Asano, K.; Watari, T. Pulmonary adenosquamous carcinoma in a dog. J. Vet. Medicine. A Physiol. Pathol. Clin. Med. 2005, 52, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Pei-Chi, H.; Jane-Fang, Y.; Lih-Chiann, W. A retrospective study of the medical status on 63 african hedgehogs (Atelerix albiventris) at the taipei zoo from 2003 to 2011. J. Exot. Pet Med. 2015, 24, 105–111. [Google Scholar] [CrossRef]
| Case | Species | Sex | Age (Years) | Body Weight (g) | Clinical History |
|---|---|---|---|---|---|
| 1 | African pygmy hedgehog (Atelerix albiventris) | F | 2 | 245 | Ataxia and lameness of the hind limbs (lasting six months) Malnutrition Skin wound in the right forelimb |
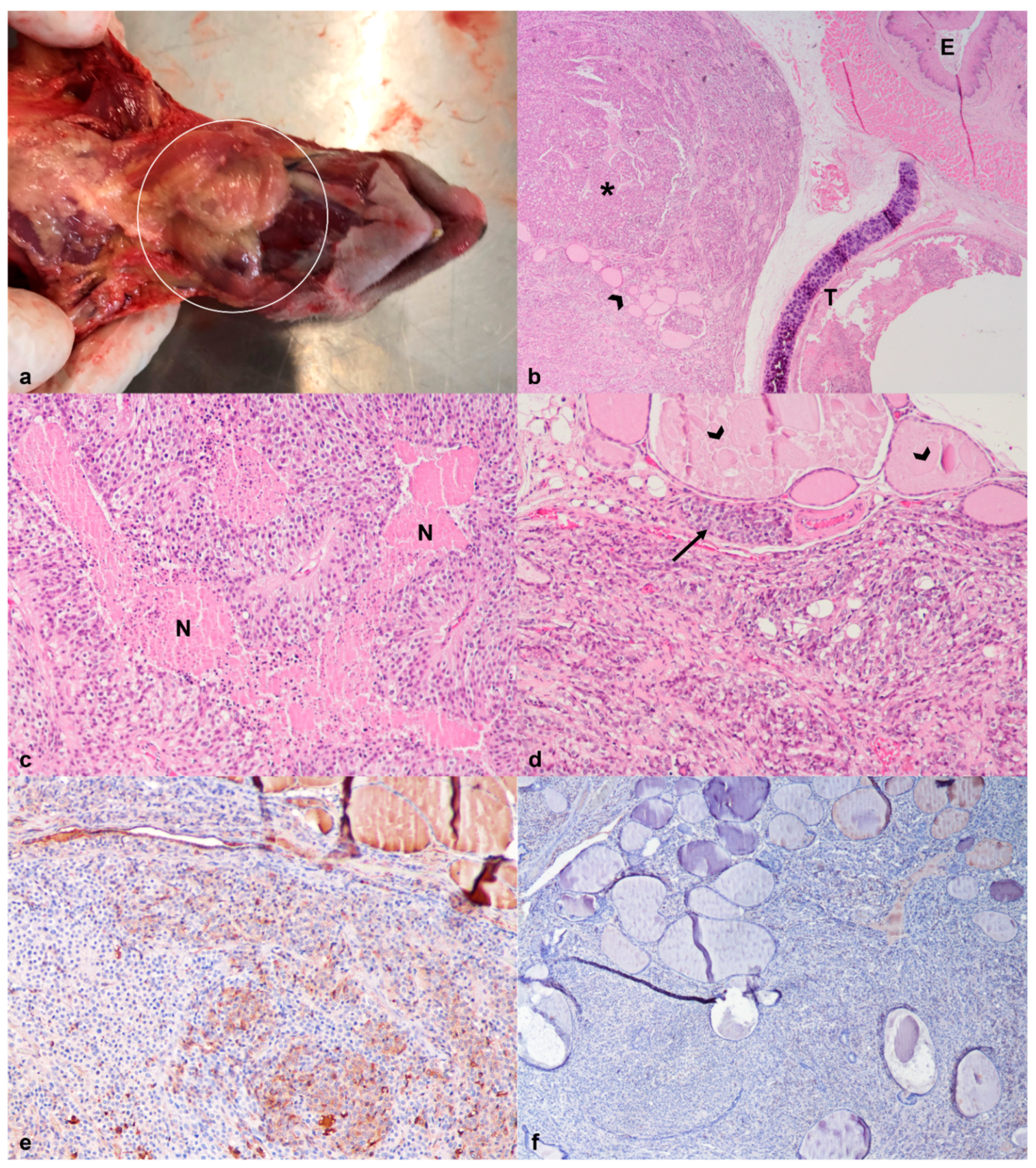
| 2 | African pygmy hedgehog (Atelerix albiventris) | F | >3 | 336 | Severe tachypnea Mass in the oral cavity Subcutaneous mass in the neck region suspected of lymphadenomegaly |
| 3 | African pygmy hedgehog (Atelerix albiventris) | F | -- | -- | Nodule in the oral cavity |
| 4 | African pygmy hedgehog (Atelerix albiventris) | F | 5 | 625 | Breathing difficulty Obesity (body condition 9 (score 1–9)) |
| 5 | African pygmy hedgehog (Atelerix albiventris) | M | -- | 240 | Ataxia and nonspecific neurological symptomatology |
| 6 | African pygmy hedgehog (Atelerix albiventris) | M | 5 | -- | Breathing difficulties |
| Antibody | Clone | Supplier | Dilution | Antigen UM | IT | Positive Control | Case |
|---|---|---|---|---|---|---|---|
| Thyroglobulin | Polyclonal | Dako, Denmark | 1:2000 | RS/WB | ON | Hedgehog normal thyroid | 2 |
| Thyroperoxidase (TPO) | Monoclonal (MoAb47) | Abcam, UK | 1:20 | EDTA pH = 9/WB | ON | Hedgehog normal thyroid | 2 |
| Neuron-specific enolase (NSE) | Monoclonal (VI-H14) | Imgenex, USA | 1:500 | RS/WB | ON | Canine normal pancreas | 2 |
| Cytokeratins (AE1/AE3) | Polyclonal | Thermo, USA | 1:300 | RS/WB | ON | Canine uterus and internal control | 2, 4 |
| c-Kit (CD117) | Polyclonal | Dako, Denmark | 1:450 | RS/WB | ON | Canine mast cell tumor | 4 |
| Synaptophysin | Monoclonal (SP11) | Thermo, USA | 1:150 | RS/WB | ON | Canine normal pancreas | 2 |
| Case | Histochemical Stain | Structure Identification | Tissue | Result |
|---|---|---|---|---|
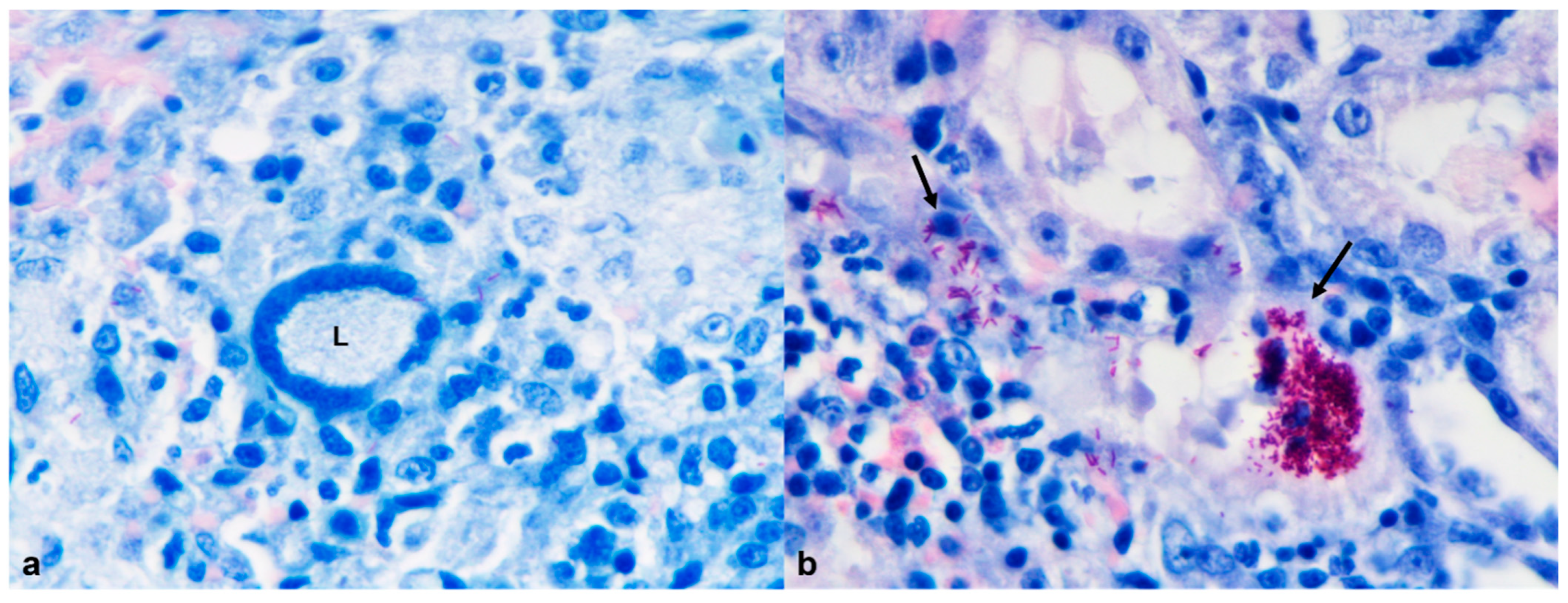
| 1 | Ziehl–Neelsen | Mycobacterium spp. (acid-fast bacilli) | Lung; spleen; liver; kidney | + |
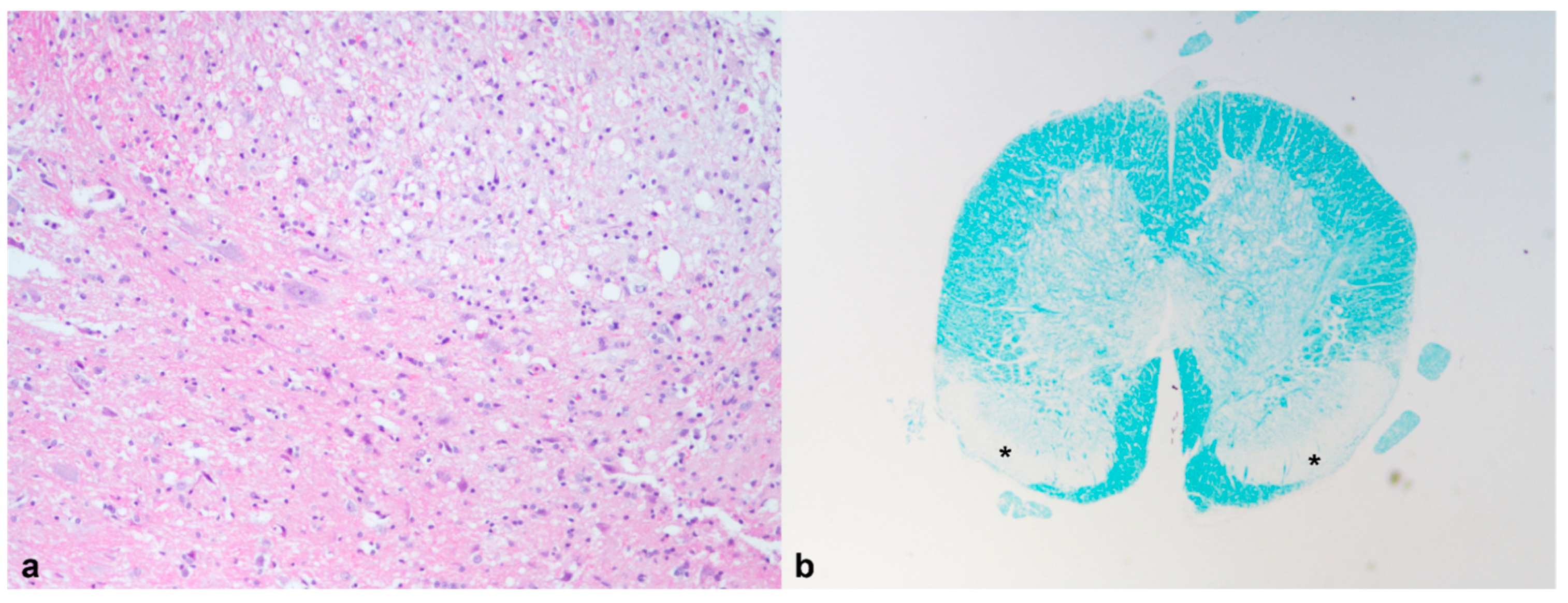
| Luxol fast blue | Myelin/loss of myelin | CNS | + | |
| 2 | Ziehl–Neelsen | Mycobacterium spp. (acid-fast bacilli) | Liver | − |
| Congo red | Amyloid | Cervical mass | − | |
| 4 | Grocott methenamine silver | Fungal organisms | Lung | − |
| Periodic acid–Schiff | Fungal hyphae | Lung | − | |
| Glycogen | Liver | + | ||
| Gram stain | Bacteria | Lung | − | |
| Giemsa stain | Mast cells | Cervical nodule | + | |
| Masson’s trichrome stain | Connective tissue (fibrosis) | Ovary, liver | + | |
| 5 | Luxol fast blue | Myelin/loss of myelin | CNS | + |
| Perl’s Prussian Blue | Hemosiderin | Liver | + | |
| Hall’s Bilirubin Stain | Bilirubin | Liver | + |
| Case | Respiratory System | Digestive Tract and Liver | Urinary/Reproductive System | Endocrine System | Lymphatic System | Central Nervous System | Skin and Subcutaneous Tissue |
|---|---|---|---|---|---|---|---|
| 1 | Granulomatous pneumonia (Mycobacterium spp.) | Granulomatous hepatitis (Mycobacterium spp.) | Granulomatous nephritis (Mycobacterium spp.) | Granulomatous splenitis (Mycobacterium spp.) | Myelin degeneration Spongiosis and microgliosis | Ulcerative and necrotizing dermatitis | |
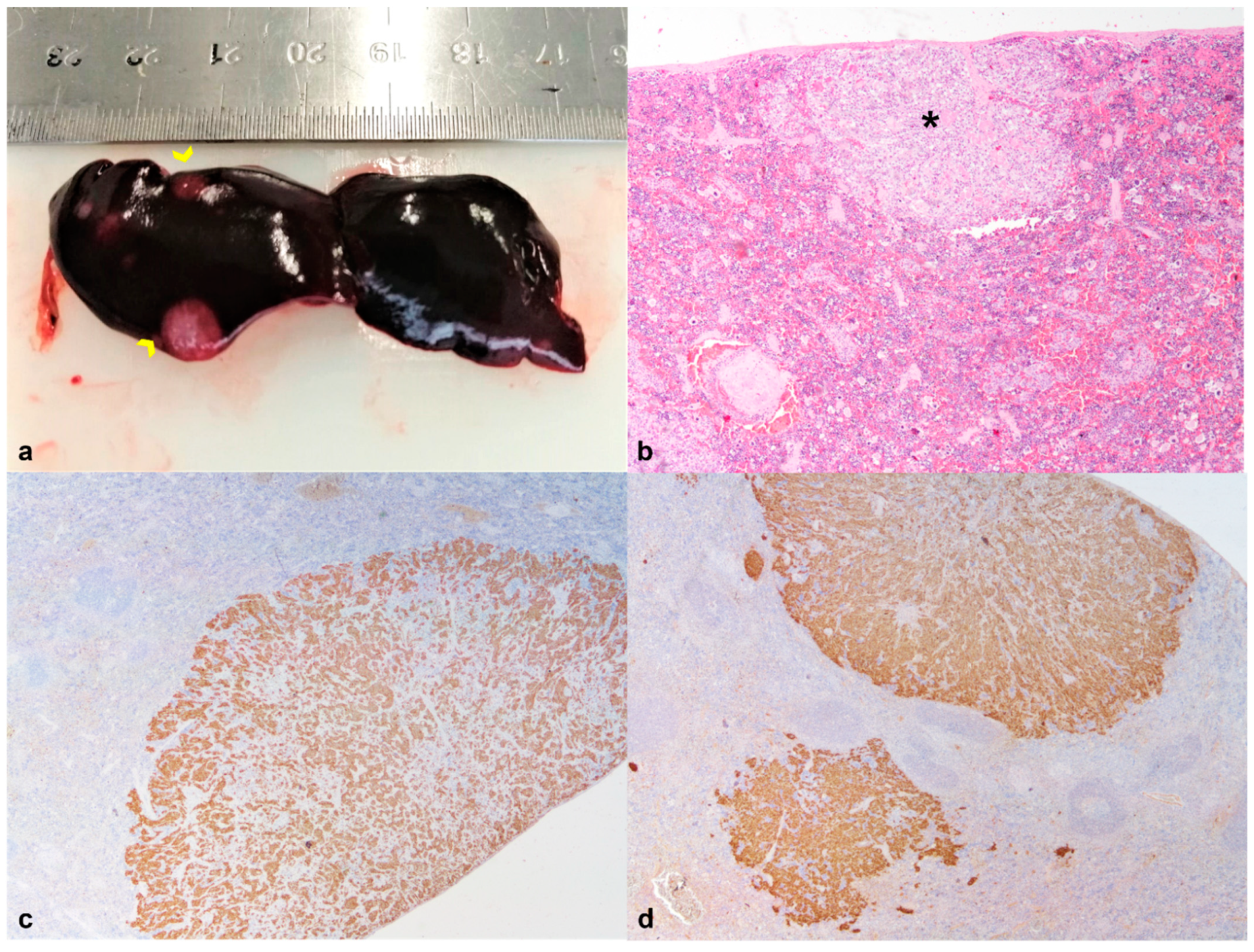
| 2 | Granulomatous hepatitis Oral squamous cell carcinoma | Thyroid Carcinoma | Metastases of thyroid carcinoma in the spleen | ||||
| 3 | Chronic lymphoplasmacytic and ulcerative gingivostomatitis | ||||||
| 4 | Lipoid pneumonia | Liver steatosis Hepatic extramedullary hematopoiesis Bile duct hyperplasia | Chronic interstitial nephritis Ovarian fibroma | Subcutaneous mast cell tumor | |||
| 5 | Acute necrotizing hepatitis Cholestasis; Hemosiderosis Hepatic extramedullary hematopoiesis | Myelin degeneration | |||||
| 6 | Pulmonary adenocarcinoma | Liver steatosis |
| Immunomarker | Results |
|---|---|
| Thyroglobulin | Mild to moderate cytoplasmic immunostaining in 70% of the neoplastic cell population (Figure 6e). |
| TPO | Weak and diffuse immunopositivity affecting 50–75% of the neoplastic cells. |
| Synaptophysin | Weak immunostaining in 30% of the neoplastic cells, more evident in the cells adjacent to the outer capsule (Figure 6f). |
| NSE | Intense and diffuse cytoplasmic immunostaining of more than 75% of the neoplastic cells. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.F.; Rêma, A.; Teixeira, S.; Pires, M.d.A.; Taulescu, M.; Amorim, I. Pathological Findings in African Pygmy Hedgehogs Admitted into a Portuguese Rehabilitation Center. Animals 2022, 12, 1361. https://doi.org/10.3390/ani12111361
Silva GF, Rêma A, Teixeira S, Pires MdA, Taulescu M, Amorim I. Pathological Findings in African Pygmy Hedgehogs Admitted into a Portuguese Rehabilitation Center. Animals. 2022; 12(11):1361. https://doi.org/10.3390/ani12111361
Chicago/Turabian StyleSilva, Gabriela Fernandes, Alexandra Rêma, Sílvia Teixeira, Maria dos Anjos Pires, Marian Taulescu, and Irina Amorim. 2022. "Pathological Findings in African Pygmy Hedgehogs Admitted into a Portuguese Rehabilitation Center" Animals 12, no. 11: 1361. https://doi.org/10.3390/ani12111361
APA StyleSilva, G. F., Rêma, A., Teixeira, S., Pires, M. d. A., Taulescu, M., & Amorim, I. (2022). Pathological Findings in African Pygmy Hedgehogs Admitted into a Portuguese Rehabilitation Center. Animals, 12(11), 1361. https://doi.org/10.3390/ani12111361






