Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice
Abstract
Simple Summary
Abstract
1. Introduction
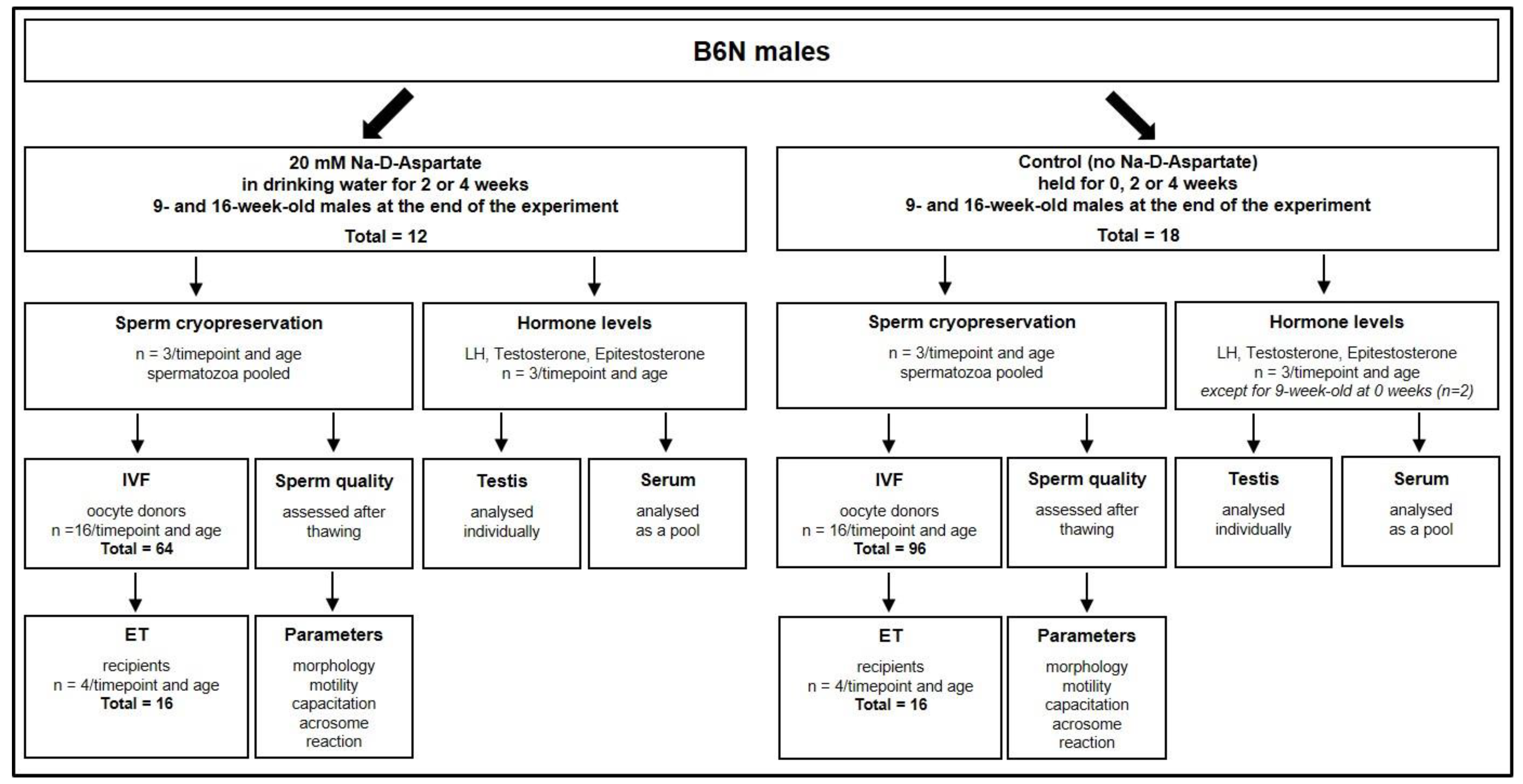
2. Materials and Methods
2.1. Mice and Husbandry
2.2. Treatment of Mice
2.3. Sperm Cryopreservation
2.4. In Vitro Fertilization (IVF)
2.5. Embryo Transfer
2.6. Measurement of Hormones
2.7. Assessment of Sperm Parameters
2.7.1. Measurement of Sperm Concentration and Motility
2.7.2. Measurement of Sperm Morphology
2.7.3. Determination of the Capacitation Rate
2.7.4. Evaluation of the Acrosome Reaction
2.8. Statistical Analysis
2.9. Ethical Review Procedure
3. Results
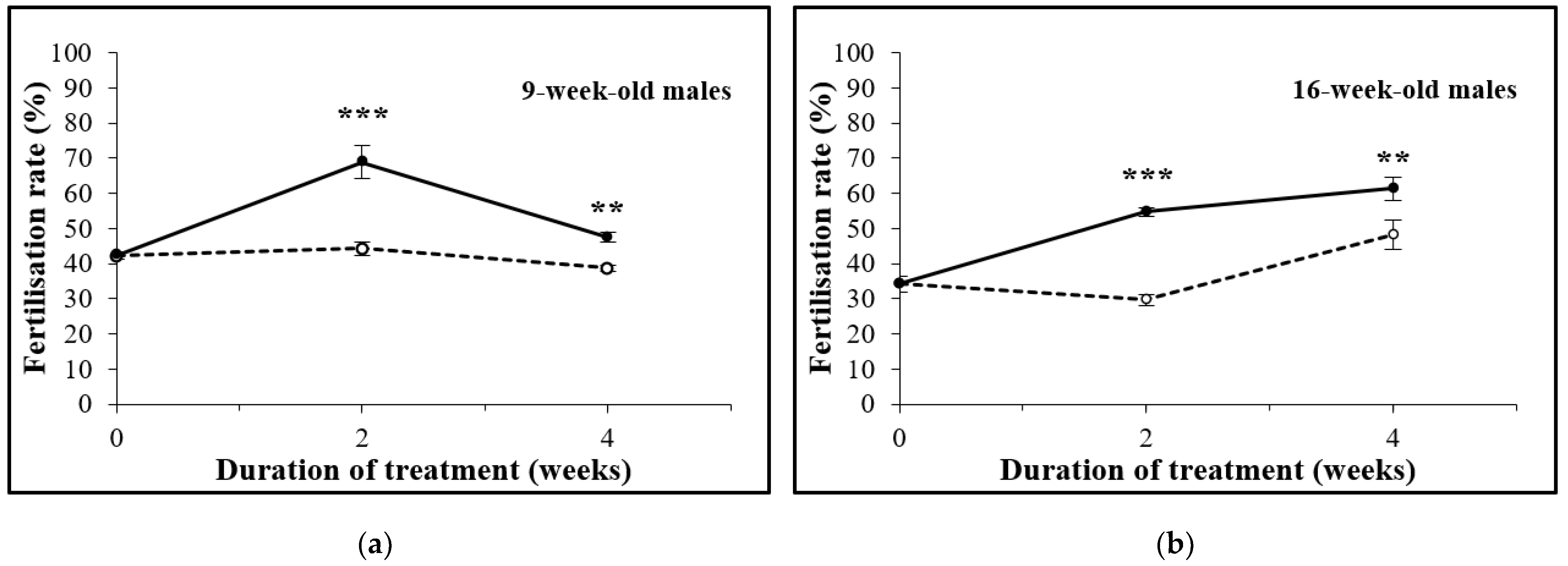
3.1. D-Asp Treatment Increases the In Vitro Fertilization (IVF) Rate
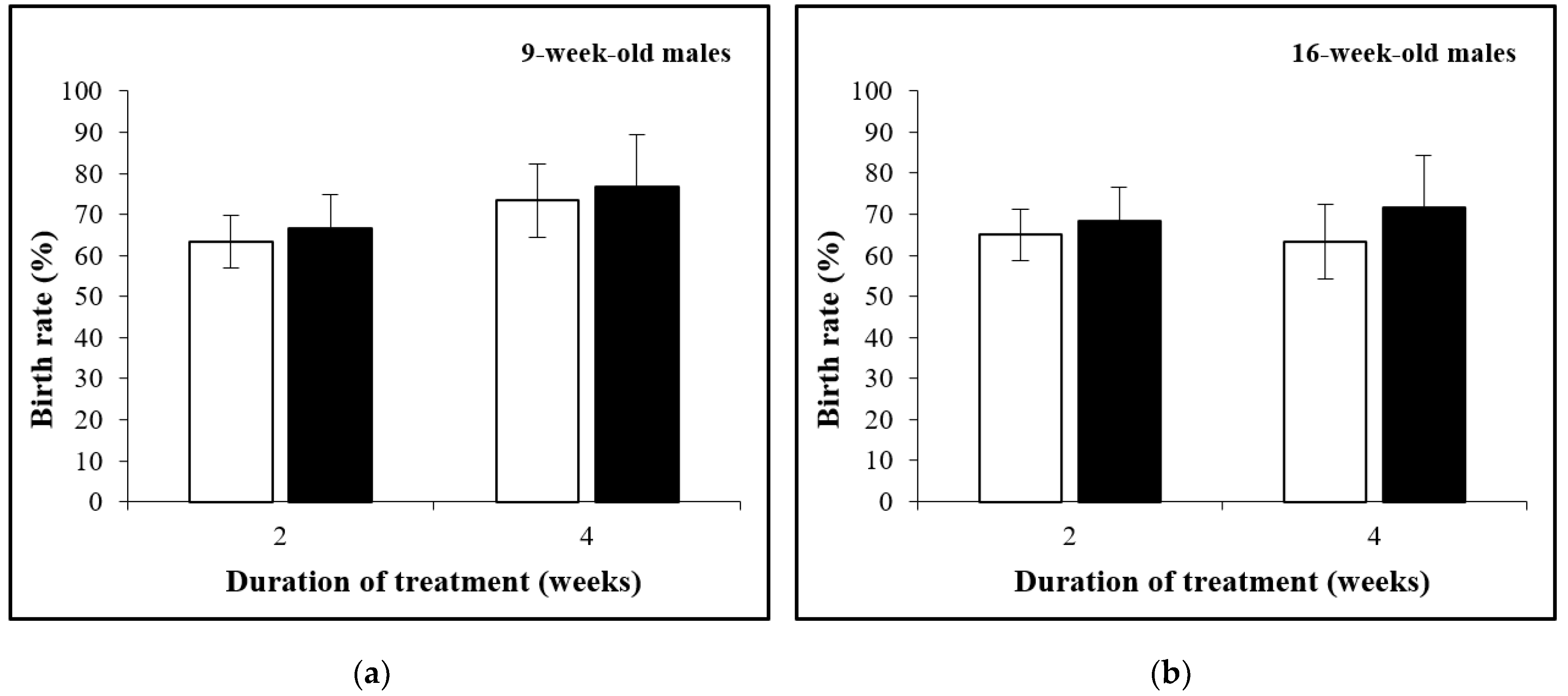
3.2. The Birth Rate Is Not Affected by D-Asp Treatment
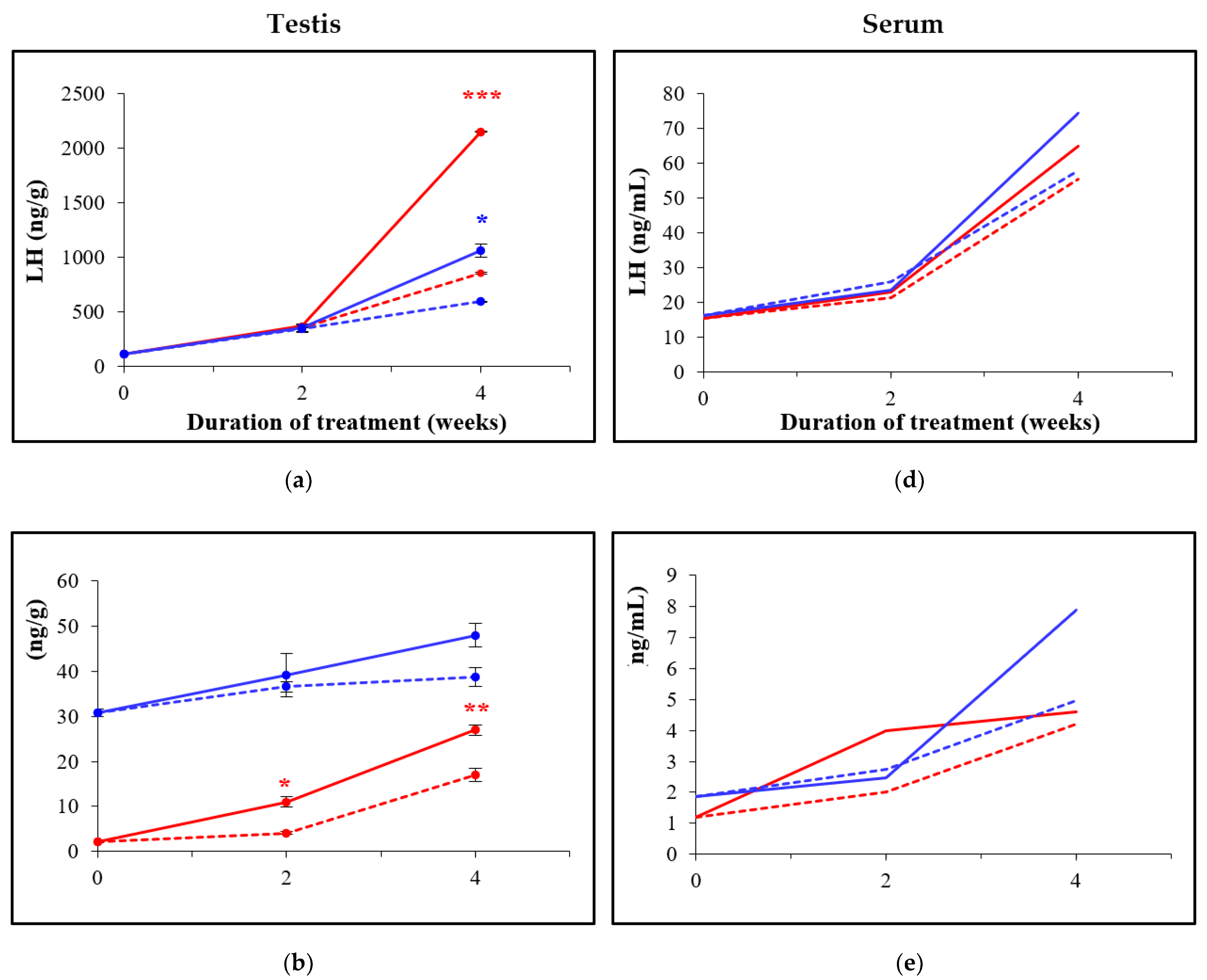
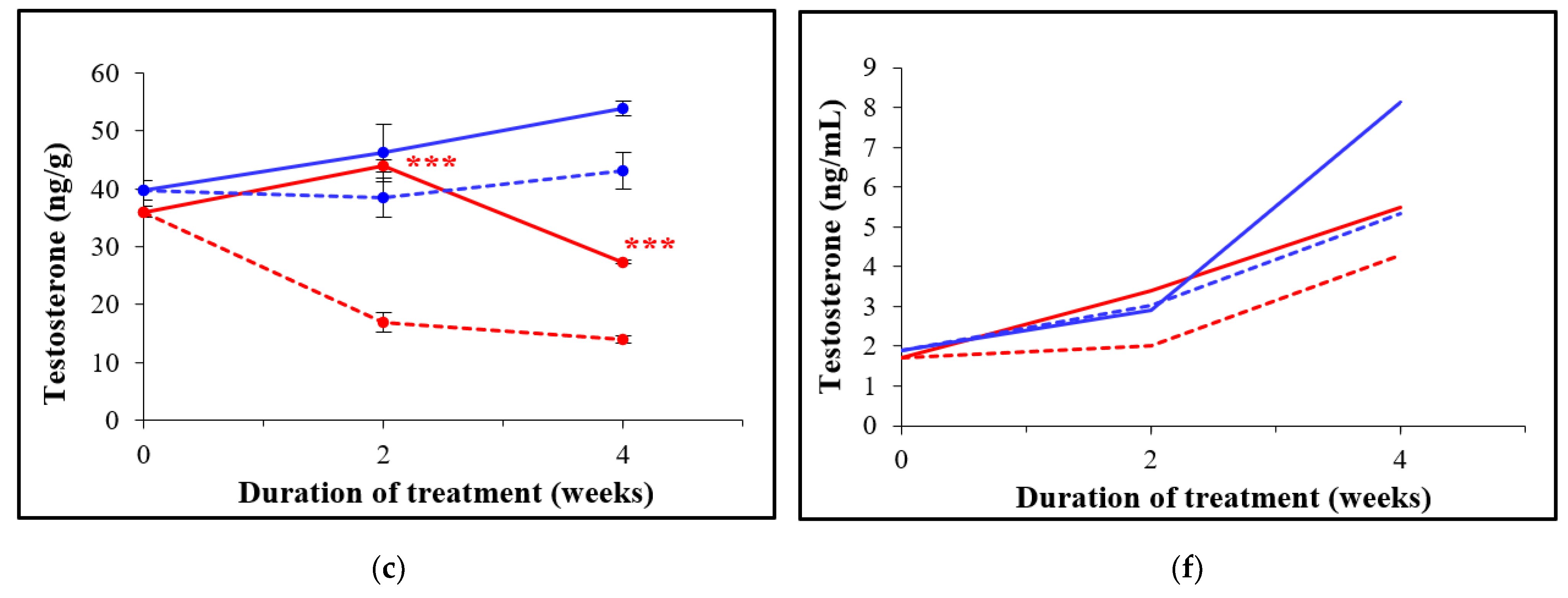
3.3. Oral Administration of D-Asp Elevates Hormone Levels in Testes and in Sera
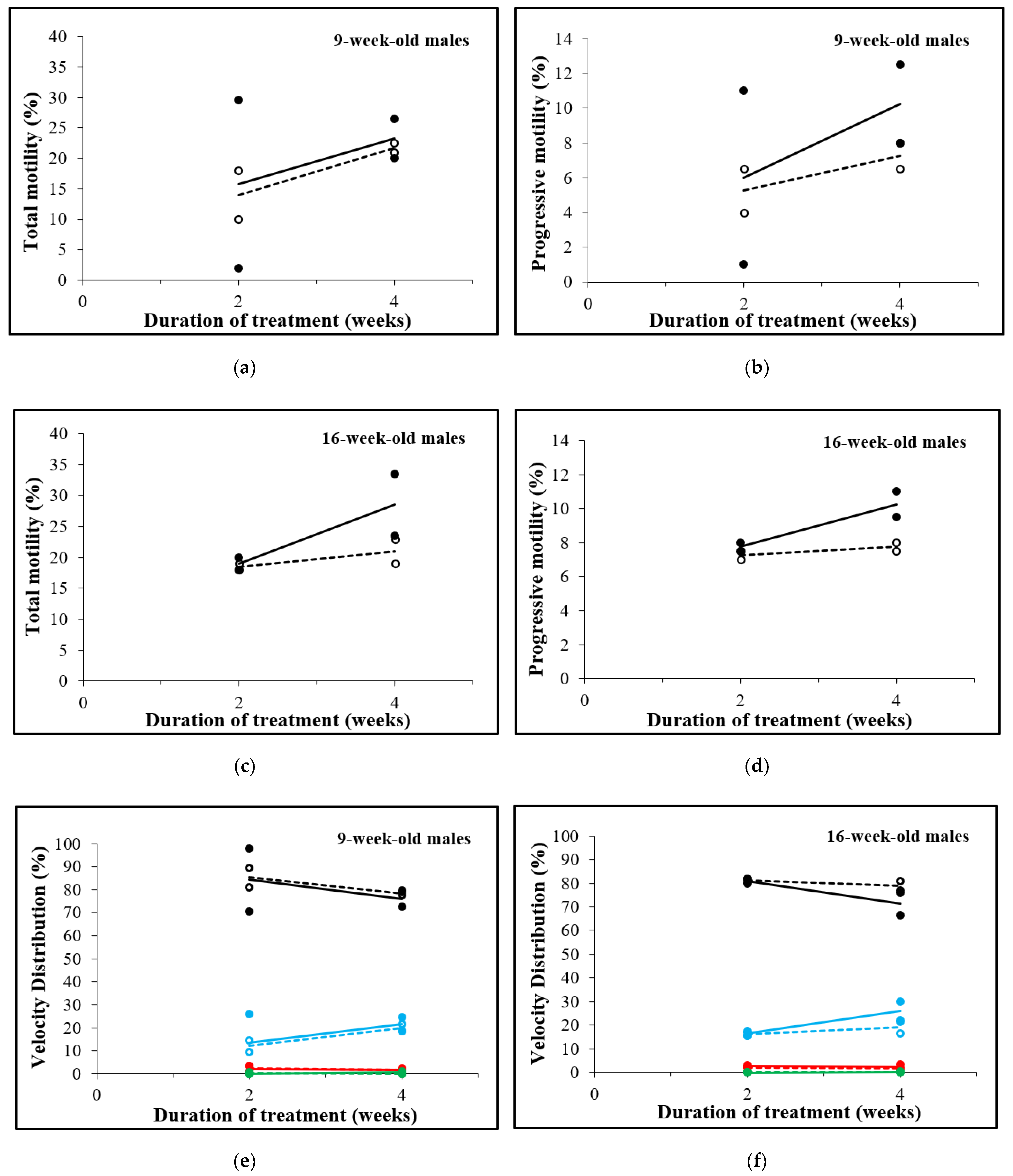
3.4. Total and Progressive Sperm Motility, but Not Concentration and Velocity Increases after D-Asp Treatment
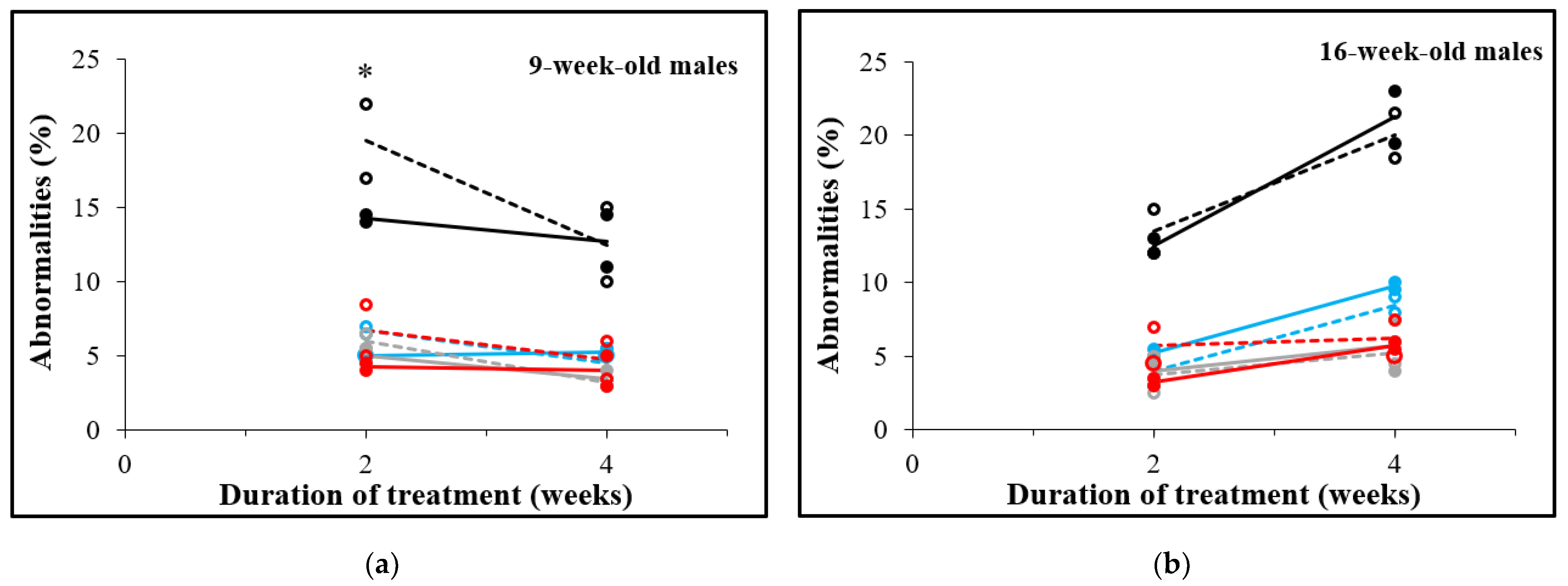
3.5. D-Asp Treatment for 2 Weeks Decreases Sperm Abnormalities in 9-Week-Old Males
3.6. The Capacitation Rate Is Increased after D-Asp Treatment
3.7. The Acrosome Reaction Increases after D-Asp Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostermeier, G.C.; Wiles, M.V.; Farley, J.S.; Taft, R.A. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE 2008, 3, e2792. [Google Scholar] [CrossRef]
- Takeo, T.; Nakagata, N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol. Reprod. 2011, 85, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Bogani, D.; Marschall, S.; Raspa, M.; Takeo, T.; Nakagata, N.; Fray, M. In vitro fertilization in mice using the MBCD-GSH protocol. Curr. Protoc. Mouse Biol. 2014, 4, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.; Guan, M.; Bogani, D.; Marschall, S.; Raspa, M.; Pickard, A.; Takeo, T.; Nakagata, N.; Fray, M. Transporting mouse embryos and germplasm as frozen or unfrozen materials. Curr. Protoc. Mouse Biol. 2014, 4, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Bogani, D.; Marschall, S.; Raspa, M.; Takeo, T.; Nakagata, N.; Fray, M. Conservation of mouse models through embryo freezing. Curr. Protoc. Mouse Biol. 2014, 4, 205–227. [Google Scholar] [CrossRef]
- Critser, J.K.; Mobraaten, L.E. Cryopreservation of murine spermatozoa. ILAR J. 2000, 41, 197–206. [Google Scholar] [CrossRef][Green Version]
- Stacy, R.; Eroglu, A.; Fowler, A.; Biggers, J.; Toner, M. Thermal characterization of Nakagata’s mouse sperm freezing protocol. Cryobiology 2006, 52, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Taft, R. Mouse Sperm Cryopreservation Using Cryoprotectant Containing Monothioglycerol (MTG). Cold Spring Harb. Protoc. 2017, 2017, pdb-prot094490. [Google Scholar] [CrossRef]
- Takeo, T.; Sztein, J.; Nakagata, N. The CARD Method for Mouse Sperm Cryopreservation and In Vitro Fertilization Using Frozen-Thawed Sperm. In Microinjection: Methods and Protocols; Liu, C., Du, Y., Eds.; Springer: New York, NY, USA, 2019; pp. 243–256. [Google Scholar]
- Raspa, M.; Paoletti, R.; Mahabir, E.; Scavizzi, F. d-aspartate treatment in vitro improves mouse sperm fertility in young B6N mice. Theriogenology 2020, 148, 60–67. [Google Scholar] [CrossRef]
- Ota, N.; Shi, T.; Sweedler, J.V. D-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012, 43, 1873–1886. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Baccari, G.C. D-Aspartic Acid in Vertebrate Reproduction: Animal Models and Experimental Designs. Biomolecules 2019, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Tanaka, H.; Kageyama, S.; Nagasawa, M.; Wada, A.; Murai, R.; Kobayashi, K.; Hanada, E.; Agata, Y.; Kawauchi, A. The Effect of D-Aspartate on Spermatogenesis in Mouse Testis. Biol. Reprod. 2016, 94, 30. [Google Scholar] [CrossRef] [PubMed]
- Raspa, M.; Mahabir, E.; Paoletti, R.; Protti, M.; Mercolini, L.; Schiller, P.; Scavizzi, F. Effects of oral d-aspartate on sperm quality in B6N mice. Theriogenology 2018, 121, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Mähler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar]
- Kawai, Y.; Hata, T.; Suzuki, O.; Matsuda, J. The relationship between sperm morphology and in vitro fertilization ability in mice. J. Reprod. Dev. 2006, 52, 561–568. [Google Scholar] [CrossRef]
- Gotoh, H. Inherited sperm head abnormalities in the B10.M mouse strain. Reprod. Fertil. Dev. 2010, 22, 1066–1073. [Google Scholar] [CrossRef]
- Raghuvanshi, P.; Mathur, M.; Sethi, R.; Bhatnagar, P. Evaluation of the changes in sperm morphology, sperm count and gonadotropic ratio of swiss albino male mice fed continuously with microwave exposed food. Adv. Biores. 2012, 3, 95–99. [Google Scholar]
- Ward, C.R.; Storey, B.T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 1984, 104, 287–296. [Google Scholar] [CrossRef]
- Choi, Y.H.; Toyoda, Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 1998, 59, 1328–1333. [Google Scholar] [CrossRef]
- Huo, L.J.; Yang, Z.M. Effects of platelet activating factor on capacitation and acrosome reaction in mouse spermatozoa. Mol. Reprod. Dev. 2000, 56, 436–440. [Google Scholar] [CrossRef]
- Larson, J.L.; Miller, D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999, 52, 445–449. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Usiello, A.; Di Fiore, M.M.; De Rosa, A.; Falvo, S.; Errico, F.; Santillo, A.; Nuzzo, T.; Chieffi Baccari, G. New Evidence on the Role of D-Aspartate Metabolism in Regulating Brain and Endocrine System Physiology: From Preclinical Observations to Clinical Applications. Int. J. Mol. Sci. 2020, 21, 8718. [Google Scholar] [CrossRef]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, L.; Noel, K.; Dutertre, M.; Belville, C.; Forest, M.G.; Burgoyne, P.S.; Josso, N.; Rey, R. Hormonal and cellular regulation of Sertoli cell anti-Mullerian hormone production in the postnatal mouse. J. Clin. Investig. 1997, 100, 1335–1343. [Google Scholar] [CrossRef]
- Machida, T.; Yonezawa, Y.; Noumura, T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm. Behav. 1981, 15, 238–245. [Google Scholar] [CrossRef]
- Osadchuk, L.V.; Kleshyov, M.A. Interstrain Differences in the Parameters of Spermatogenesis in Inbred Mice. Morfologiia 2016, 2, 54–57. [Google Scholar]
- Kemp, C.J.; Drinkwater, N.R. Genetic variation in liver tumor susceptibility, plasma testosterone levels, and androgen receptor binding in six inbred strains of mice. Cancer Res. 1989, 49, 5044–5047. [Google Scholar]
- Brouillette, J.; Rivard, K.; Lizotte, E.; Fiset, C. Sex and strain differences in adult mouse cardiac repolarization: Importance of androgens. Cardiovasc. Res. 2005, 65, 148–157. [Google Scholar] [CrossRef]
- Chen, H.; Hardy, M.P.; Huhtaniemi, I.; Zirkin, B.R. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 1994, 15, 551–557. [Google Scholar]
- D’Aniello, A.; Di Cosmo, A.; Di Cristo, C.; Annunziato, L.; Petrucelli, L.; Fisher, G. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996, 59, 97–104. [Google Scholar] [CrossRef]
- Santillo, A.; Falvo, S.; Chieffi, P.; Di Fiore, M.M.; Senese, R.; Chieffi Baccari, G. D-Aspartate Induces Proliferative Pathways in Spermatogonial GC-1 Cells. J. Cell Physiol. 2016, 231, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Falvo, S.; Santillo, A.; Chieffi Baccari, G.; Cioffi, F.; Di Fiore, M.M. d-aspartate and N-methyl-d-aspartate promote proliferative activity in mouse spermatocyte GC-2 cells. Reprod. Biol. 2022, 22, 100601. [Google Scholar] [CrossRef] [PubMed]
- Lasiene, K.; Gedrimas, V.; Vitkus, A.; Glinskyte, S.; Lasys, V.; Valanciute, A.; Sienkiewicz, W. Evaluation of morphological criteria of sperm quality before in vitro fertilization and intracytoplasmic sperm injection. Pol. J. Vet. Sci. 2013, 16, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Macchia, G.; Topo, E.; Mangano, N.; D’Aniello, E.; Boni, R. DL-Aspartic acid administration improves semen quality in rabbit bucks. Anim. Reprod. Sci. 2010, 118, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Zhandi, M.; Kohram, H.; Zaghari, M.; Sadeghi, M.; Sharafi, M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology 2017, 92, 69–74. [Google Scholar] [CrossRef]
- D’Aniello, G.; Ronsini, S.; Notari, T.; Grieco, N.; Infante, V.; D’Angel, N.; Mascia, F.; Di Fiore, M.M.; Fisher, G.; D’Aniello, A. D-Aspartate, a key element for the improvement of sperm quality. Adv. Sex. Med. 2012, 2, 47–53. [Google Scholar] [CrossRef]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef]
- Gualtieri, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Rizos, D.; Longobardi, S.; Talevi, R. Treatment with zinc, d-aspartate, and coenzyme Q10 protects bull sperm against damage and improves their ability to support embryo development. Theriogenology 2014, 82, 592–598. [Google Scholar] [CrossRef]
- Giacone, F.; Condorelli, R.A.; Mongioi, L.M.; Bullara, V.; La Vignera, S.; Calogero, A.E. In vitro effects of zinc, d-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine 2017, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Nikolettos, N.; Kupker, W.; Demirel, C.; Schopper, B.; Blasig, C.; Sturm, R.; Felberbaum, R.; Bauer, O.; Diedrich, K.; Al-Hasani, S. Fertilization potential of spermatozoa with abnormal morphology. Hum. Reprod. 1999, 14 (Suppl. 1), 47–70. [Google Scholar] [CrossRef] [PubMed]
- Demarco, I.A.; Espinosa, F.; Edwards, J.; Sosnik, J.; De La Vega-Beltran, J.L.; Hockensmith, J.W.; Kopf, G.S.; Darszon, A.; Visconti, P.E. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J. Biol. Chem. 2003, 278, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marin-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M. Mammalian fertilization: Molecular aspects of gamete adhesion, exocytosis, and fusion. Cell 1999, 96, 175–183. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Esteves, S.C.; Verza, S.J. Relationship of in Vitro Acrosome Reaction to Sperm Function: An Update. Open Reprod. Sci. J. 2011, 3, 72–84. [Google Scholar] [CrossRef]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm membrane potential: Hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166. [Google Scholar] [CrossRef]
- Aitken, R.J.; Paterson, M.; Fisher, H.; Buckingham, D.W.; van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995, 108 Pt 5, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 1998, 111 Pt 5, 645–656. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Interaction between Ca2+, cyclic 3′,5′ adenosine monophosphate, the superoxide anion, and tyrosine phosphorylation pathways in the regulation of human sperm capacitation. J. Androl. 1998, 19, 434–443. [Google Scholar]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef]
- Kia, H.D.; Vatankhah, S. Effect of Antioxidant d-Aspartic Acid and Thawing Rate on the Freeze-Thawing Process of Ram Semen. Iran. J. Appl. Anim. Sci. 2019, 9, 265–273. [Google Scholar]
- Venditti, M.; Romano, M.Z.; Aniello, F.; Minucci, S. Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis. Animals 2021, 11, 133. [Google Scholar] [CrossRef]

| 9-Week-Old Males | 16-Week-Old Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | D-Asp Treatment | Control | D-Asp Treatment | |||||
| Duration of Treatment (Weeks) | No. of Oocytes | No. of 2-Cell Embryos | No. of Oocytes | No. of 2-Cell Embryos | No. of Oocytes | No. of 2-Cell Embryos | No. of Oocytes | No. of 2-Cell Embryos |
| 0 | 283 | 120 | n.d. | n.d. | 318 | 108 | n.d. | n.d. |
| 2 | 242 | 108 | 260 | 175 | 316 | 93 | 273 | 149 |
| 4 | 346 | 134 | 349 | 166 | 333 | 158 | 322 | 194 |
| Duration of Treatment (Weeks) | 9-Week-Old Males | 16-Week-Old Males | ||
|---|---|---|---|---|
| Control | D-Asp | Control | D-Asp | |
| 2 | 13.8 ± 1.3 | 13.0 ± 4.7 | 30.8 ± 0.2 | 22.4 ± 0 |
| 4 | 7.4 ± 1.1 | 9.1 ± 1.5 | 25.5 ± 3 | 21.7 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raspa, M.; Paoletti, R.; Peltier, M.; Majjouti, M.; Protti, M.; Mercolini, L.; Mahabir, E.; Scavizzi, F. Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice. Animals 2022, 12, 1350. https://doi.org/10.3390/ani12111350
Raspa M, Paoletti R, Peltier M, Majjouti M, Protti M, Mercolini L, Mahabir E, Scavizzi F. Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice. Animals. 2022; 12(11):1350. https://doi.org/10.3390/ani12111350
Chicago/Turabian StyleRaspa, Marcello, Renata Paoletti, Manon Peltier, Mohamed Majjouti, Michele Protti, Laura Mercolini, Esther Mahabir, and Ferdinando Scavizzi. 2022. "Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice" Animals 12, no. 11: 1350. https://doi.org/10.3390/ani12111350
APA StyleRaspa, M., Paoletti, R., Peltier, M., Majjouti, M., Protti, M., Mercolini, L., Mahabir, E., & Scavizzi, F. (2022). Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice. Animals, 12(11), 1350. https://doi.org/10.3390/ani12111350








