Acoustic and Genetic Data Can Reduce Uncertainty Regarding Populations of Migratory Tree-Roosting Bats Impacted by Wind Energy
Abstract
Simple Summary
Abstract
1. Introduction
2. Population Monitoring Using Acoustic Surveys
3. Population Monitoring Using Genetic Approaches
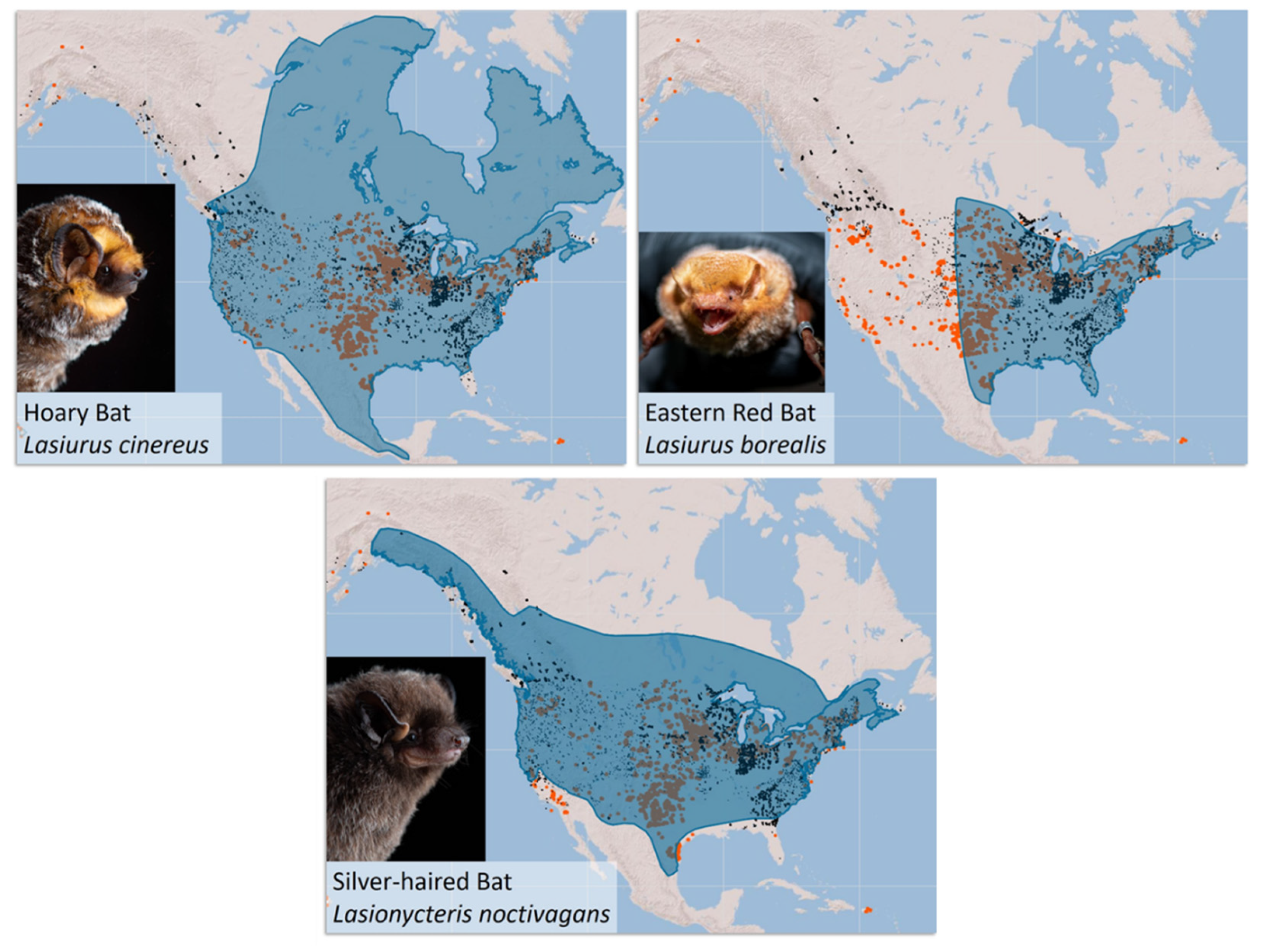
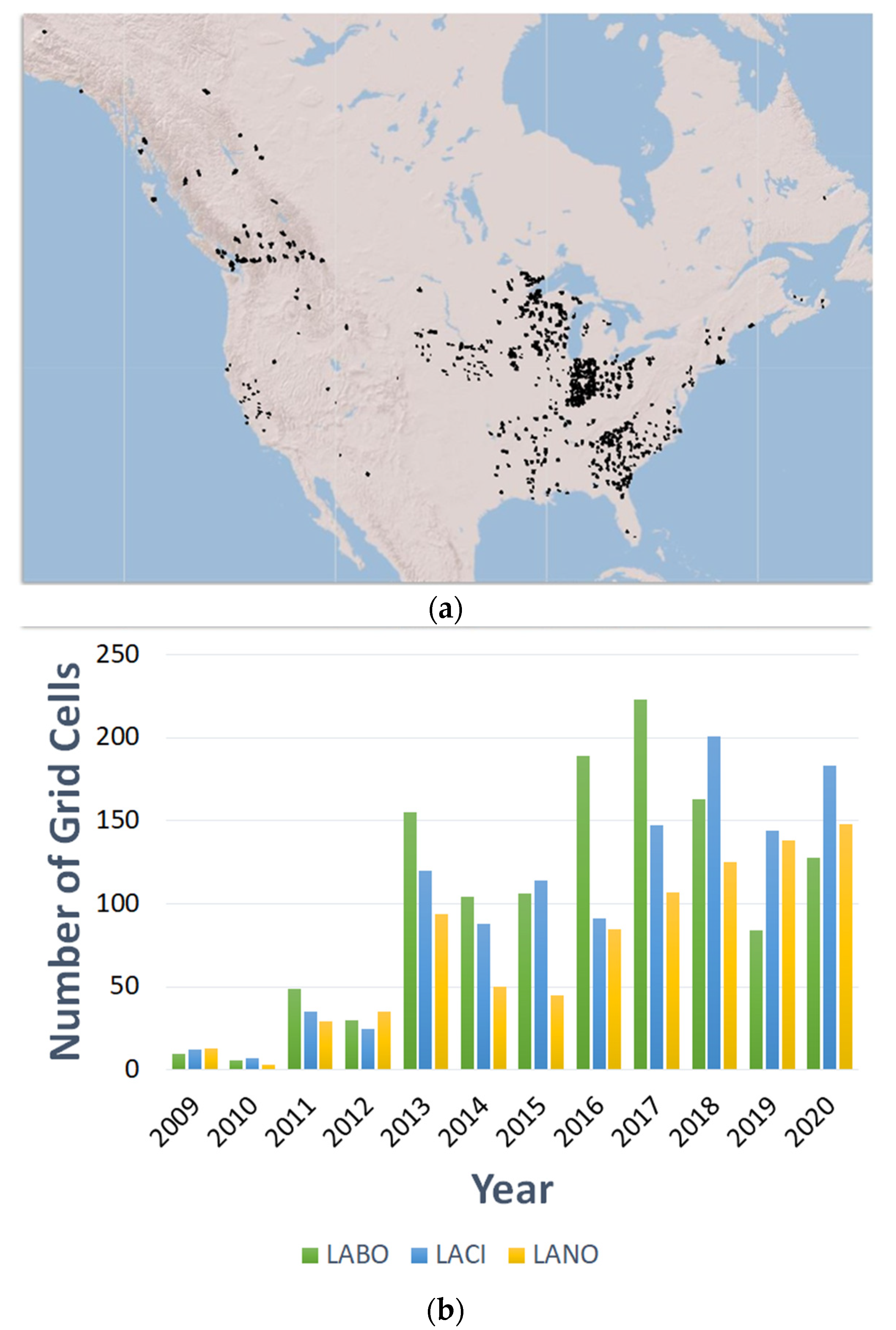
Population Genetic Studies of Migratory Tree-Roosting Bats
4. Recommendations
4.1. Acoustic Monitoring Recommendations
4.2. Genetic Recommendations
4.2.1. Comparative Studies
4.2.2. Monitoring Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.S.; Plowright, R.K.; Steicker, D.G. Multiple mortality events in bats: A global review. Mammal. Rev. 2016, 46, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2019, 1469, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.M.R.; Harder, L.D. Life histories of bats: Life in the slow lane. In Bat Ecology; Kunz, T.H., Fenton, M.B., Eds.; The University of Chicago Press: Chicago, IL, USA, 2003; pp. 209–253. [Google Scholar]
- Podlutsky, A.J.; Khritankov, A.M.; Ovodov, N.D.; Austad, S.N. A new field record for bat longevity. J. Geront. 2005, 60, 1366–1368. [Google Scholar] [CrossRef] [PubMed]
- Findley, J.S. Bats: A Community Perspective; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Henderson, L.E.; Broders, H.G. Movements and resource selection of the northern long-eared myotis (Myotis septentrionalis) in a forest-agriculture landscape. J. Mammal. 2008, 89, 952–963. [Google Scholar] [CrossRef]
- Simmons, N.B.; Cirranello, A.L. Bat Species of the World: A Taxonomic and Geographic Database. 2020. Available online: https://www.batnames.org/ (accessed on 28 September 2021).
- Baird, A.B.; Braun, J.K.; Mares, M.A.; Morales, J.C.; Patton, J.C.; Tran, C.Q.; Bickham, J.W. Molecular systematic revision of tree bats (Lasiurini): Doubling the native mammals of the Hawaiian Islands. J. Mammal. 2015, 96, 1255–1274. [Google Scholar] [CrossRef]
- Baird, A.B.; Braun, J.K.; Engstrom, M.D.; Holbert, A.C.; Huerta, M.G.; Lim, B.K.; Mares, M.A.; Patton, J.C.; Bickham, J.W. Nuclear and mtDNA phylogenetic analyses clarify the evolutionary history of two species of native Hawaiian bats and the taxonomy of Lasiurini (Mammalia: Chiroptera). PLoS ONE 2017, 12, e0186085. [Google Scholar] [CrossRef] [PubMed]
- Arnett, E.B.; Baerwald, E.F. Impacts of wind energy development on bats: Implications for conservation. In Bat Evolution, Ecology and Conservation; Adams, R.A., Pedersen, S.C., Eds.; Springer: New York, NY, USA, 2013; pp. 435–456. [Google Scholar]
- CanWEA. Available online: https://canwea.ca/wind-energy/installed-capacity/ (accessed on 2 November 2021).
- American Clean Power. Available online: https://cleanpower.org/facts/wind-power/ (accessed on 2 November 2021).
- Larson, E.; Greig, C.; Jenkins, J.; Mayfield, E.; Pascale, A.; Zhang, C.; Drossman, J.; Williams, R.; Pacala, S.; Socolow, R.; et al. Net-Zero America: Potential Pathways, Infrastructure, and Impacts, Interim Report; Princeton University: Princeton, NJ, USA, 15 December 2020. [Google Scholar]
- Williams, J.H.; Jones, R.A.; Haley, B.; Kwok, G.; Hargreaves, J.; Farbes, J.; Torn, M.S. Carbon neutral pathways for the United States. AGU Adv. 2021, 2, e2020AV000284. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Bogan, M.A. (Eds.) Monitoring Trends in Bat Populations of the United States and Territories: Problems and Prospects; U. S. Geological Survey, Biological Resources Division, Information and Technology Report: Washington, DC, USA, 2003. [Google Scholar]
- Fleming, T.H. Bat migration. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: New York, NY, USA, 2019; pp. 605–610. [Google Scholar]
- Frick, W.F.; Cheng, T.L.; Langwig, K.E.; Hoyt, J.R.; Janicki, A.F.; Parise, K.L.; Foster, J.T.; Kilpatrick, A.M. Pathogen dynamics during invasion and establishment of white-nose syndrome explain mechanisms of host persistence. Ecology 2017, 98, 624–631. [Google Scholar] [CrossRef]
- Friedenberg, N.A.; Frick, W.F. Assessing fatality minimization for hoary bats amid continued wind energy development. Biol. Cons. 2021, 262, 109309. [Google Scholar] [CrossRef]
- The 2035 Report. Available online: https://www.2035report.com/ (accessed on 2 November 2021).
- O’Donnell, C.F.J. Population dynamics and survivorship in bats. In Ecological and Behavioral Methods for the Study of Bats, 2nd ed.; Kunz, T.H., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 158–176. [Google Scholar]
- Erickson, R.A.; Thogmartin, W.E.; Szymanski, J.A. BatTool: An R package with GUI for assessing the effect of White-nose syndrome and other take events on Myotis spp. of bats. Source Code Biol. Med. 2014, 9, 9. Available online: https://scfbm.biomedcentral.com/articles/10.1186/1751-0473-9-9 (accessed on 27 September 2021). [CrossRef] [PubMed]
- Loeb, S.C.; Rodhouse, T.J.; Ellison, L.E.; Lausen, C.L.; Reichard, J.D.; Irvine, K.M.; Ingersoll, T.E.; Coleman, J.T.H.; Thogmartin, W.E.; Sauer, J.R.; et al. A plan for the North American bat monitoring program (NABat). In U. S. Forest Service General Technical Report SRS-208; United States Department of Agriculture Forest Service, Southern Research Station: Ashville, NC, USA, 2015. [Google Scholar]
- Hoen, B.D.; Diffendorfer, J.E.; Rand, J.T.; Kramer, L.A.; Garrity, C.P.; Hunt, H.E. American Clean Power Association, and Lawrence Berkeley National Laboratory Data Release. U.S. Geol. Surv. 2021. [Google Scholar] [CrossRef]
- North American Bat Monitoring Program (NABat). Database v7.0.0 (Provisional Release): U.S. Geological Survey. 2021. Available online: https://sciencebase.usgs.gov/nabat/#/home (accessed on 27 September 2021).
- NABat Data Inventory. Available online: https://sciencebase.usgs.gov/nabat/#/data/inventory (accessed on 2 November 2021).
- Hein, C.D.; Hale, A.M. Wind energy effects on bats. In Renewable Energy and Wildlife Conservation; Moorman, C.E., Grodsky, S.M., Rupp, S.P., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2019; pp. 122–145. [Google Scholar]
- Hein, C.; Hale, A.; Straw, B. Acoustic and Genetic Approaches for Informing Population Status and Trends of Migratory Tree Bats; NREL/TP-5000-78563; National Renewable Energy Laboratory: Golden, CO, USA, 2019. Available online: https://www.nrel.gov/docs/fy21osti/78563.pdf (accessed on 27 September 2021).
- Loeb, S.C.; Britzke, E.R.; Coleman, J.T.H.; Ellison, L.; Rodhouse, T.; Vonhof, M.; Amelon, S.; Cryan, P.; Hein, C.; Kilpatrick, M.; et al. Bat Population Monitoring and Modeling Workshop: Final Report of Proceedings and Recommendations; U. S. Fish and Wildlife Service: Hadley, MA, USA, 2012. [Google Scholar]
- The North American Bat Monitoring Program. Available online: https://www.nabatmonitoring.org/about-1 (accessed on 2 November 2021).
- Reichert, B.E.; Bayless, M.; Cheng, T.L.; Coleman, J.T.H.; Francis, C.M.; Frick, W.F.; Gotthold, B.S.; Irvine, K.M.; Lausen, C.; Li, H.; et al. NABat: A top-down, bottom-up solution to collaborative continental-scale monitoring. Ambio 2021, 50, 901–913. [Google Scholar] [CrossRef]
- Cochran, W.G. Sampling Techniques, 3rd ed.; Wiley Press: New York, NY, USA, 1977. [Google Scholar]
- Green, R.H. Sampling Design and Statistical Methods for Environmental Biologists; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Olsen, A.R.; Sedransk, J.; Edwards, D.; Gotway, C.A.; Liggett, W.; Rathbun, S.; Reckhow, K.H.; Young, J.J. Statistical issues for monitoring ecological and natural resources in the United States. Environ. Monit. Assess. 1999, 54, 1–45. [Google Scholar] [CrossRef]
- Thompson, S.K. Sampling, 2nd ed.; Wiley Press: New York, NY, USA, 2002. [Google Scholar]
- Gitzen, R.A.; Millspaugh, J.J. Ecological monitoring: The heart of the matter. In Design and Analysis of Long-Term Ecological Monitoring Studies; Gitzen, R.A., Millspaugh, J.J., Cooper, A.B., Licht, D.S., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 3–22. [Google Scholar]
- Stevens, D.L.; Olsen, A.R. Spatially balanced sampling of natural resources. J. Am. Stat. Assoc. 2004, 99, 262–278. [Google Scholar] [CrossRef]
- Banner, K.M.; Irvine, K.M.; Rodhouse, T.J.; Donner, D.; Litt, A.R. Statistical power of dynamic occupancy models to identify temporal change: Informing the North American Bat Monitoring Program. Ecol. Indic. 2019, 150, 166–176. [Google Scholar] [CrossRef]
- Roche, N.; Langton, S.; Aughney, T.; Russ, J.M.; Marnell, F.; Lynn, D.; Catto, C. A car-based monitoring method reveals new information on bat populations and distributions in Ireland. Anim. Conserv. 2011, 14, 642–651. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Academic Press: New York, NY, USA, 2006. [Google Scholar]
- Rodhouse, T.J.; Rodriguez, R.M.; Banner, K.M.; Ormsbee, P.C.; Barnett, J.; Irvine, K.M. Evidence of region-wide bat population decline from long-term monitoring and Bayesian occupancy models with empirically informed priors. Ecol. Evol. 2019, 9, 11078–11088. [Google Scholar] [CrossRef]
- Roche, N.; Catto, C.; Langton, S.; Aughney, T.; Russ, J. Development of a Car-Based Bat Monitoring Protocol for the Republic of Ireland. Irish Wildlife Manuals, No.19; National Parks and Wildlife Service, Department of Environment, Heritage and Local Government: Dublin, Ireland, 2005; 42p. [Google Scholar]
- Hayward, B.; Davis, R. Flight speeds in western bats. J. Mammal. 1964, 45, 236–242. [Google Scholar] [CrossRef]
- Patterson, A.P.; Hardin, J.W. Flight speeds of five species of vespertilionid bats. J. Mammal. 1969, 50, 152–153. [Google Scholar] [CrossRef]
- Kennedy, M.L.; Best, T.L. Flight speed of the gray bat, Myotis grisescens. J. Mammal. 1972, 88, 254–255. [Google Scholar] [CrossRef]
- Schaub, A.; Schnitzler, H.U. Flight and echolocation behavior of three vespertilionid bat species while commuting on flyways. J. Comp. Physiol. A 2007, 193, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Grodzinski, U.; Spiegel, O.; Korine, C.; Holderied, M.W. Context-dependent flight speed: Evidence for energetically optimal flight speed in the bat Pipistrellus kuhlii? J. Anim. Ecol. 2009, 78, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Doser, J.W.; Finley, A.O.; Weed, A.S.; Zipkin, E.F. Integrating automated acoustic vocalization data and point count surveys for estimation of bird abundance. Methods Ecol. Evol. 2021, 12, 1040–1049. [Google Scholar] [CrossRef]
- Kéry, M.; Royle, J. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS, 1st ed.; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Neece, B.D.; Jachowski, D.S.; Loeb, S.C. Implementing and assessing the efficacy of the North American Bat Monitoring Program. J. Fish Wildl. Manag. 2019, 10, 391–409. [Google Scholar] [CrossRef]
- Whitby, M.D.; Carter, T.C.; Britzke, E.R.; Bergeson, S.M. Evaluation of mobile acoustic techniques for bat population monitoring. Acta Chiropterol. 2014, 16, 223–230. [Google Scholar] [CrossRef]
- Fisher-Phelps, M.; Schwilk, D.; Kingston, T. Mobile acoustic transects detect more bat activity than stationary acoustic point counts in a semi-arid and agricultural landscape. J. Arid Environ. 2017, 136, 38–44. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Koepfli, K.-P.; Gooley, R.M. A modern synthesis of mammal conservation genetics. In Conservation Genetics in Mammals—Integrative Research Using Novel Approaches; Ortega, J., Maldonado, J.E., Eds.; Springer: Basel, Switzerland, 2020; pp. 3–12. [Google Scholar]
- McCartney-Melstad, E.; Vu, J.K.; Shaffer, H.B. Genomic data from an endangered amphibian reveal unforeseen consequences of fragmentation by roads. bioRxiv 2018, 306340. [Google Scholar] [CrossRef]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet. Res. Camb. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Schwartz, M.K.; Luikart, G.; Waples, R.S. Genetic monitoring as a promising tool for conservation and management. Trends Ecol. Evol. 2007, 22, 25–33. [Google Scholar] [CrossRef]
- Lage, C.; Kornfield, I. Reduced genetic diversity and effective population size in an endangered Atlantic salmon (Salmo salar) population from Maine, USA. Conserv. Genet. 2006, 7, 91–104. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Allendorf, F.W. Genetics and the conservation of natural populations: Allozymes to genomes. Mol. Ecol. 2017, 26, 420–430. [Google Scholar] [CrossRef]
- Ortega, J.; Maldonado, J.E. (Eds.) Conservation Genetics in Mammals—Integrative Research Using Novel Approaches; Springer: Basel, Switzerland, 2020. [Google Scholar]
- Morin, P.A.; Luikart, G.; Wayne, R.K.; SNP Workshop Group. SNPs in ecology, evolution and conservation. Trends Ecol. Evol. 2004, 19, 208–216. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef]
- Ellegren, H. Genome sequencing and population genomics in non-model organisms. Trends Ecol. Evol. 2014, 29, 51–63. [Google Scholar] [CrossRef]
- Supple, M.A.; Shapiro, B. Conservation of biodiversity in the genomics era. Genome Biol. 2018, 19, 131. [Google Scholar] [CrossRef]
- Forcina, G.; Leonard, J.A. Tools for monitoring genetic diversity in mammals: Past, present, and future. In Conservation Genetics in Mammals—Integrative Research Using Novel Approaches; Ortega, J., Maldonado, J.E., Eds.; Springer: Basel, Switzerland, 2020; pp. 13–28. [Google Scholar]
- Coates, D.J.; Byrne, M.; Moritz, C. Genetic diversity and conservation units: Dealing with the species-population continuum in the age of genomics. Front. Ecol. Evol. 2018, 6, 165. [Google Scholar] [CrossRef]
- Korstian, J.M.; Hale, A.M.; Williams, D.A. High genetic diversity, large historic population size, and lack of population structure in 2 North American tree bats. J. Mammal. 2015, 96, 972–980. [Google Scholar] [CrossRef]
- Vonhof, M.J.; Russell, A.L. Genetic approaches to the conservation of migratory bats: A study of the eastern red bat (Lasiurus borealis). PeerJ 2015, 3, e983. [Google Scholar] [CrossRef] [PubMed]
- Pylant, C.L.; Nelson, D.M.; Fitzpatrick, M.C.; Gates, J.E.; Keller, S.R. Geographic origins and population genetics of bats killed at wind-energy facilities. Ecol. Appl. 2016, 26, 1381–1395. [Google Scholar] [CrossRef]
- Sovic, M.G.; Carstens, B.C.; Gibbs, H.L. Genetic diversity in migratory bats: Results from RADseq data for three tree bat species at an Ohio windfarm. PeerJ 2016, 4, e1647. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, L.K.; Lee, D.N.; Jones, B.A.; Holt, M.P.; Harrison, S.J.; Decker, S.K. High frequency of multiple paternity in eastern red bats, Lasiurus borealis, based on microsatellite analysis. J. Heredity 2019, 110, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Sugg, D.W.; Chesser, R.K. Effective population sizes with multiple paternity. Genetics 1994, 137, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.E.; Anderson, E.C. Multiple paternity increases effective population size. Mol. Ecol. 2009, 18, 3124–3127. [Google Scholar] [CrossRef]
- Gregory, A.J.; Kaler, R.S.A.; Prebyl, T.J.; Sandercock, B.K.; Wisely, S.M. Influence of translocation strategy and mating system on the genetic structure of a newly established population of island ptarmigan. Conserv. Genet. 2012, 3, 465–474. [Google Scholar] [CrossRef]
- Cornman, R.S.; Fike, J.A.; Oyler-McCance, S.J.; Cryan, P.M. Historical effective population size of North American hoary bat (Lasiurus cinereus) and challenges to estimating trends in contemporary effective breeding population size from archived samples. PeerJ 2021, 9, e11285. [Google Scholar] [CrossRef]
- Wang, J.; Santiago, E.; Caballero, A. Prediction and estimation of effective population size. Heredity 2016, 117, 193–206. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.M.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef] [PubMed]
- U.S. Fish and Wildlife Service. U.S. Fish and Wildlife Service Land-Based Wind Energy Guidelines. 2012. Available online: https://www.fws.gov/ecological-services/es-library/pdfs/WEG_final.pdf (accessed on 27 September 2021).
- Mc Overton, J.C.; Young, T.C.; Overton, W.S. Using ‘found’ data to augment a probability sample: Procedure and case study. Environ. Monit. Assess. 1993, 26, 65–83. [Google Scholar] [CrossRef]
- New York State Energy and Research Development Authority (NYSERDA). Wildlife Data Standardization and Sharing: Environmental Data Transparency for New York State Offshore Wind Energy. NYSERDA Report 21-11; Biodiversity Research Institute: Portland, ME, USA, 2021. Available online: www.nyserda.ny.gov/publications (accessed on 27 September 2021).
- Solick, D.; Pham, D.; Nasman, K.; Bay, K. Bat activity rates do not predict bat fatality rates at wind energy facilities. Acta Chiropterologica 2020, 22, 135–146. [Google Scholar] [CrossRef]
- Richardson, S.M.; Lintott, P.R.; Hosken, D.J.; Economou, T.; Mathews, F. Peaks in bat activity at turbines and the implications for mitigating the impact of wind energy developments on bats. Sci. Rep. 2021, 11, 3636. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Range-Wide Indiana Bat Survey Guidelines. 2020. Available online: https://www.fws.gov/midwest/Endangered/mammals/inba/inbasummersurveyguidance.html (accessed on 27 September 2021).
- Bergner, L.M.; Dussex, N.; Jamieson, I.G.; Robertson, B.C. European colonization, not Polynesian arrival, impacted population size and genetic diversity in the critically endangered New Zealand kākāpō. J. Hered. 2016, 107, 597–602. [Google Scholar] [CrossRef]
- Cammen, K.M.; Vincze, S.; Heller, A.S.; McLeod, B.A.; Wood, S.A.; Bowen, W.D.; Hammill, M.O.; Puryear, W.B.; Runstadler, J.; Wenzel, F.W.; et al. Genetic diversity from pre-bottleneck to recovery in two sympatric pinniped species in the Northwest Atlantic. Conserv. Genet. 2018, 19, 555–569. [Google Scholar] [CrossRef]
- Chipps, A.S.; Hale, A.M.; Weaver, S.P.; Williams, D.A. Genetic diversity, population structure, and effective population size in two yellow bats species in south Texas. PeerJ 2020, 8, e10348. [Google Scholar] [CrossRef]
- Chipps, A.S.; Hale, A.M.; Weaver, S.P.; Williams, D.A. Genetic approaches are necessary to accurately understand bat-wind turbine impacts. Diversity 2020, 12, 236. [Google Scholar] [CrossRef]
- Korstian, J.M.; Hale, A.M.; Bennett, V.J.; Williams, D.A. Advances in sex determination in bats and its utility in wind-wildlife studies. Mol. Ecol. Resour. 2013, 13, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Nagel, J.; Trott, R.; Campbell, C.J.; Pruitt, L.; Good, R.E.; Iskali, G.; Gugger, P.F. Carcass age and searcher identity affect morphological assessment of sex of bats. J. Wild. Man. 2018, 82, 1582–1587. [Google Scholar] [CrossRef]
- Korstian, J.M.; Hale, A.M.; Bennett, V.J.; Williams, D.A. Using DNA barcoding to improve bat carcass identification at wind farms in the United States. Conserv. Genet. Resour. 2016, 8, 27–34. [Google Scholar] [CrossRef]
- Geluso, K.; Valdez, E.W. First Records of the Eastern Red Bat (Lasiurus borealis) in Arizona, Utah, and western New Mexico; Museum of Texas Tech University: Lubbock, TX, USA, 2019; Occasional Papers; Number 361. [Google Scholar]
- Zabriskie, J.E.; Cutler, P.L.; Stuart, J.N. Range extension of the western yellow bat (Dasypterus xanthinus) in New Mexico. West. Wildl. 2019, 6, 1–4. [Google Scholar]
- Decker, S.K.; Krejsa, D.M.; Lindsey, L.L.; Amoateng, R.P.; Ammerman, L.K. Updated distributions of three species of yellow bat (Dasypterus) in Texas based on specimen records. West. Wildl. 2020, 7, 2–8. [Google Scholar]
- Solick, D.I.; Barclay, R.M.R.; Bishop-Boros, L.; Hays, Q.R.; Lausen, C.L. Distributions of eastern and western red bats in western North America. West. NA Nat. 2020, 80, 90–97. [Google Scholar] [CrossRef]
- Andersen, B.R.; Geluso, K.; Otto, H.W.; Bishop-Boros, L. Westward expansion of the evening bat (Nycticeius humeralis) in the United States, with notes on the first record from New Mexico. West. N. Am. Nat. 2017, 77, 223–229. [Google Scholar] [CrossRef][Green Version]
- Ommundsen, P.; Lausen, C.; Matthias, L. First acoustic records of the Brazilian free-tailed bat (Tadarida brasiliensis) in British Columbia. Northwestern Nat. 2017, 98, 132–136. [Google Scholar] [CrossRef]
- McCracken, G.F.; Bernard, R.F.; Gamba-Rios, M.; Wolfe, R.; Krauel, J.J.; Jones, D.N.; Russell, A.L.; Brown, V.A. Rapid range expansion of the Brazilian free-tailed bat in the southeastern United States, 2008–2016. J. Mammal. 2018, 99, 312–320. [Google Scholar] [CrossRef]
- Perry, R. Migration and recent range expansion of Seminole bats (Lasiurus seminolus) in the United States. J. Mammal. 2018, 99, 1478–1485. [Google Scholar] [CrossRef]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal Rev. 2012, 43, 171–182. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conser. 2019, 232, 8–27. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hale, A.M.; Hein, C.D.; Straw, B.R. Acoustic and Genetic Data Can Reduce Uncertainty Regarding Populations of Migratory Tree-Roosting Bats Impacted by Wind Energy. Animals 2022, 12, 81. https://doi.org/10.3390/ani12010081
Hale AM, Hein CD, Straw BR. Acoustic and Genetic Data Can Reduce Uncertainty Regarding Populations of Migratory Tree-Roosting Bats Impacted by Wind Energy. Animals. 2022; 12(1):81. https://doi.org/10.3390/ani12010081
Chicago/Turabian StyleHale, Amanda M., Cris D. Hein, and Bethany R. Straw. 2022. "Acoustic and Genetic Data Can Reduce Uncertainty Regarding Populations of Migratory Tree-Roosting Bats Impacted by Wind Energy" Animals 12, no. 1: 81. https://doi.org/10.3390/ani12010081
APA StyleHale, A. M., Hein, C. D., & Straw, B. R. (2022). Acoustic and Genetic Data Can Reduce Uncertainty Regarding Populations of Migratory Tree-Roosting Bats Impacted by Wind Energy. Animals, 12(1), 81. https://doi.org/10.3390/ani12010081





