First Integrative Morphological and Genetic Characterization of Tremoctopus violaceussensu stricto in the Mediterranean Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphometric and Biological Analysis
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Molecular Analyses
3. Results
3.1. Morphometric and Biological Results
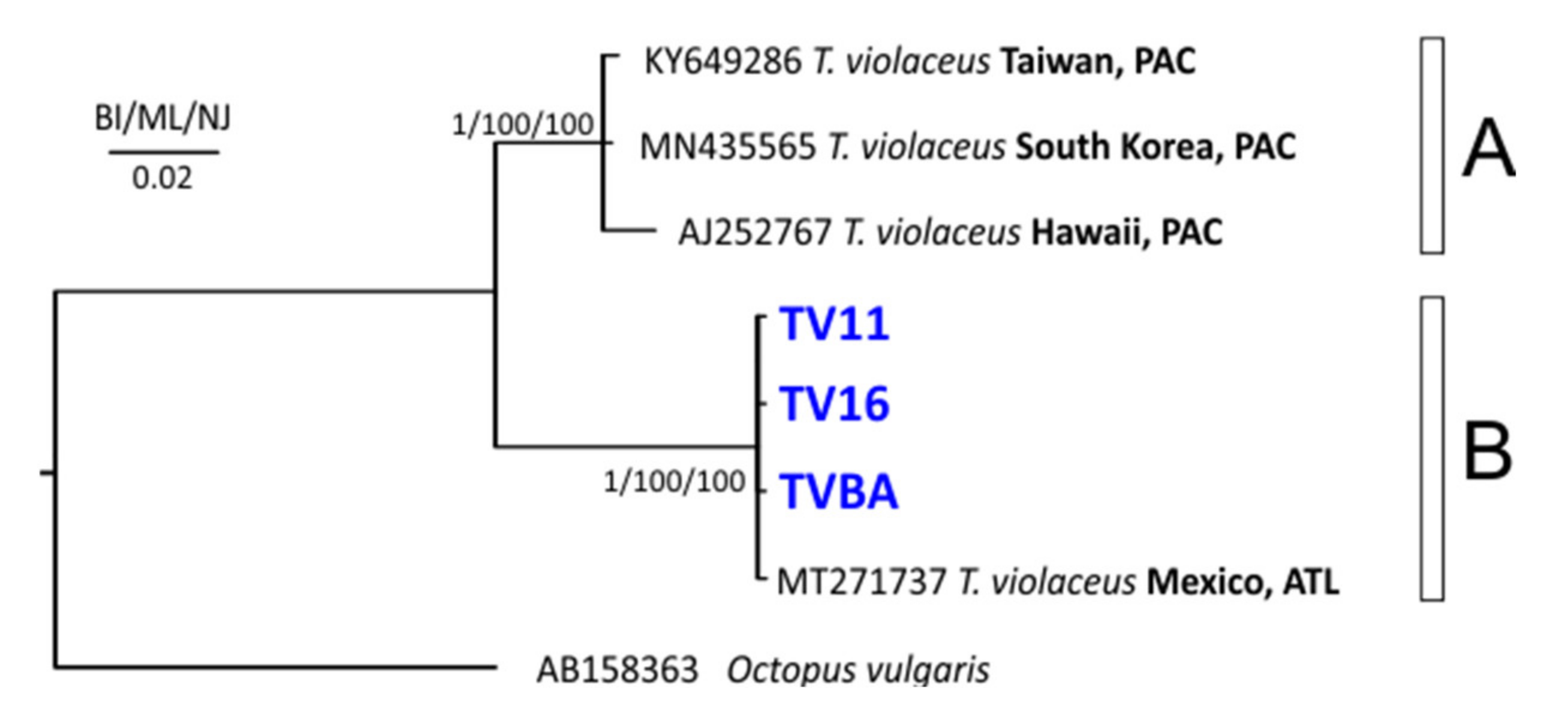
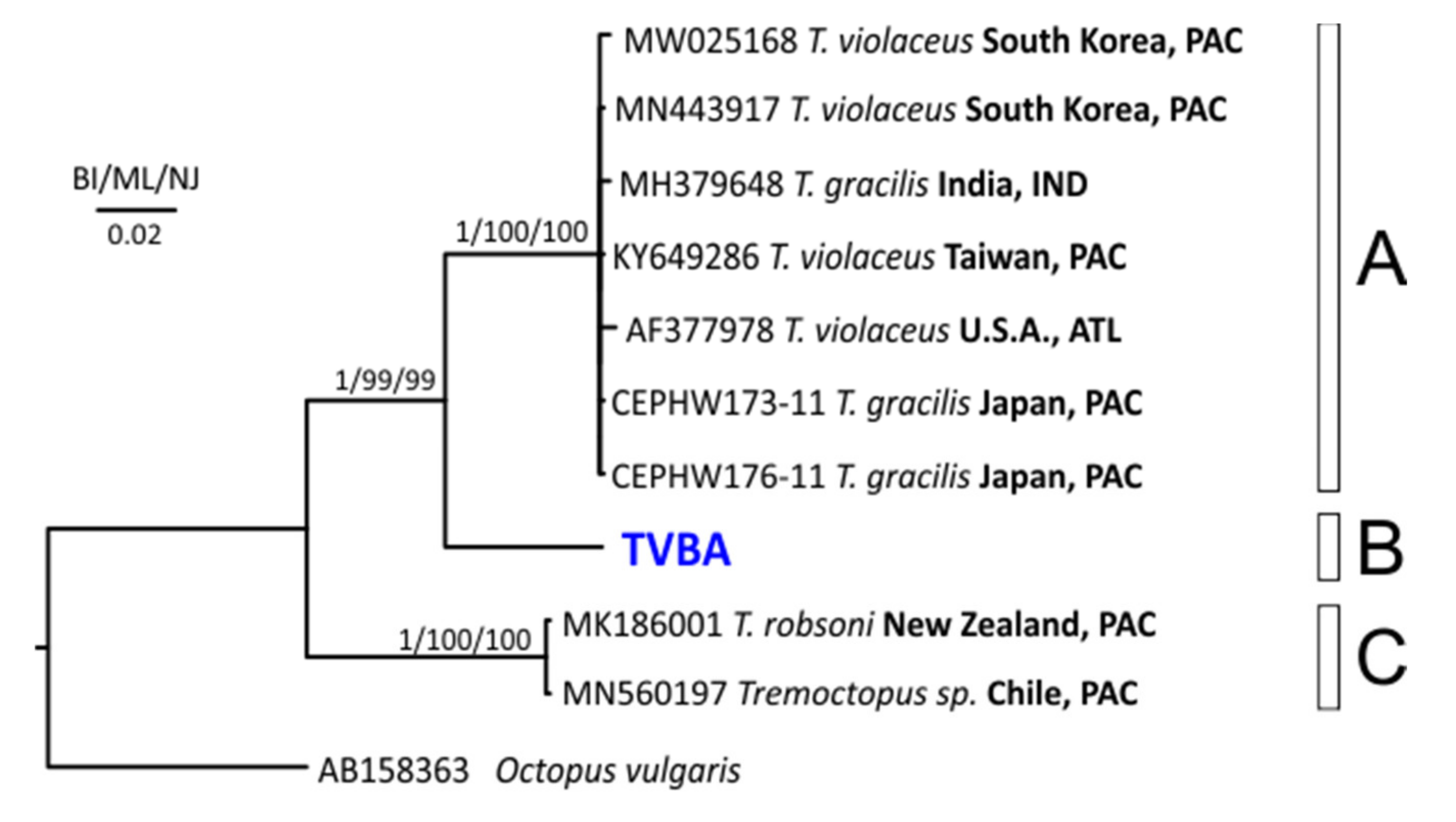
3.2. Molecular Analysis
4. Discussion
4.1. Morphometric and Biological Results
4.2. Molecular Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portmann, A. Les bras dorsaux de Tremoctopus violaceus Delle Chiaje. Rev. Suisse. Zool. 1952, 59, 288–293. [Google Scholar] [CrossRef]
- Thomas, R.F. Systematics, distribution, and biology of cephalopods of the genus Tremoctopus (Octopoda: Tremoctopodidae). Bull. Mar. Sci. 1977, 27, 353–392. [Google Scholar]
- Naef, A. Cephalopoda. Fauna and Flora of the Bay of Naples; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 1923; pp. 1–917. [Google Scholar]
- Norman, M. Cephalopods. A World Guide: Octopuses. Argonauts. Cuttlefish. Squid. Nautilus; Conchbooks: Harxheim, Germany, 2000; p. 320. [Google Scholar]
- Finn, J.K. Family Tremoctopodidae. In Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date (Vol. 3). Octopods and Vampire Squids; Jereb, P., Roper, C.F.E., Norman, M.D., Finn, J.K., Eds.; FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2016; pp. 240–243. Available online: http://www.fao.org/3/a-i3489e (accessed on 30 August 2021).
- Mangold, V. Tremoctopodidae Tryon, 1879. Tremoctopus Chiaje, 1830. Blanket octopus. Version 29 March 2018. Available online: http://tolweb.org/Tremoctopus/20202/2018.03.29 (accessed on 30 August 2021).
- Jiménez-Badillo, M.L.; Meiners-Mandujano, C.; Galindo-Crtes, G.; Morillo-Velarde, P.S.; González-Gómez, R.; Barriga-Sosa, I.A.; Pliego-Cárdenas, R. The first record of Tremoctopus violaceus sensu stricto Delle Chiaje, 1830 in southwestern Gulf of Mexico gives a hint of the taxonomic status of Tremoctopus gracilis. ZooKeys 2021, 1012, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Orsi Relini, L.; Belluscio, A.; Ardizzone, G.D. Tracking the Indopacific pelagic octopus Tremoctopus gracilis in the Mediterranean. Rapp. Comm. Int. Mer Médit. 2004, 37, 415. [Google Scholar]
- Orsi Relini, L. Notes about colour display observed in female specimens of Tremoctopus (Cephalopoda: Octopoda) and their taxonomic value. Boll. Malacol. 2009, 45, 13–16. [Google Scholar]
- Bello, G.; Andaloro, F.; Battaglia, P. Non-indigenous cephalopods in the Mediterranean Sea: A review. Acta Adriat. 2020, 61, 113–134. [Google Scholar] [CrossRef]
- Zenetos, A.; Galanidi, M. Mediterranean non indigenous species at the start of the 2020s: Recent changes. Mar. Biodivers. Rec. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Biagi, V. Spiaggiamenti di cefalopodi sulla costa livornese. Quad. Mus. Stor. Nat. Livorno 1984, 5, 99–115. [Google Scholar]
- Biagi, V.; Bertozzi, A. Presenza stagionale di Tremoctopus violaceus Delle Chiaje, 1830 (Cephalopoda: Octopoda) nel mare di Piombino. Boll. Malacol. 1992, 28, 47–54. [Google Scholar]
- Laptikhovsky, V.; Salman, A. On reproductive strategies of the epipelagic octopods of the superfamily Argonautoidea (Cephalopoda: Octopoda). Mar. Biol. 2003, 142, 321–326. [Google Scholar] [CrossRef]
- Mereu, M.; Agus, B.; Cau, A.L.; Cuccu, D. On a female of Tremoctopus sp. (Octopoda: Tremoctopodidae) caught in the Sardinian Sea. Biol. Mar. Mediterr. 2012, 19, 214–215. [Google Scholar]
- Bello, G. Tremoctopus violaceus (Cephalopoda: Tremoctopodidae) in the stomach content of a swordfish from the Adriatic Sea. Boll. Malacol. 1993, 29, 45–48. [Google Scholar]
- Kramer, C. Einige Beobachten an Tremoctopus violaceus. Note Ist. Italo-Germanico Biol. Mar. Rovigno 1937, 25, 1–11. [Google Scholar]
- Belluscio, A.; Ardizzone, G.; Conticelli, M.; Pellicciari, C. Prima documentazione fotografica di una femmina ovigera di Tremoctopus sp. (Octopoda, Tremoctopodidae) nel Mediterraneo. Biol. Mar. Mediterr. 2004, 11, 556–559. [Google Scholar]
- Rifi, M.; Ben Souissi, J. Première mention du poulpe palmée Tremoctopus gracilis (Eydoux and Souleyet, 1852) dans le Golfe de Tunis. In Proceedings of the 4ème Congrès Franco-Maghrébin et 5èmes Journées FrancoTunisiennes de Zoologie 2014, Korba, Tunisia, 19 November 2014. [Google Scholar]
- Ounifi-Ben Amor, K.; Rifi, M.; Ghanem, R.; Draeif, I.; Zaouali, J.; Ben Souissi, J. Update of alien fauna and new records from Tunisian marine waters. Mediterr. Mar. Sci. 2016, 17, 124–143. [Google Scholar] [CrossRef]
- Roper, C.F.E.; Voss, G.L. Guidelines for taxonomic descriptions of cephalopod species. Mem. Natl. Mus. Victoria 1983, 44, 49–63. [Google Scholar] [CrossRef]
- AAVV. MEDITS-Handbook. MEDITS Working Group. Version n°9 2017. Available online: https://www.sibm.it/MEDITS%202011/principaledownload.htm (accessed on 2 November 2020).
- Follesa, M.C.; Agus, B.; Bellodi, A.; Cannas, R.; Capezzuto, F.; Casciaro, L.; Cau, A.L.; Cuccu, D.; Donnaloia, M.; Fernandez-Arcaya, U.; et al. The MEDITS maturity scales as a useful tool for investigating the reproductive traits of key species in the Mediterranean Sea. Sci. Mar. 2019, 83 (Suppl. 1), 235–256. [Google Scholar] [CrossRef]
- Nigmatullin, C.H.M.; Markaida, U. Oocyte development, fecundity and spawning strategy of large sized jumbo squid Dosidicus gigas. J. Mar. Biol. Assoc. U.K. 2009, 89, 789–801. [Google Scholar] [CrossRef]
- Rasero, M.; González, Á.F.; Castro, B.G.; Guerra, Á. Predatory relationships of two sympatric squid, Todaropsis eblanae and Illex coindetii (Cephalopoda: Ommastrephidae) in Galician waters. J. Mar. Biol. Assoc. U. K. 1996, 76, 73–87. [Google Scholar] [CrossRef][Green Version]
- Clarke, M.R. A Handbook for The Identification of Cephalopod Beaks; Clarendon Press: Oxford, UK, 1986; p. 273. [Google Scholar]
- Hernández-López, J.L.; Castro-Hernández, J.J.; Hernández-García, V. Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fish. Bull. 2001, 99, 679–684. [Google Scholar]
- Chang, W.Y.B. A Statistical Method for Evaluating the Reproducibility of Age Determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A Method for Comparing the Precision of a Set of Age Determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0.; Department of Zoology and Kewalo Marine Laboratory, University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Hӧhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Yokobori, S.; Fukuda, N.; Nakamura, M.; Aoyama, T.; Oshima, T. Long-term conservation of six duplicated structural genes in cephalopod mitochondrial genomes. Mol. Biol. Evol. 2004, 21, 2034–2046. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Dai, L.; Zheng, X.; Kong, L.; Li, Q. DNA barcoding analysis of Coleoidea (Mollusca: Cephalopoda) from Chinese waters. Mol. Ecol. Resour. 2012, 12, 437–447. [Google Scholar] [CrossRef]
- Nesis, K. Cephalopods of the World; TFH Publications: Neptune City, NJ, USA, 1987; p. 351. [Google Scholar]
- Voss, G.L.; Williamson, G.R. Cephalopods of Hong Kong; Hong Kong Government Press: Hong Kong, China, 1971; p. 138.
- Quetglas, A.; Ordines, F.; González, M.; Zaragoza, N.; Mallol, S.; Valls, M.; De Mesa, A. Uncommon pelagic and deep-sea cephalopods in the Mediterranean: New data and literature review. Mediterr. Mar. Sci. 2013, 14, 69–85. [Google Scholar] [CrossRef][Green Version]
- Robson, G.C. A Monograph of The Recent Cephalopoda. Part II. The Octopoda; Oxford University Press: Oxford, UK, 1932; p. 359. [Google Scholar]
- Cuccu, D.; Mereu, M.; Cannas, R.; Follesa, M.C.; Cau, A.; Jereb, P. Morphology, biology and molecular characterizations of Opisthoteuthis calypso (Cephalopoda: Octopoda) from the Sardinian Channel (Central Western Mediterranean). J. Mar. Biol. Assoc. 2009, 89, 1709–1715. [Google Scholar] [CrossRef]
- Cuccu, D.; Mereu, M.; Follesa, M.C.; Deiana, A.M.; Cau, A. Bathypolypus sponsalis (Cephalopoda: Octopoda) from the central western Mediterranean Sea. J. Mar. Biol. Assoc. 2011, 91, 549–553. [Google Scholar] [CrossRef]
- Cuccu, D.; Mereu, M.; Porcu, C.; Follesa, M.C.; Cau, A.L.; Cau, A. Development of sexual organs and fecundity in Octopus vulgaris Cuvier, 1797 from the Sardinian waters (Mediterranean Sea). Mediterr. Mar. Sci. 2013, 14, 270–277. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, A.; Rosas, C.; Méndez-Loeza, I.; Markaida, U. Validation of growth increments in stylet, beaks and lenses as ageing tools in Octopus maya. J. Exp. Mar. Biol. Ecol. 2013, 449, 194–199. [Google Scholar] [CrossRef]
- Guerra, A.; Roura, A.; Gonzalez, A.F.; Pascual, S.; Cherel, Y.; Perez-Losada, M. Morphological and genetic evidence that Octopus vulgaris Cuvier, 1797 inhabits Amsterdam and Saint Paul Islands (southern Indian Ocean). ICES J. Mar. Sci. 2010, 67, 1401–1407. [Google Scholar] [CrossRef]
- Pliego-Cárdenas, R.; Hochberg, F.G.; León, F.J.G.D.; Barriga-Sosa, I.D.L.A. Close Genetic Relationships between Two American Octopuses: Octopus hubbsorum Berry, 1953, and Octopus mimus Gould, 1852. J. Shellfish Res. 2014, 33, 293–303. [Google Scholar] [CrossRef]
- Jereb, P.; Cannas, R.; Maiorano, P.; Bello, G.; Garibaldi, F.; Mereu, M.; Ancona, F.G.; Ammendolia, G.; Battaglia, P.; Duysak, Ö.; et al. The deep-water squid Octopoteuthis sicula Ruppell, 1844 (Cephalopoda: Octopoteuthidae) as the single species of the genus occurring in the Mediterranean Sea. Mar. Biol. 2016, 163, 1–18. [Google Scholar] [CrossRef]
- Melis, R.; Vacca, L.; Cuccu, D.; Mereu, M.; Cau, A.L.; Follesa, M.C.; Cannas, R. Genetic population structure and phylogeny of the common octopus Octopus vulgaris Cuvier, 1797 in the western Mediterranean Sea through nuclear and mitochondrial markers. Hydrobiologia 2018, 807, 277–296. [Google Scholar] [CrossRef]
- Agus, B.; Carugati, L.; Bellodi, A.; Cannas, R.; Cau, A.L.; Cera, J.; Coluccia, E.; Melis, R.; Ruiu, S.; Cuccu, D. Molecular and Biological Analysis on Ommastrephes caroli Findings in the Central Western Mediterranean Sea (Sardinian Waters) Including First Age Investigation Using Eye Lenses and Beaks. Front. Mar. Sci. 2021, 8, 683856. [Google Scholar] [CrossRef]
- Delle Chiaje, S. Memorie Sulla Storia e Notomia Degli Animali Senza Vertebre del Regno di Napoli. Vol. 3. Stamp; Società Tipografica: Napoli, Italy, 1830; p. 273. [Google Scholar]
- Braid, H.E.; Bolstad, K.S. Cephalopod biodiversity of the Kermadec Islands: Implications for conservation and some future taxonomic priorities. Invertebr. Syst. 2019, 33, 402–425. [Google Scholar] [CrossRef]
- Carugati, L.; Melis, R.; Cariani, A.; Cau, A.; Crobe, V.; Ferrari, A.; Follesa, M.C.; Geraci, M.L.; Iglésias, S.P.; Pesci, P.; et al. Combined COI barcode-based methods to avoid mislabelling of threatened species of deep-sea skates. Anim. Conserv. 2021. [Google Scholar] [CrossRef]
- Pereira, R.J.; Ruiz-Ruano, F.J.; Thomas, C.J.; Pérez-Ruiz, M.; Jiménez-Bartolomé, M.; Liu, S.; De la Torre, J.; Bella, J.L. Mind the numt: Finding informative mitochondrial markers in a giant grasshopper genome. J. Zool. Syst. Evol. Res. 2021, 59, 635–645. [Google Scholar] [CrossRef]
- Carlini, D.B.; Young, R.E.; Vecchione, M. A Molecular Phylogeny of the Octopoda (Mollusca: Cephalopoda) Evaluated in Light of Morphological Evidence. Mol. Phylogenet. Evol. 2001, 21, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-W.; Chang, C.-W.; Shen, K.-N.; Ju, Y.-M.; Lin, H.-D. Complete mitochondrial genome and the phylogenetic position of the pelagic octopus Tremoctopus violaceus (Mollusca: Tremoctopodidae). Mitochondrial DNA B Resour. 2018, 3, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.A.; Varela, A.I.; Ibáñez, C.M.; Sellanes, J.; Thiel, M. Paralarval and juvenile stages as a proxy for cephalopod diversity in the Juan Fernández and Desventuradas ecoregion, southeast Pacific Ocean. Bull. Mar. Sci. 2020, 96, 263–280. [Google Scholar] [CrossRef]

| Sample code | TV11 | TV16 | TVBA |
|---|---|---|---|
| Locality | Sardinian waters | Sardinian waters | Adriatic Sea |
| Date | 15 September 2011 | 24 November 2015 | 16 June 2017 |
| Total weight | 875.0 | 93.0 | 106.0 |
| Sex | Female | Female | Female |
| Dorsal mantle length | 165.0 | 81.5 | 76.6 |
| Ventral mantle length | 100.0 | 53.2 | 62.5 |
| Mantle width (index %) | 130.0 (78.8) | 48.5 (59.5) | 49.9 (65.1) |
| Head length (index %) | 61.0 (37.0) | 22.6 (27.7) | 28.1 (36.7) |
| Head width (index %) | 105.0 (63.6) | 44.6 (54.7) | 40.5 (52.9) |
| Dorsal pore size (left/right) | 15.5 × 11.8/15.5 × 10.1 | 7.6 × 4.6/7.2 × 4.6 | 7.4 × 5.1/7.2 × 4.9 |
| Ventral pore size (left/right) | 8.8 × 5.1/8.8 × 4.9 | 6.5 × 4.3/6.1 × 4.3 | 4.5 × 3.8/4.1 × 3.6 |
| Arm length I (left/right) | 80 **/84 ** | 128 */127 * | 92 */103 * |
| Arm length II (left/right) | -/140 ** | 231/241 | 159 */189 |
| Arm length III (left/right) | 86 **/88 ** | 99/98 | 93/90 |
| Arm length IV (left/right) | 70 **/90 ** | 137/137 | 114/113 |
| Number gill lamellae | 13 | 13 | 13 |
| Maturity condition | Maturing (mated) | Immature | Developing |
| Ovary oocytes size (median) | 0.06–0.70 (0.35) | 0.14–0.46 (0.26) | 0.16–0.41 (0.26) |
| Ovary potential fecundity | 105,758 | 20,140 | 11,237 |
| Stylets size | 12.81 × 2.20 | 12.78 × 2.45 | 10.10 × 2.00 |
| Crest length (upper/lower beaks) | 16.9/11.03 | 9.37/5.28 | 9.13/5.71 |
| Hood length (upper/lower beaks) | 12/8.27 | 5.53/2.53 | 5.05/3.18 |
| Wing length (upper/lower beaks) | 8.22/10 | 4.38/3.8 | 4.31/4.0 |
| LWA (upper beak) | 13.2 | 6.16 | 7.17 |
| JAD (upper/lower beaks) | 9.52/7.3 | 4.33/4.45 | 5.16/4.72 |
| BL (lower beak) | 13.8 | 9.53 | 9.31 |
| Stomach fullness index | 0 | 1 | 1 |
| Beak increments (mean ± SD; unit: day) Coefficient of variation (%) Average percent error | 161 ± 0.84 0.1 0.40 | 92 ± 1.40 0.1 0.87 | 74 ± 1.00 0.1 1.08 |
| COI | Clade A | Clade B | Clade C |
|---|---|---|---|
| Clade A | 0.00198 | ||
| Clade B | 0.08867 | na | |
| Clade C | 0.14282 | 0.14661 | 0.00000 |
| 16S | Clade A | Clade B | |
| Clade A | 0.00576 | ||
| Clade B | 0.06393 | 0.00000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agus, B.; Carbonara, P.; Melis, R.; Cannas, R.; Carugati, L.; Cera, J.; Donnaloia, M.; Mulas, A.; Pais, A.; Ruiu, S.; et al. First Integrative Morphological and Genetic Characterization of Tremoctopus violaceussensu stricto in the Mediterranean Sea. Animals 2022, 12, 80. https://doi.org/10.3390/ani12010080
Agus B, Carbonara P, Melis R, Cannas R, Carugati L, Cera J, Donnaloia M, Mulas A, Pais A, Ruiu S, et al. First Integrative Morphological and Genetic Characterization of Tremoctopus violaceussensu stricto in the Mediterranean Sea. Animals. 2022; 12(1):80. https://doi.org/10.3390/ani12010080
Chicago/Turabian StyleAgus, Blondine, Pierluigi Carbonara, Riccardo Melis, Rita Cannas, Laura Carugati, Jacopo Cera, Marilena Donnaloia, Antonello Mulas, Antonio Pais, Stefano Ruiu, and et al. 2022. "First Integrative Morphological and Genetic Characterization of Tremoctopus violaceussensu stricto in the Mediterranean Sea" Animals 12, no. 1: 80. https://doi.org/10.3390/ani12010080
APA StyleAgus, B., Carbonara, P., Melis, R., Cannas, R., Carugati, L., Cera, J., Donnaloia, M., Mulas, A., Pais, A., Ruiu, S., Vinci, G., & Cuccu, D. (2022). First Integrative Morphological and Genetic Characterization of Tremoctopus violaceussensu stricto in the Mediterranean Sea. Animals, 12(1), 80. https://doi.org/10.3390/ani12010080









