Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
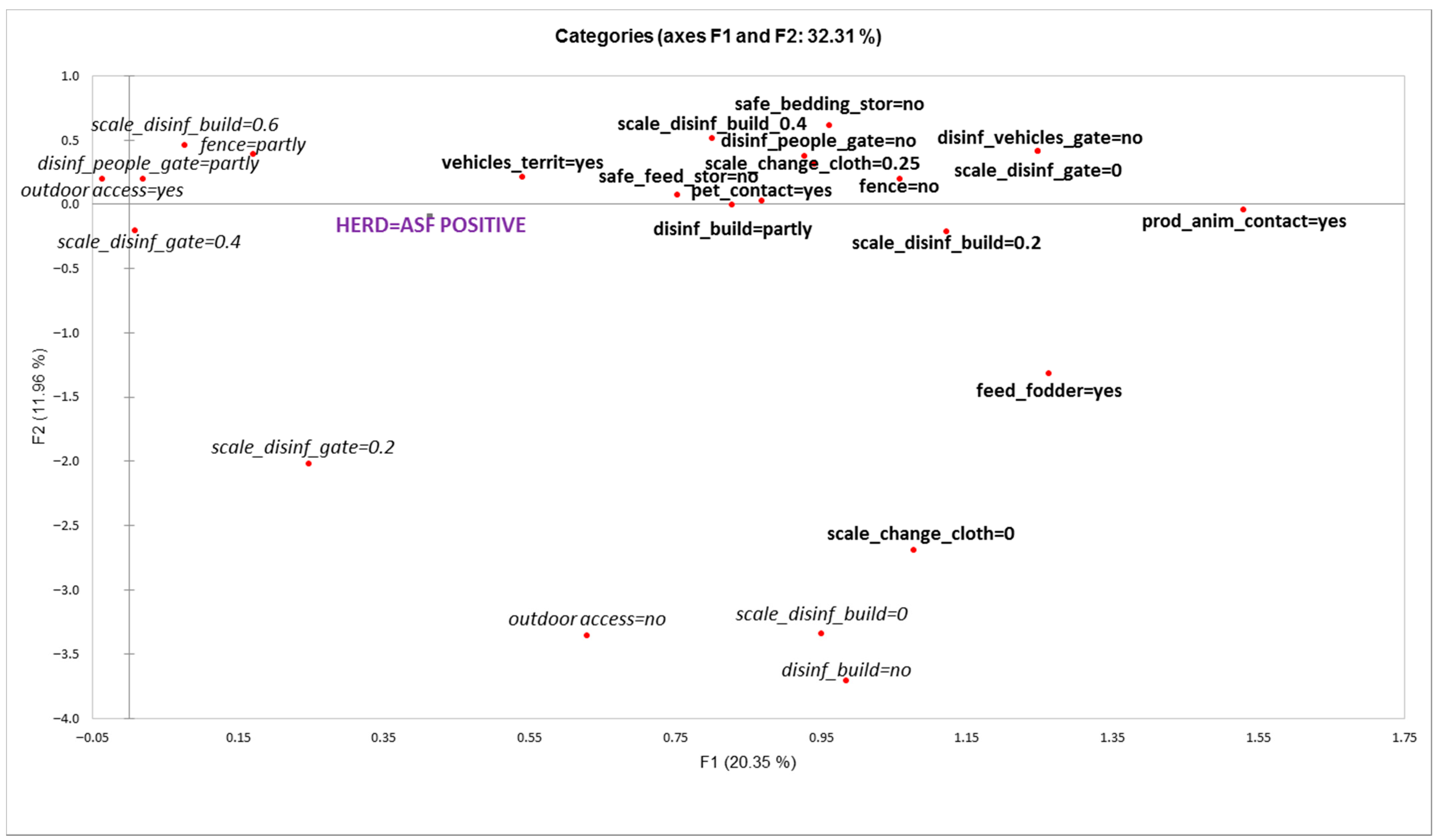
Statistical Analysis
3. Results
- Other vehicles on territory (vehicles_territ = yes; 4.083)
- Pets in contact with pigs (pet_contact = yes; 4.102)
- Other production animals in contact with pigs (prod_anim_cont = yes; 4.296)
- Feeding freshly cut fodder (feed_fodder = yes; 4.108)
- Storing feed in bins or piles (safe_feed_stor = no; 4.900)
- Unsafe storage of bedding materials (safe_bedding_stor = no; 2.916)
- No fence (fence = no; 4.775)
- No disinfection of people at the entrance of the territory (disinf_people_gate = no; 5.042)
- No disinfection of vehicles at the entrance of the territory (disinf_vehicles_gate = no; 6.152)
- Dysfunctional disinfection at the entrance of the territory (scale_disinf_gate = 0; 6.152)
- Disinfection barrier at every entrance of the building is partly present (disinf_build = partly; 3.047)
- Poor functionality of disinfection barrier at the entrance to the building:
- ○
- Score value 0.2 (scale_disinf_build = 0.2; 3.121)
- ○
- Score value 0.4 (scale_disinf_build = 0.4; 2.606)
- Low safety of changing clothes before entering the pig facility:
- ○
- Score value 0 (scale_change_cloth = 0; 2.218)
- ○
- Score value 0.25 (scale_change_cloth = 0.25; 5.171)
4. Discussion
5. Conclusions
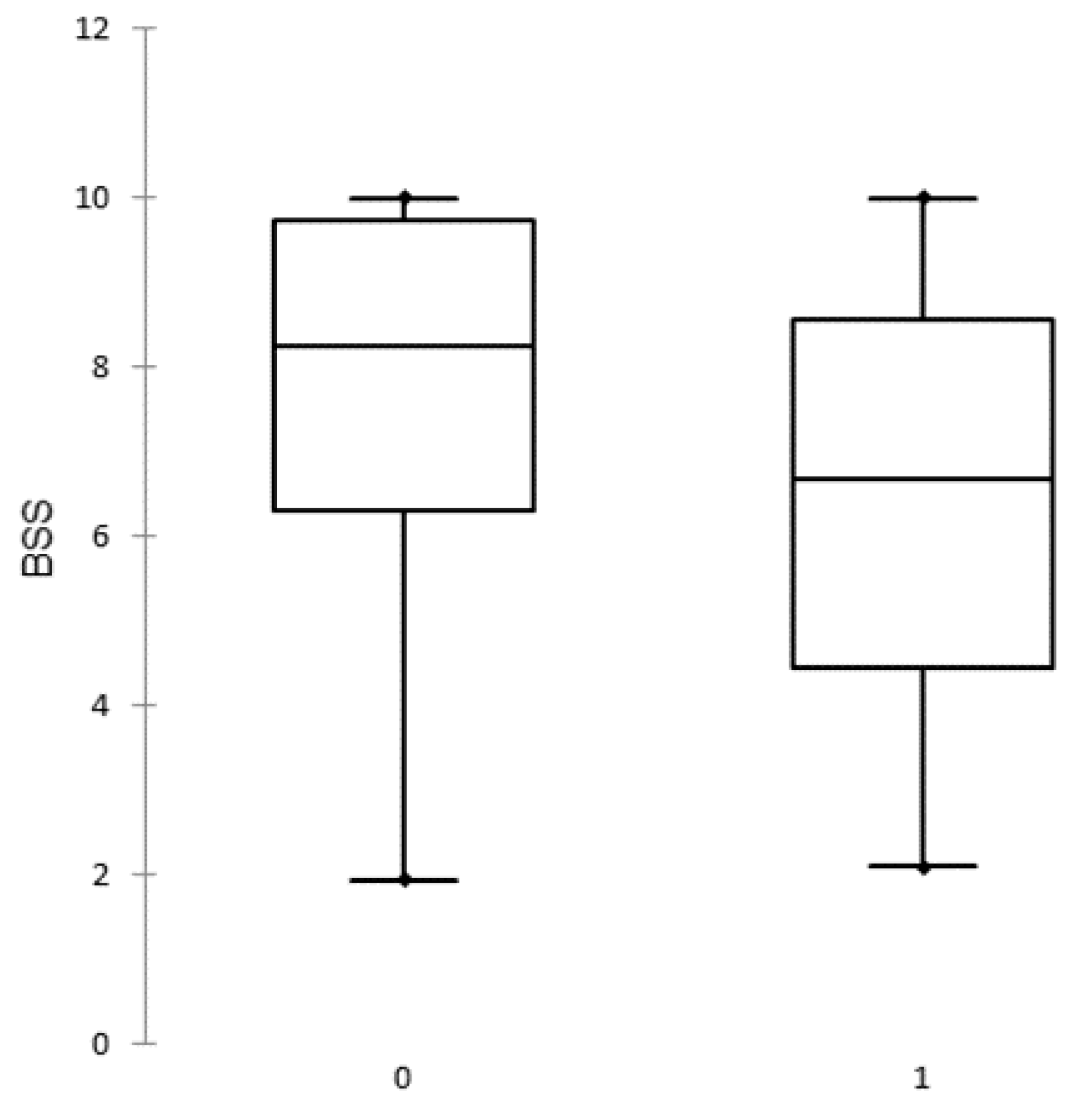
- The biosecurity level in ASF outbreak herds (6.34; SD 2.42) was significantly lower than in uninfected herds (7.86; SD 2.09) in Estonia. However, this may reflect a general improvement in the application of biosecurity measures in pig farms over a three-year period.
- The factors associated with the ‘outbreak’ status of a herd included unsafe contacts with the outside farm environment, such as lack of fencing, lack of or poor disinfection barriers at the entrance of the farm territory for people and vehicles, poor safety procedures for changing clothes before entering the pig facility, contacts with other domestic animals, and entering of unnecessary vehicles on the territory of the farm.
- Another distinctive group of factors associated with outbreak status of the herd related to feed and bedding, such as feeding freshly cut fodder, storing feed in bins or piles, and unsafe storage of bedding materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016, 58, 82. [Google Scholar] [CrossRef]
- Asambe, A.; Sackey, A.K.B.; Tekdek, L.B. Sanitary measures in piggeries, awareness, and risk factors of African swine fever in Benue state, Nigeria. Trop. Anim. Health Prod. 2019, 51, 997–1001. [Google Scholar] [CrossRef]
- Fasina, F.O.; Agbaje, M.; Ajani, F.L.; Talabi, O.A.; Lazarus, D.D.; Gallardo, C.; Thompson, P.N.; Bastos, A.D. Risk factors for farm-level African swine fever infection in major pig-producing areas in Nigeria, 1997–2011. Prev. Vet. Med. 2012, 107, 65–75. [Google Scholar] [CrossRef]
- Dione, M.M.; Akol, J.; Roesel, K.; Kungu, J.; Ouma, E.A.; Wieland, B.; Pezo, D. Risk factors for African swine fever in smallholder pig production systems in Uganda. Transbound. Emerg. Dis. 2017, 64, 872–882. [Google Scholar] [CrossRef]
- Okoth, E.; Gallardo, C.; Macharia, J.M.; Omore, A.; Pelayo, V.; Bulimo, D.W.; Arias, M.; Kitala, P.; Baboon, K.; Lekolol, I.; et al. Comparison of African swine fever virus prevalence and risk in two contrasting pig-farming systems in south-west and central Kenya. Prev. Vet. Med. 2013, 110, 198–205. [Google Scholar] [CrossRef]
- Mannelli, A.; Sotgia, S.; Patta, C.; Sarria, A.; Madrau, P.; Sanna, L.; Firinu, A.; Laddomada, A. Effect of husbandry methods on seropositivity to African swine fever virus in Sardinian swine herds. Prev. Vet. Med. 1997, 32, 235–241. [Google Scholar] [CrossRef]
- Nurmoja, I.; Motus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Prev. Vet. Med. 2020, 181, 104556. [Google Scholar] [CrossRef]
- Boklund, A.; Dhollander, S.; Chesnoiu Vasile, T.; Abrahantes, J.C.; Botner, A.; Gogin, A.; Gonzalez Villeta, L.C.; Gortazar, C.; More, S.J.; Papanikolaou, A.; et al. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef]
- Alarcón, L.V.; Allepuz, A.; Mateu, E. Biosecurity in pig farms: A review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef]
- Gelaude, P.; Schlepers, M.; Verlinden, M.; Laanen, M.; Dewulf, J. Biocheck.Ugent: A quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult. Sci. 2014, 93, 2740–2751. [Google Scholar] [CrossRef]
- Grabkowsky, B.; Conraths, F.; Globig, A.; Wilke, A.; Denzin, N. A self-assessment tool to improve poultry farm biosecurity regarding avian influenza. Berl. Und Münchener Tierärztliche Wochenschr. 2020, 133. [Google Scholar] [CrossRef]
- Zang, Y.-T.; Tan, Y.-P.; Hu, Y.-N.; Lu, C.-H. Construction of index system for external risk factors of disease on large-scale farm based on the analytic hierarchy process. Procedia Eng. 2012, 37, 274–280. [Google Scholar] [CrossRef][Green Version]
- Silva, G.S.; Corbellini, L.G.; Linhares, D.L.C.; Baker, K.L.; Holtkamp, D.J. Development and validation of a scoring system to assess the relative vulnerability of swine breeding herds to the introduction of prrs virus. Prev. Vet. Med. 2018, 160, 116–122. [Google Scholar] [CrossRef]
- Holtkamp, D.J.; Lin, H.; Wang, C.; O′Connor, A.M. Identifying questions in the American association of swine veterinarian’s PRRS risk assessment survey that are important for retrospectively classifying swine herds according to whether they reported clinical PRRS outbreaks in the previous 3 years. Prev. Vet. Med. 2012, 106, 42–52. [Google Scholar] [CrossRef]
- Silva, G.S.; Leotti, V.B.; Castro, S.M.J.; Medeiros, A.A.R.; Silva, A.; Linhares, D.C.L.; Corbellini, L.G. Assessment of biosecurity practices and development of a scoring system in swine farms using item response theory. Prev. Vet. Med. 2019, 167, 128–136. [Google Scholar] [CrossRef]
- Vechta, U.O. ASP-Risikoampel. Available online: https://risikoampel.uni-vechta.de/ (accessed on 12 December 2021).
- Saaty, T.L. Mathematical Principles of Decision Making (Principia MATHEMATICA Decernendi); RWS Publications: Pittsburgh, PA, USA, 2010. [Google Scholar]
- Goepel, K.D. Free Web Based AHP Software. Available online: Http://bpmsg.Com (accessed on 19 July 2019).
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Dohoo, I.R.; Ducrot, C.; Fourichon, C.; Donald, A.; Hurnik, D. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Prev. Vet. Med. 1997, 29, 221–239. [Google Scholar] [CrossRef]
- Greenacre, M.J.; Blasius, J. Multiple Correspondence Analysis; Chapman & Hall/CRC: London, UK, 2006. [Google Scholar]
- Addinsoft. Xlstat Statistical and Data Analysis Solution; Addinsoft: Boston, MA, USA, 2019. [Google Scholar]
- Pisati, M. Spmap: Stata Module to Visualize Spatial Data; Statistical Software Components S456812; Boston College Department of Economics: Chestnut Hill, MA, USA, 2007. [Google Scholar]
- Estonian Land Board. Map of Municipal Counties before Public Administration Reform: 2019. Available online: Https://geoportaal.Maaamet.Ee/docs/haldus_asustus/maakond_20171015_shp.Zip?T=20171015133716 (accessed on 10 October 2019).
- Anonymous. Sigade Klassikalise Katku ja Sigade Aafrika Katku Tõrje Eeskiri; Põllumajandusministri määrus nr 179; RTL, Riigikantselei: Tallinn, Estonia, 2004; Volume 150, p. 2277. [Google Scholar]
- Correia-Gomes, C.; Henry, M.K.; Auty, H.K.; Gunn, G.J. Exploring the role of small-scale livestock keepers for national biosecurity-the pig case. Prev. Vet. Med. 2017, 145, 7–15. [Google Scholar] [CrossRef]
- Noremark, M.; Frossling, J.; Lewerin, S.S. Application of routines that contribute to on-farm biosecurity as reported by swedish livestock farmers. Transbound. Emerg. Dis. 2010, 57, 225–236. [Google Scholar] [CrossRef]
- Ribbens, S.; Dewulf, J.; Koenen, F.; Mintiens, K.; De Sadeleer, L.; de Kruif, A.; Maes, D. A survey on biosecurity and management practices in belgian pig herds. Prev. Vet. Med. 2008, 83, 228–241. [Google Scholar] [CrossRef]
- Forth, J.H.; Amendt, J.; Blome, S.; Depner, K.; Kampen, H. Evaluation of blowfly larvae (diptera: Calliphoridae) as possible reservoirs and mechanical vectors of African swine fever virus. Transbound. Emerg. Dis. 2018, 65, e210–e213. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Hansen, M.F.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Botner, A.; Bodker, R. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans). Transbound. Emerg. Dis. 2018, 65, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Turcinaviciene, J.; Petrasiunas, A.; Bernotiene, R.; Masiulis, M.; Jonusaitis, V. The contribution of insects to African swine fever virus dispersal: Data from domestic pig farms in Lithuania. Med. Vet. Entomol. 2021, 35, 484–489. [Google Scholar] [CrossRef]
| Criterion | Scoring | Weight Values |
|---|---|---|
| 1. Outdoor access for pigs | 0/1 (yes/no) | 17 |
| 2. Direct contact with other farm animals | 0/1 (yes/no) | 13 |
| 3. Direct contact with dog or cat | 0/1 (yes/no) | 13 |
| 4. Fencing of the farm territory | 0 to 1 (no/partly yes/yes) with 0.5 increment | 4 |
| 5. Allowing unnecessary vehicles to enter the territory of the farm | 0/1 (yes/no) | 4 |
| 6. Disinfection barrier at the gate of the territory for vehicles | 0 to 1 (no/partly yes/yes) with 0.5 increment | 5 |
| 7. Disinfection barrier at the gate of the territory for people | 0 to 1 (no/partly yes/yes) with 0.5 increment | 5 |
| 7.1 Functionality of the disinfection barrier at the gate | 0 (least) to 1 (most) with 0.2 increment | NA * |
| 8. Disinfection barrier upon entry into the building for people and vehicles | 0 to 1 (no/partly yes/yes) with 0.5 increment | 10 |
| 8.1 Functionality of the disinfection barrier at the entrance to the building | 0 (least) to 1 (most) with 0.2 increment | NA * |
| 9. Secure change of clothes before entering the pig facility 1 | 0 (least) to 1 (most) with 0.25 increment | 12 |
| 10. Safe storage of feed (incl. safe water source) 2 | 0 (least) to 1 (most) with 0.5 increment | 12 |
| 11. Safe storage of litter 3 | 0 (least) to 1 (most) with 0.5 increment | 8 |
| 12. Feeding of freshly cut fodder to pigs | 0/1 (yes/no) | 15 |
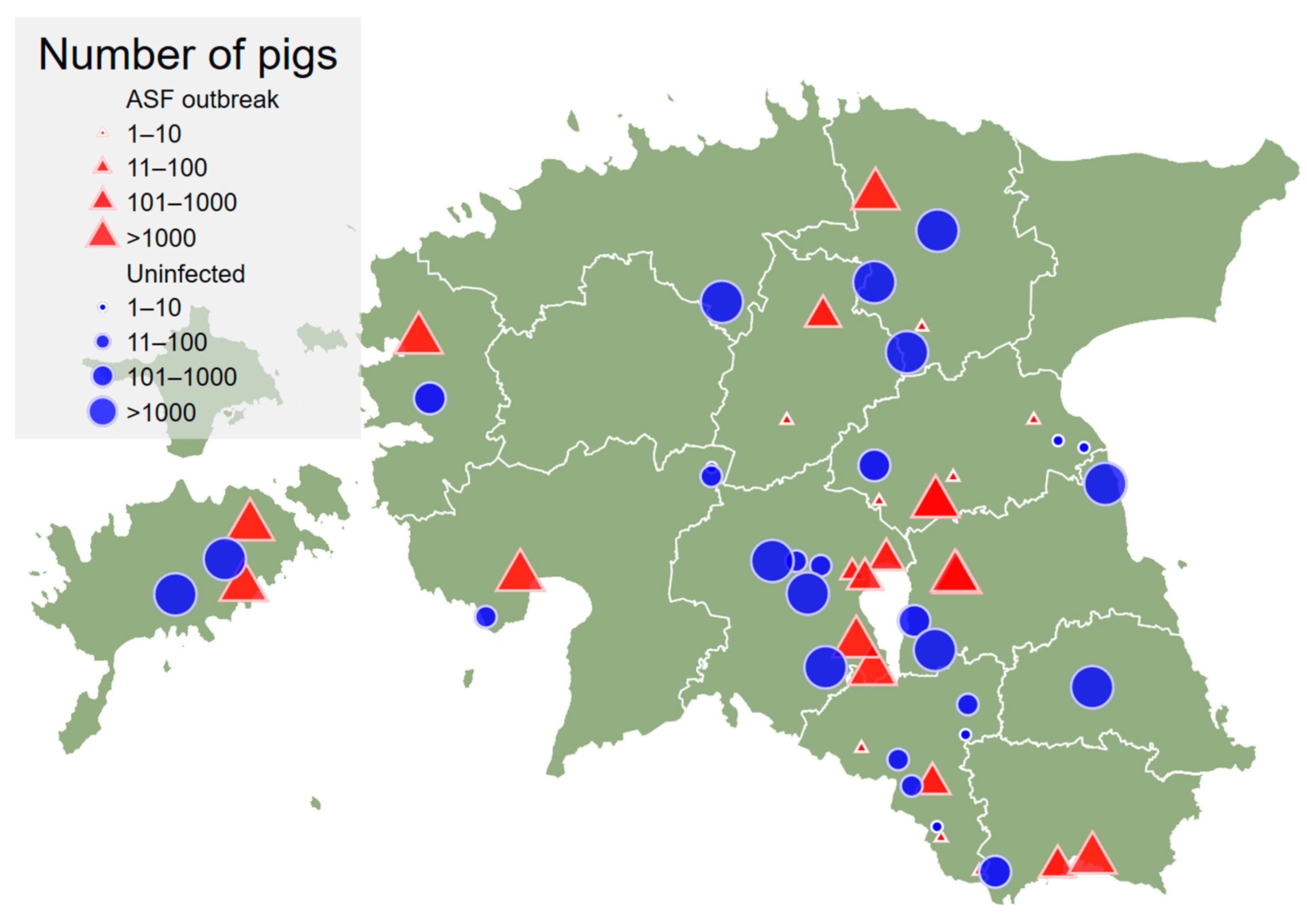
| A | Herd size Categories (n Pigs) | Outbreak Herds | Uninfected Herds | Total |
| 1–10 | 8 | 5 | 13 | |
| 11–100 | 1 | 7 | 8 | |
| 101–1000 | 5 | 4 | 9 | |
| >1000 | 12 | 12 | 24 | |
| Total | 26 | 28 | 54 | |
| B | Production Type | Outbreak Herds | Uninfected Herds | Total |
| Farrow to finish | 9 | 12 | 21 | |
| Fattener | 14 | 13 | 27 | |
| Breeders | 3 | 3 | 6 | |
| Total | 26 | 28 | 54 |
| Herd Category | Observations | Minimum | Maximum | Median | Mean | SD 1 |
|---|---|---|---|---|---|---|
| Uninfected herds | 28 | 1.95 | 10.00 | 8.26 | 7.86 | 2.09 |
| Outbreak herds | 26 | 2.10 | 10.00 | 6.68 | 6.34 | 2.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viltrop, A.; Reimus, K.; Niine, T.; Mõtus, K. Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them? Animals 2022, 12, 68. https://doi.org/10.3390/ani12010068
Viltrop A, Reimus K, Niine T, Mõtus K. Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them? Animals. 2022; 12(1):68. https://doi.org/10.3390/ani12010068
Chicago/Turabian StyleViltrop, Arvo, Kaari Reimus, Tarmo Niine, and Kerli Mõtus. 2022. "Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them?" Animals 12, no. 1: 68. https://doi.org/10.3390/ani12010068
APA StyleViltrop, A., Reimus, K., Niine, T., & Mõtus, K. (2022). Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them? Animals, 12(1), 68. https://doi.org/10.3390/ani12010068






