Variations in HIF-1α Contributed to High Altitude Hypoxia Adaptation via Affected Oxygen Metabolism in Tibetan Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objects and Blood Gas Indicators Measure
2.2. PCR Amplification and Genotyping
2.3. Statistical Analyses
3. Results
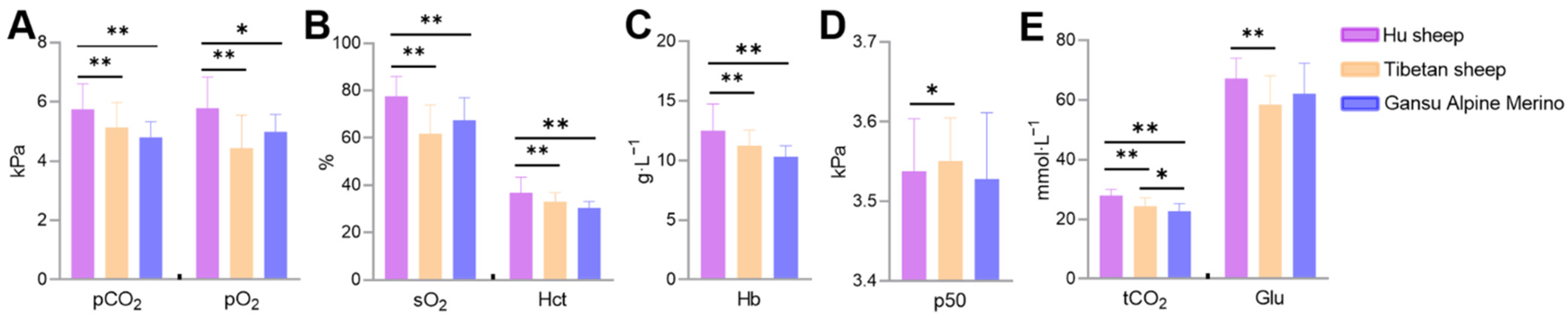
3.1. Differences in Blood Gas Indicators between Breeds
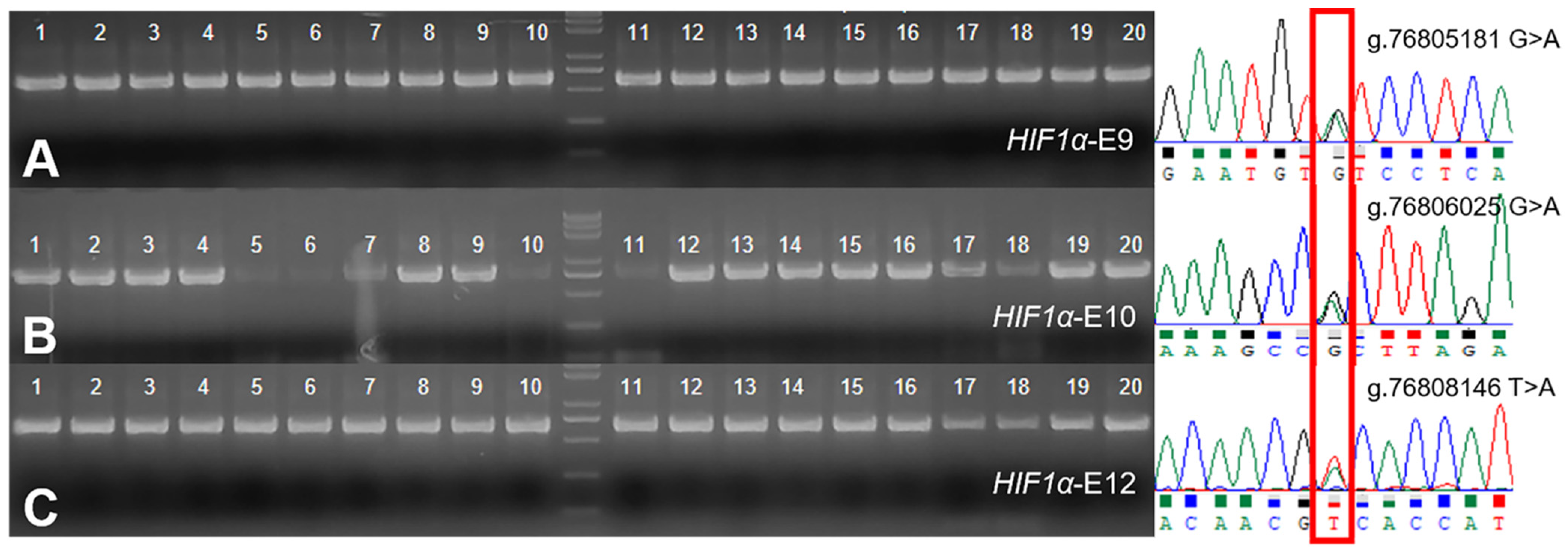
3.2. Variation of HIF-1α in Tibetan Sheep and Hu Sheep
3.3. Association Analysis of Genotype and Haplotype Combinations with Blood Gas Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, L.G.; Yao, T.; Mosley, T.E.; Davis, M.E.; Henderson, K.A.; Lin, P.N. A High-Resolution Millennial Record of the South Asian Monsoon from Himalayan Ice Cores. Science 2000, 289, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M. Adaptation to High Altitude: Phenotypes and Genotypes. Annu. Rev. Anthropol. 2014, 43, 251–272. [Google Scholar] [CrossRef]
- Wei, C.; Wang, H.; Liu, G.; Zhao, F.; Kijas, J.W.; Ma, Y.; Lu, J.; Zhang, L.; Cao, J.; Wu, M.; et al. Genome-wide analysis reveals adaptation to high altitudes in Tibetan sheep. Sci. Rep. 2016, 6, 26770. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and Maladaptive Cardiorespiratory Responses to Continuous and Intermittent Hypoxia Mediated by Hypoxia-Inducible Factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High Altitude Adaptability and Meat Quality in Tibetan Pigs: A Reference for Local Pork Processing and Genetic Improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, C.; Guo, F.; Wang, Y.; Zeng, X.; Ding, Z.; Lu, Z.; Renqing, D.; Zhang, H.; Xu, X.; et al. Genetic signatures of high-altitude adaptation and geographic distribution in Tibetan sheep. Sci. Rep. 2020, 10, 18332. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, X.; Liu, Y.; Abied, A.; Ding, Y.; Zhao, S.; Wang, W.; Ma, L.; Guo, J.; Guan, W.; et al. Genome-wide comparative analyses reveal selection signatures underlying adaptation and production in Tibetan and Poll Dorset sheep. Sci. Rep. 2021, 11, 2466. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M.; Cavalleri, G.L.; Deng, L.; Elston, R.C.; Gao, Y.; Knight, J.; Li, C.; Li, J.C.; Liang, Y.; McCormack, M.; et al. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. USA 2010, 107, 11459–11464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Murphy, M.S.; Adamson, J.W. Detection of mutant hemoglobins with altered affinity for oxygen. A simplified technique. Ann. Intern. Med. 1976, 84, 517–520. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [CrossRef]
- Simonson, T.S.; Yang, Y.; Huff, C.D.; Yun, H.; Qin, G.; Witherspoon, D.J.; Bai, Z.; Lorenzo, F.R.; Xing, J.; Jorde, L.B.; et al. Genetic Evidence for High-Altitude Adaptation in Tibet. Science 2010, 329, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, Z.; Chen, J.; Huang, X.; Li, X.; Liang, Y.; Mao, J.-Y.; Chen, X.; Zheng, Z.; Bakshi, A.; et al. Genetic signatures of high-altitude adaptation in Tibetans. Proc. Natl. Acad. Sci. USA 2017, 114, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, Y.; Pan, J.; Wang, D.; Chen, W.; Zheng, Z.; He, X.; Zhao, Q.; Pu, Y.; et al. EPAS1 gain-of-function mutation contributes to high-altitude adaptation in Tibetan horses. Mol. Biol. Evol. 2019, 36, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hemann, C.; Hille, R.; Stuehr, D.J.; et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Otten, E.J. High altitude: An exploration of human adaptation. J. Emerg. Med. 2003, 25, 345–346. [Google Scholar] [CrossRef]
- Ge, R.L.; Simonson, T.S.; Cooksey, R.C.; Tanna, U.; Qin, G.; Huff, C.D.; Witherspoon, D.J.; Xing, J.; Zhengzhong, B.; Prchal, J.T.; et al. Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Mol. Genet. Metab. 2012, 106, 244–247. [Google Scholar] [CrossRef]
- Horscroft, J.A.; Kotwica, A.O.; Laner, V.; West, J.A.; Hennis, P.J.; Levett, D.Z.H.; Howard, D.J.; Fernandez, B.O.; Burgess, S.L.; Ament, Z.; et al. Metabolic basis to Sherpa altitude adaptation. Proc. Natl. Acad. Sci. USA 2017, 114, 6382–6387. [Google Scholar] [CrossRef]
- Kelly, K.R.; Williamson, D.L.; Fealy, C.E.; Kriz, D.A.; Krishnan, R.K.; Huang, H.; Ahn, J.; Loomis, J.L.; Kirwan, J.P. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism 2010, 59, 200–205. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Y.; Alessandro, A.D.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef]
- Zhuang, J.; Droma, T.; Sun, S.; Janes, C.; Mccullough, R.E.; Mccullough, R.G.; Cymerman, A.; Huang, S.Y.; Reeves, J.T.; Moore, L.G. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3658 m. J. Appl. Physiol. 1993, 74, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, Y.; Luo, Y. Expression of OPA1 and Mic60 genes and their association with mitochondrial cristae morphology in Tibetan sheep. Cell. Tissue Res. 2019, 376, 273–279. [Google Scholar] [CrossRef]
- Ivy, C.M.; Scott, G.R. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta. Physiol. 2017, 221, 266–282. [Google Scholar] [CrossRef]
- Tashi, T.; Feng, T.; Koul, P.; Amaru, R.; Hussey, D.; Lorenzo, F.R.; RiLi, G.; Prchal, J.T. High altitude genetic adaptation in Tibetans: No role of increased hemoglobin–oxygen affinity. Blood Cells Mol. Dis. 2014, 53, 27–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beall, C.M. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar] [CrossRef]
- Rao, M.; Li, J.; Qin, J.; Zhang, J.; Gao, X.; Yu, S.; Yu, J.; Chen, G.; Xu, B.; Li, H.; et al. Left Ventricular Function during Acute High-Altitude Exposure in a Large Group of Healthy Young Chinese Men. PLoS ONE 2015, 10, e116936. [Google Scholar] [CrossRef]
- Stembridge, M.; Williams, A.M.; Gasho, C.; Dawkins, T.G.; Drane, A.; Villafuerte, F.C.; Levine, B.D.; Shave, R.; Ainslie, P.N. The overlooked significance of plasma volume for successful adaptation to high altitude in Sherpa and Andean natives. Proc. Natl. Acad. Sci. USA 2019, 116, 16177–16179. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Li, M.; Frid, M.G.; Flockton, A.R.; McKeon, B.A.; Yeager, M.E.; Fini, M.A.; Morrell, N.W.; Pullamsetti, S.S.; et al. MicroRNA-124 Controls the Proliferative, Migratory, and Inflammatory Phenotype of Pulmonary Vascular Fibroblasts. Circ. Res. 2013, 114, 67–78. [Google Scholar] [CrossRef]




| Gene | Exon | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|---|
| HIF-1α | 9 | TCAGAGTCCTCCTCCTCCAA | GGCCACAATGTCCAAATGAT |
| HIF-1α | 10 | TGCAGCAGCCATAAGTTGAG | CCTGAAACATGGGACTGAGG |
| HIF-1α | 12 | TCTCAAGTGGCTGTGGGTTT | GGGTTGGAAAGAGTTGGACA |
| Locus | Genotype | Genotype Frequency | p | Allele | Allele Frequency | ||
|---|---|---|---|---|---|---|---|
| TS (n) | HS(n) | TS | HS | ||||
| g.76805181 G > A | GG | 0.319 a (120) | 0.241 b (77) | 0.022 | G | 0.569 | 0.491 |
| GA | 0.500 (188) | 0.499 (159) | 0.967 | A | 0.431 | 0.509 | |
| AA | 0.181 b (68) | 0.260 a (83) | 0.012 | ||||
| g.76806025 G > A | GG | 0.273 (102) | 0.240 (80) | 0.302 | G | 0.544 | 0.485 |
| GA | 0.542 (202) | 0.491 (164) | 0.180 | A | 0.456 | 0.515 | |
| AA | 0.185 B (69) | 0.269 A (90) | 0.008 | ||||
| g.76808146 T > A | TT | 0.749 B (293) | 0.834 A (281) | 0.005 | T | 0.859 | 0.911 |
| TA | 0.220 a (86) | 0.154 b (52) | 0.023 | A | 0.141 | 0.089 | |
| AA | 0.031 (12) | 0.012 (4) | 0.075 | ||||
| Locus | Breed | PIC 1 | He 2 | Ho 3 | Ne 4 | HWE 5 |
|---|---|---|---|---|---|---|
| g.76805181 G > A | TS | 0.370 | 0.490 | 0.510 | 1.963 | p > 0.05 |
| HS | 0.375 | 0.500 | 0.500 | 1.999 | p > 0.05 | |
| g.76806025 G > A | TS | 0.373 | 0.496 | 0.504 | 1.985 | p > 0.05 |
| HS | 0.475 | 0.500 | 0.500 | 1.998 | p > 0.05 | |
| g.76808146 T > A | TS | 0.213 | 0.242 | 0.758 | 1.320 | p > 0.05 |
| HS | 0.149 | 0.162 | 0.838 | 1.194 | p > 0.05 |
| Haplotype | SNP1 | SNP2 | SNP3 | Frequency/% | Haplotype Combination | Frequency/% | ||
|---|---|---|---|---|---|---|---|---|
| TS | HS | TS | HS | |||||
| H1 (AAT) | A | A | T | 0.416 | 0.473 | H1H1 | 0.173 | 0.224 |
| H2 (GGT) | G | G | T | 0.403 | 0.422 | H1H2 | 0.168 | 0.200 |
| H3 (GGA) | G | G | A | 0.138 | 0.052 | H1H3 | 0.057 | 0.025 |
| H4 (GAT) | G | A | T | 0.020 | 0.010 | H2H2 | 0.162 | 0.178 |
| H5 (AGT) | A | G | T | 0.020 | 0.007 | H2H3 | 0.056 | 0.022 |
| H6 (AAA) | A | A | A | 0.029 | ||||
| H7 (GAA) | G | A | A | 0.002 | 0.006 | |||
| H8 (AGA) | A | G | A | 0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; He, Z.; Xi, Q.; Sun, H.; Luo, Y.; Wang, J.; Liu, X.; Zhao, Z.; Li, S. Variations in HIF-1α Contributed to High Altitude Hypoxia Adaptation via Affected Oxygen Metabolism in Tibetan Sheep. Animals 2022, 12, 58. https://doi.org/10.3390/ani12010058
Zhao P, He Z, Xi Q, Sun H, Luo Y, Wang J, Liu X, Zhao Z, Li S. Variations in HIF-1α Contributed to High Altitude Hypoxia Adaptation via Affected Oxygen Metabolism in Tibetan Sheep. Animals. 2022; 12(1):58. https://doi.org/10.3390/ani12010058
Chicago/Turabian StyleZhao, Pengfei, Zhaohua He, Qiming Xi, Hongxian Sun, Yuzhu Luo, Jiqing Wang, Xiu Liu, Zhidong Zhao, and Shaobin Li. 2022. "Variations in HIF-1α Contributed to High Altitude Hypoxia Adaptation via Affected Oxygen Metabolism in Tibetan Sheep" Animals 12, no. 1: 58. https://doi.org/10.3390/ani12010058
APA StyleZhao, P., He, Z., Xi, Q., Sun, H., Luo, Y., Wang, J., Liu, X., Zhao, Z., & Li, S. (2022). Variations in HIF-1α Contributed to High Altitude Hypoxia Adaptation via Affected Oxygen Metabolism in Tibetan Sheep. Animals, 12(1), 58. https://doi.org/10.3390/ani12010058






