High Numbers of CD163-Positive Macrophages in the Fibrotic Region of Exuberant Granulation Tissue in Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Wounds
2.2.1. Experimental Wounds
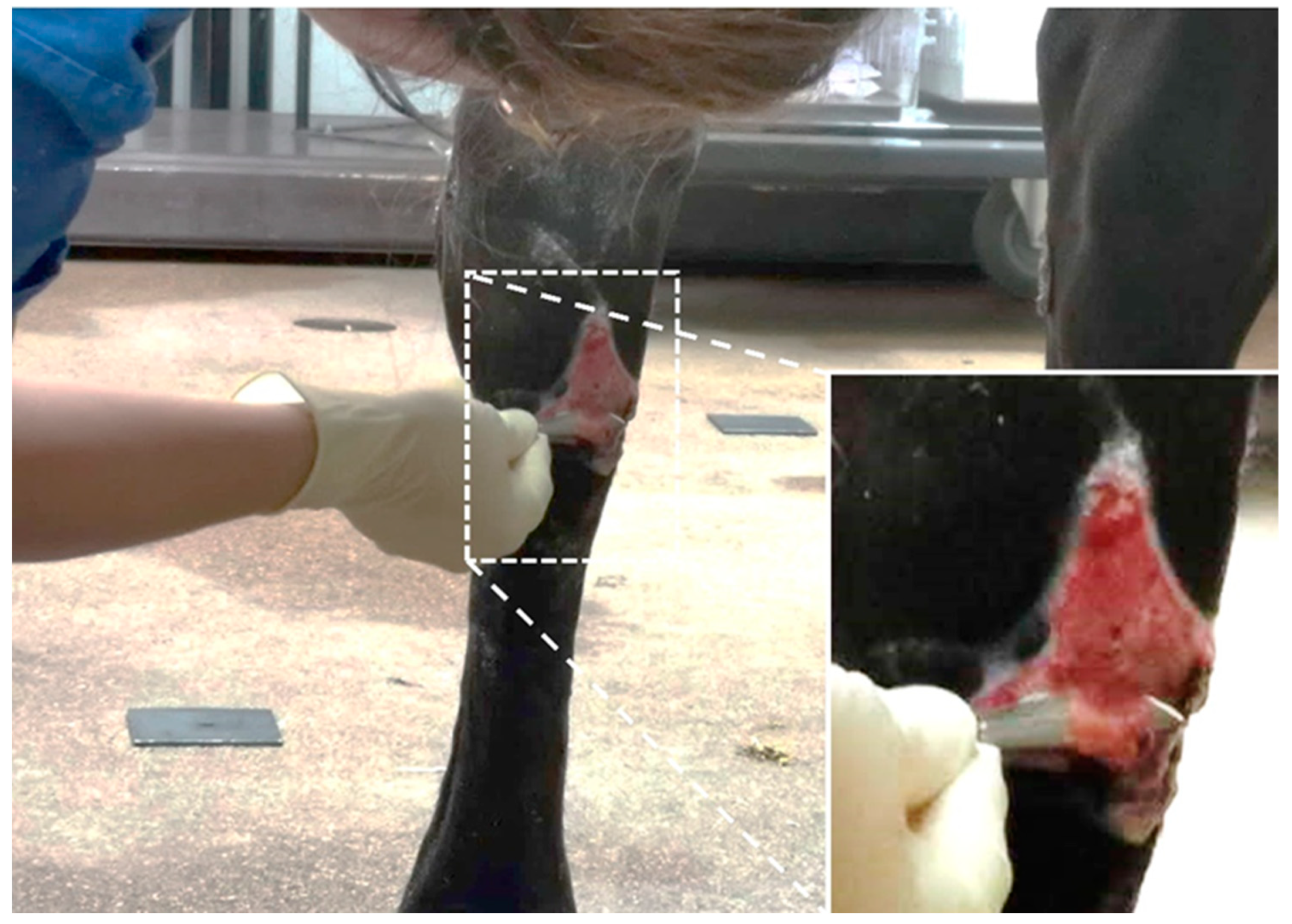
2.2.2. EGT Wounds
2.3. Histology
2.3.1. Histological Staining
2.3.2. Immunohistochemical Stainings
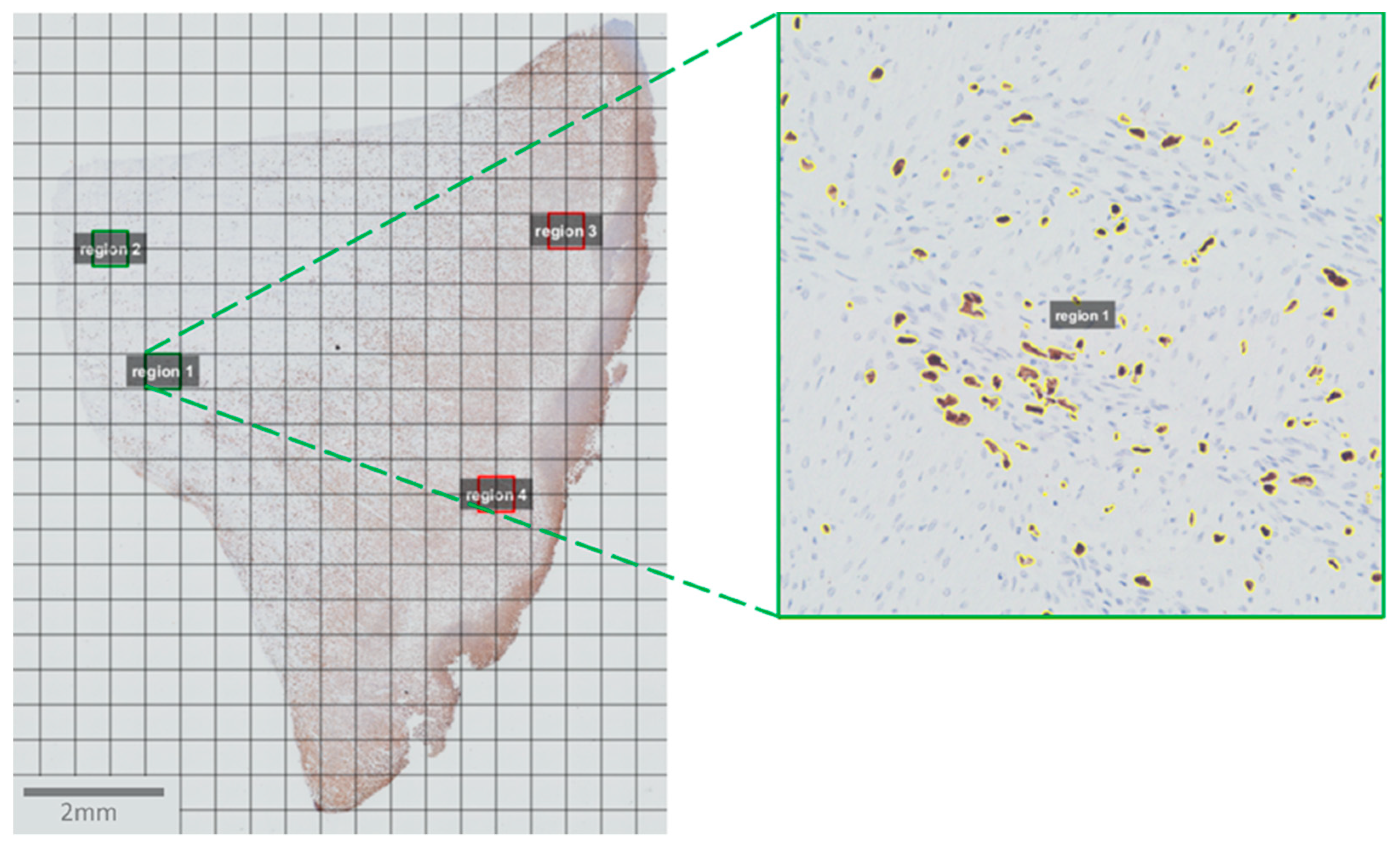
2.4. Image Acquisition and Analysis
2.5. Statistics
3. Results
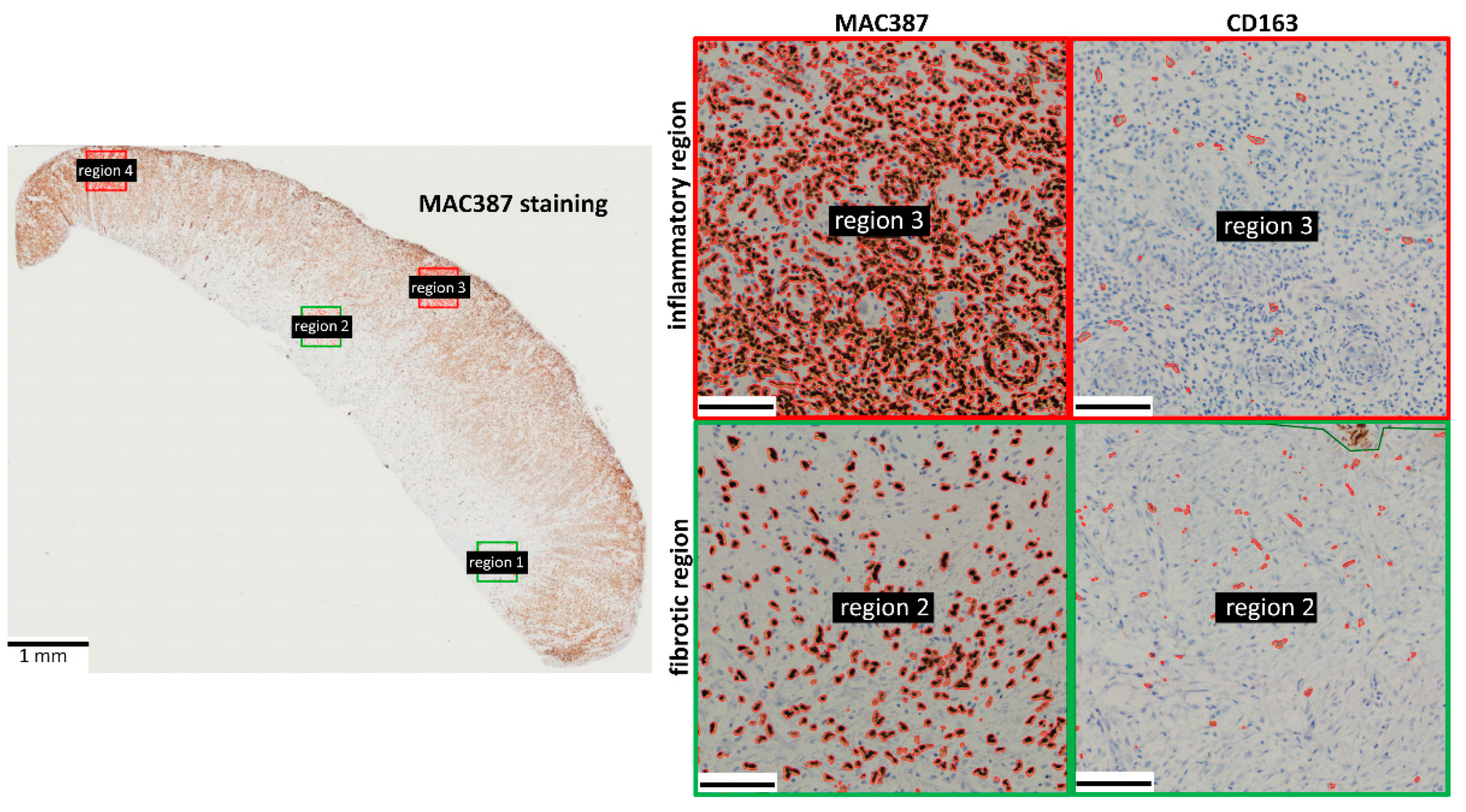
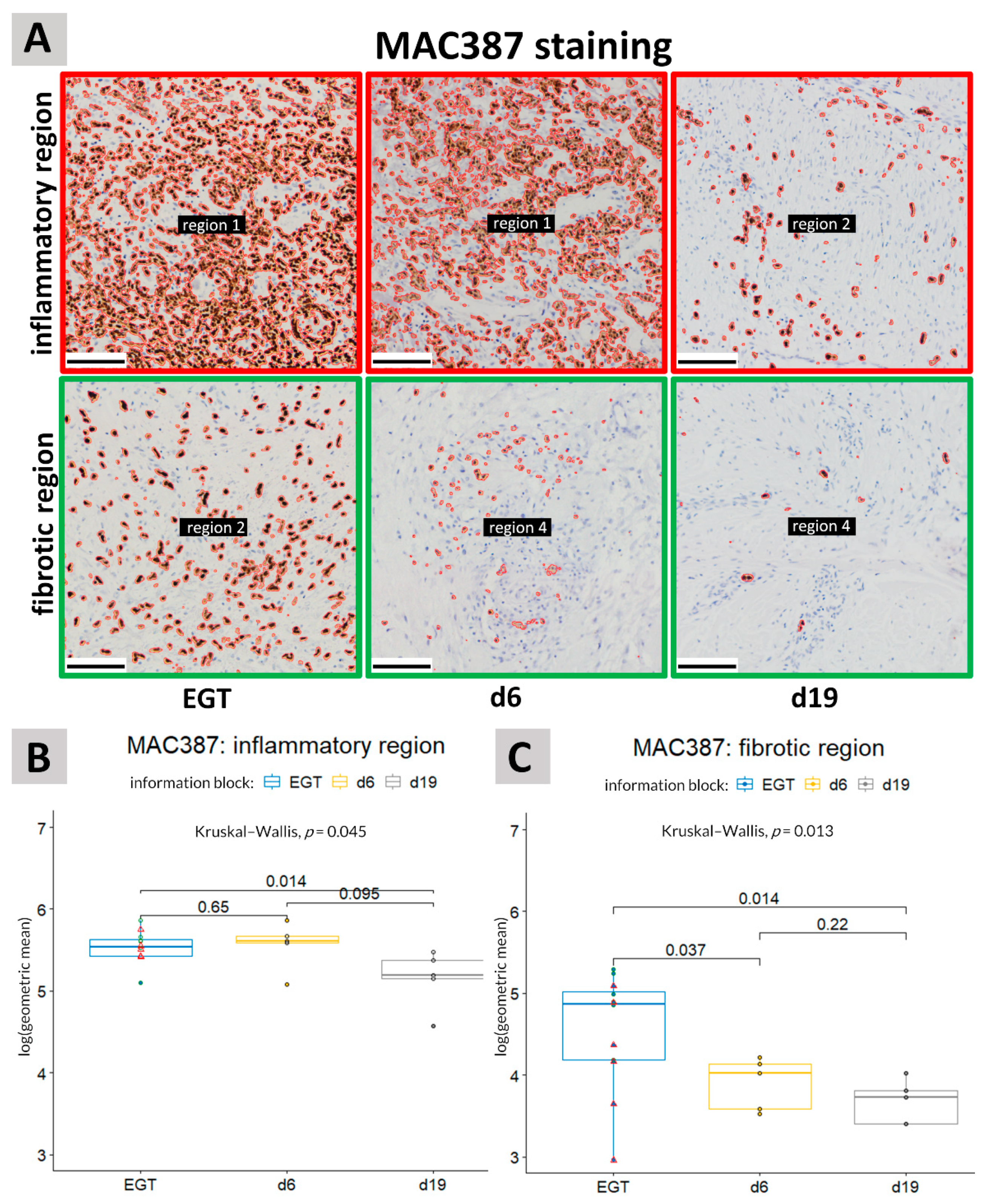
3.1. MAC387 Staining
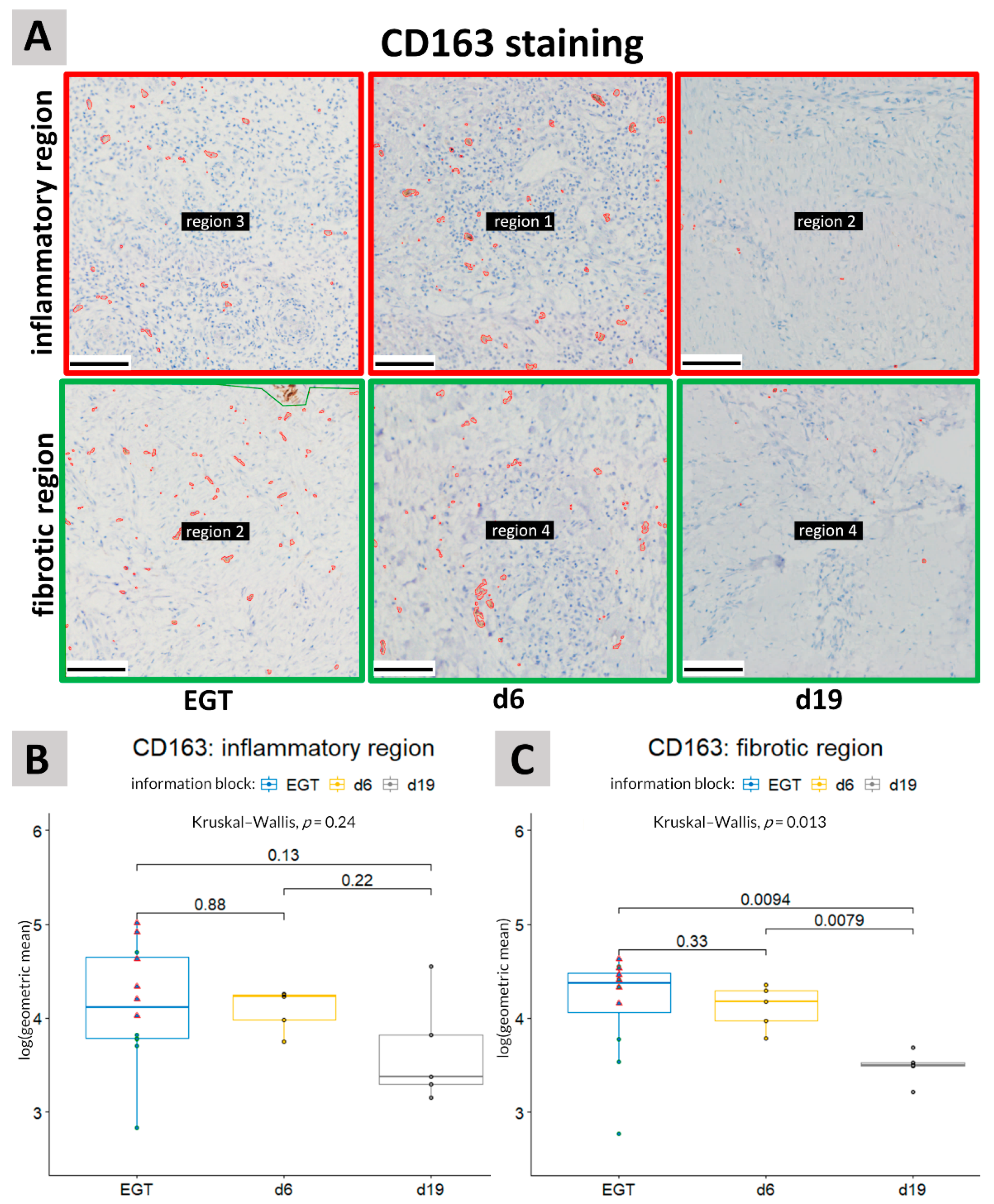
3.2. CD163 Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, H.D.; Sigaeva, T.; Tarraf, S.; Mandla, S.; Pope, H.; Hee, O.; Di Martino, E.S.; Biernaskie, J.; Radisic, M.; Scott, W.M. Biomechanics of Wound Healing in an Equine Limb Model: Effect of Location and Treatment with a Peptide-Modified Collagen-Chitosan Hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 265–278. [Google Scholar] [CrossRef]
- Lepault, E.; Celeste, C.; Dore, M.; Martineau, D.; Theoret, C.L. Comparative study on microvascular occlusion and apoptosis in body and limb wounds in the horse. Wound Repair Regen. 2005, 13, 520–529. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Silver, I.A. The mechanics of wound healing. Equine Vet. J. 1979, 11, 93–96. [Google Scholar] [CrossRef]
- Wilmink, J.M.; Van Weeren, P.R.; Stolk, P.W.T.; Van Mil, F.N.; Barneveld, A. Differences in second-intention wound healing between horses and ponies: Histological aspects. Equine Vet. J. 1999, 31, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.A.; Leach, D.H.; Fretz, P.B.; Townsend, H.G.G. Comparative Aspects of the Healing of Excisional Wounds on the Leg and Body of Horses. Vet. Surg. 1984, 13, 83–90. [Google Scholar] [CrossRef]
- Miller, C.B.; Wilson, D.A.; Keegan, K.G.; Kreeger, J.M.; Adelstein, E.H.; Ganjam, V.K. Growth characteristics of fibroblasts isolated from the trunk and distal aspect of the limb of horses and ponies. Vet. Surg. 2000, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knottenbelt, D.C. Equine wound management: Are there significant differences in healing at different sites on the body? Vet. Dermatol. 1997, 8, 273–290. [Google Scholar] [CrossRef]
- Theoret, C.; Schumacher, J. Physiology of Wound Healing. In Equine Wound Management, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 2. [Google Scholar]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, C. The molecular biology in wound healing & non-healing wound. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2017, 20, 189–193. [Google Scholar] [PubMed]
- Du Cheyne, C.; Tay, H.; De Spiegelaere, W. The complex TIE between macrophages and angiogenesis. Anat. Histol. Embryol. 2020, 49, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Ferreira, D.W.; Ulecia-Morón, C.; Alvarado-Vázquez, P.A.; Cunnane, K.; Moracho-Vilriales, C.; Grosick, R.L.; Cunha, T.M.; Romero-Sandoval, E.A. CD163 overexpression using a macrophage-directed gene therapy approach improves wound healing in ex vivo and in vivo human skin models. Immunobiology 2020, 225, 151862. [Google Scholar] [CrossRef]
- Haspeslagh, M.; Van Hecke, L.L.; Hermans, K.; Chiers, K.; Pint, E.; Wilmink, J.M.; Martens, A.M. Limited added value of negative pressure wound therapy compared with calcium alginate dressings for second intention healing in a noncontaminated and contaminated equine distal limb wound model. Equine Vet. J. 2021. [CrossRef]
- Van Hecke, L. The Influence of Negative Pressure Wound Therapy on Second Intention Wound Healing in the Equine Distal Limb; Ghent University: Merelbeke, Belgium, 2017. [Google Scholar]
- Komohara, Y.; Hirahara, J.; Horikawa, T.; Kawamura, K.; Kiyota, E.; Sakashita, N.; Araki, N.; Takeya, M. AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J. Histochem. Cytochem. 2006, 54, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Soulas, C.; Conerly, C.; Kim, W.K.; Burdo, T.H.; Alvarez, X.; Lackner, A.A.; Williams, K.C. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am. J. Pathol. 2011, 178, 2121–2135. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Nouno, T.; Okamoto, M.; Ohnishi, K.; Kaieda, S.; Tominaga, M.; Zaizen, Y.; Ichiki, M.; Momosaki, S.; Nakamura, M.; Fujimoto, K.; et al. Elevation of pulmonary CD163(+) and CD204(+) macrophages is associated with the clinical course of idiopathic pulmonary fibrosis patients. J. Thorac. Dis. 2019, 11, 4005–4017. [Google Scholar] [CrossRef]
- Van den Boom, R.; Wilmink, J.M.; O’Kane, S.; Wood, J.; Ferguson, M.W.J. Transforming growth factor-beta levels during second-intention healing are related to the different course of wound contraction in horses and ponies. Wound Repair Regen. 2002, 10, 188–194. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar] [PubMed]
- Montanaro, M.; Meloni, M.; Anemona, L.; Giurato, L.; Scimeca, M.; Izzo, V.; Servadei, F.; Smirnov, A.; Candi, E.; Mauriello, A.; et al. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int. J. Low. Extrem. Wounds 2020, 1534734620945559. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, L.; Nabzdyk, C.; Andersen, N.D.; LoGerfo, F.W.; Veves, A. Inflammation and neuropeptides: The connection in diabetic wound healing. Expert Rev. Mol. Med. 2009, 11, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Bannon, P.; Wood, S.; Restivo, T.; Campbell, L.; Hardman, M.J.; Mace, K.A. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis. Model. Mech. 2013, 6, 1434–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Tie, G.; Wang, S.; Tutto, A.; DeMarco, N.; Khair, L.; Fazzio, T.G.; Messina, L.M. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat. Commun. 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Yuan, B.; Yang, H.; Qiao, L. Status of M1 and M2 type macrophages in keloid. Int. J. Clin. Exp. Pathol. 2017, 10, 11098–11105. [Google Scholar] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoret, C.L.; Olutoye, O.O.; Parnell, L.K.S.; Hicks, J. Equine Exuberant Granulation Tissue and Human Keloids: A Comparative Histopathologic Study. Vet. Surg. 2013, 42, 783–789. [Google Scholar] [CrossRef] [PubMed]
| Horse | Wound Age (Days) | Affected Limb | Localization EGT | Horse Age | Horse Weight (kg) |
|---|---|---|---|---|---|
| 1 | 23 | RH | Distal dorsal tarsus | ~8 years | 600 |
| 2 | 26 | LH | Distal plantar tarsus | ~2 years | 405 |
| 3 | 16 | RH | Distal dorsal tarsus | ~6 years | 610 |
| 4 | 26 | LH | Metatarsus both lateral and medial | ~2 months | / |
| 5 | Presented at the clinic with an old wound, exact age of wound was unknown | RH | Medial tibia | ~1 year | 340 |
| 6 | 30 | RF | Carpus | ~11 years | 635 |
| 7 | Presented at the clinic with an old wound, exact age of wound was unknown | RH | Tarsus + metatarsus | ~11 years | 593 |
| 8 | 66 | LF | Fetlock | ~14 years | 480 |
| 9 | 17 | LH | Fetlock | ~11 years | / |
| 10 | Presented at the clinic with an old wound, exact age of wound was unknown | RF | Metacarpus | ~9 years | / |
| 11 | 9 | LH | Metacarpus | ~5 years | 520 |
| 12 | 20 | RH | Tarsus | ~5 years | 465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du Cheyne, C.; Martens, A.; De Spiegelaere, W. High Numbers of CD163-Positive Macrophages in the Fibrotic Region of Exuberant Granulation Tissue in Horses. Animals 2021, 11, 2728. https://doi.org/10.3390/ani11092728
Du Cheyne C, Martens A, De Spiegelaere W. High Numbers of CD163-Positive Macrophages in the Fibrotic Region of Exuberant Granulation Tissue in Horses. Animals. 2021; 11(9):2728. https://doi.org/10.3390/ani11092728
Chicago/Turabian StyleDu Cheyne, Charis, Ann Martens, and Ward De Spiegelaere. 2021. "High Numbers of CD163-Positive Macrophages in the Fibrotic Region of Exuberant Granulation Tissue in Horses" Animals 11, no. 9: 2728. https://doi.org/10.3390/ani11092728
APA StyleDu Cheyne, C., Martens, A., & De Spiegelaere, W. (2021). High Numbers of CD163-Positive Macrophages in the Fibrotic Region of Exuberant Granulation Tissue in Horses. Animals, 11(9), 2728. https://doi.org/10.3390/ani11092728






