Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Confirmation of Hypothyroidism
2.3. mRNA Isolation and Real-Time PCR
2.4. Protein Isolation and Western Blotting
2.5. The Extraction of Prostaglandins and the Measurement of Their Concentrations in the Uterine Tissue
2.6. Statistical Analyses
3. Results
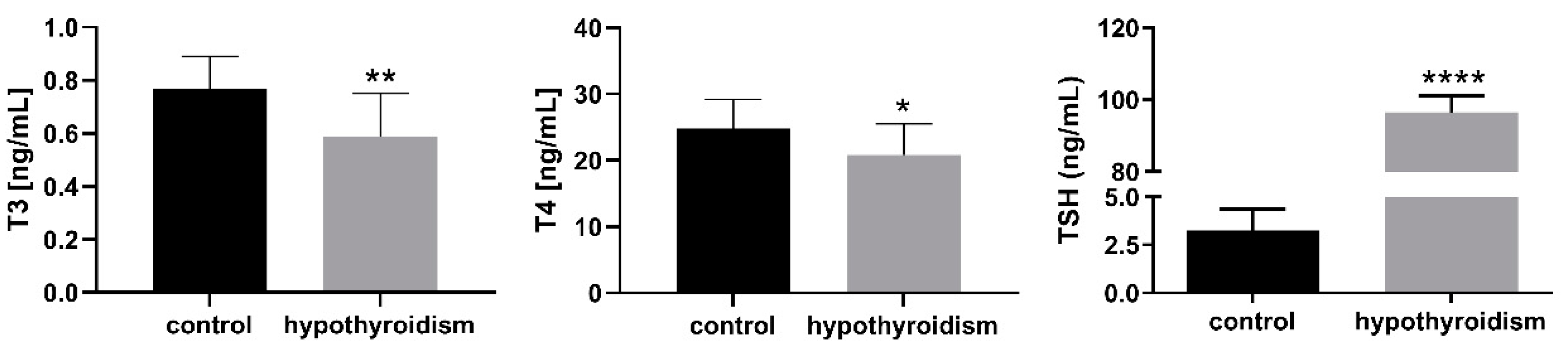
3.1. The Levels of Thyroid Hormones in the Blood Serum Samples
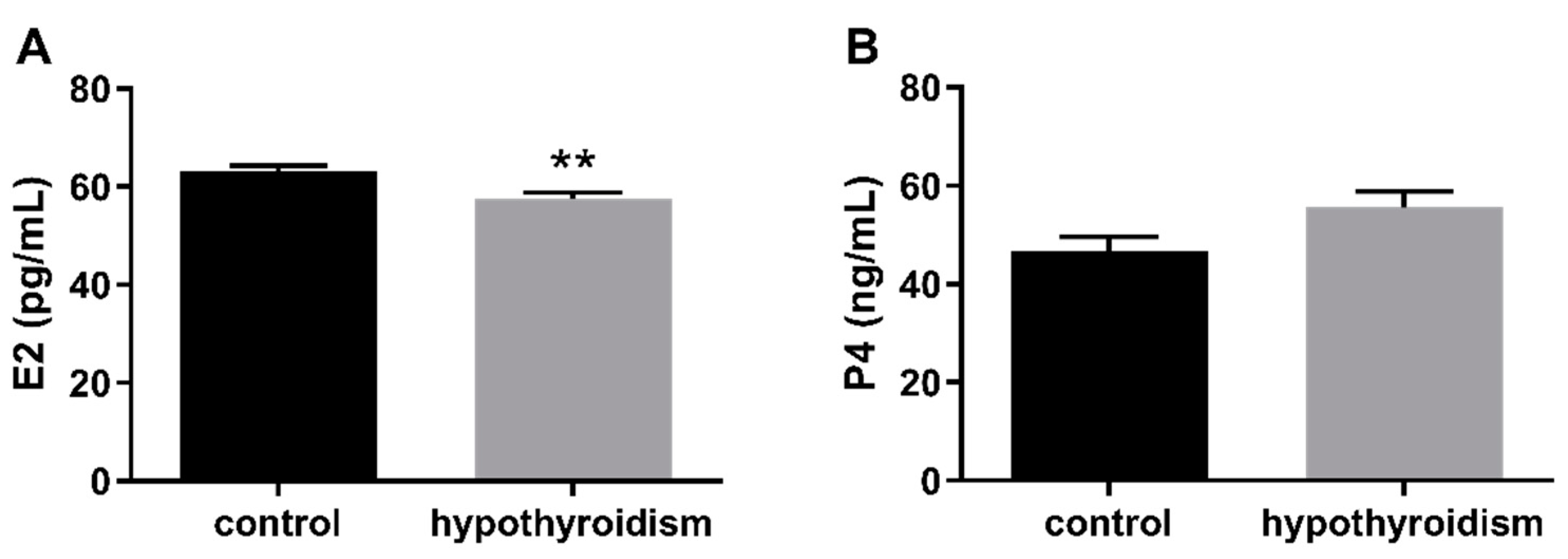
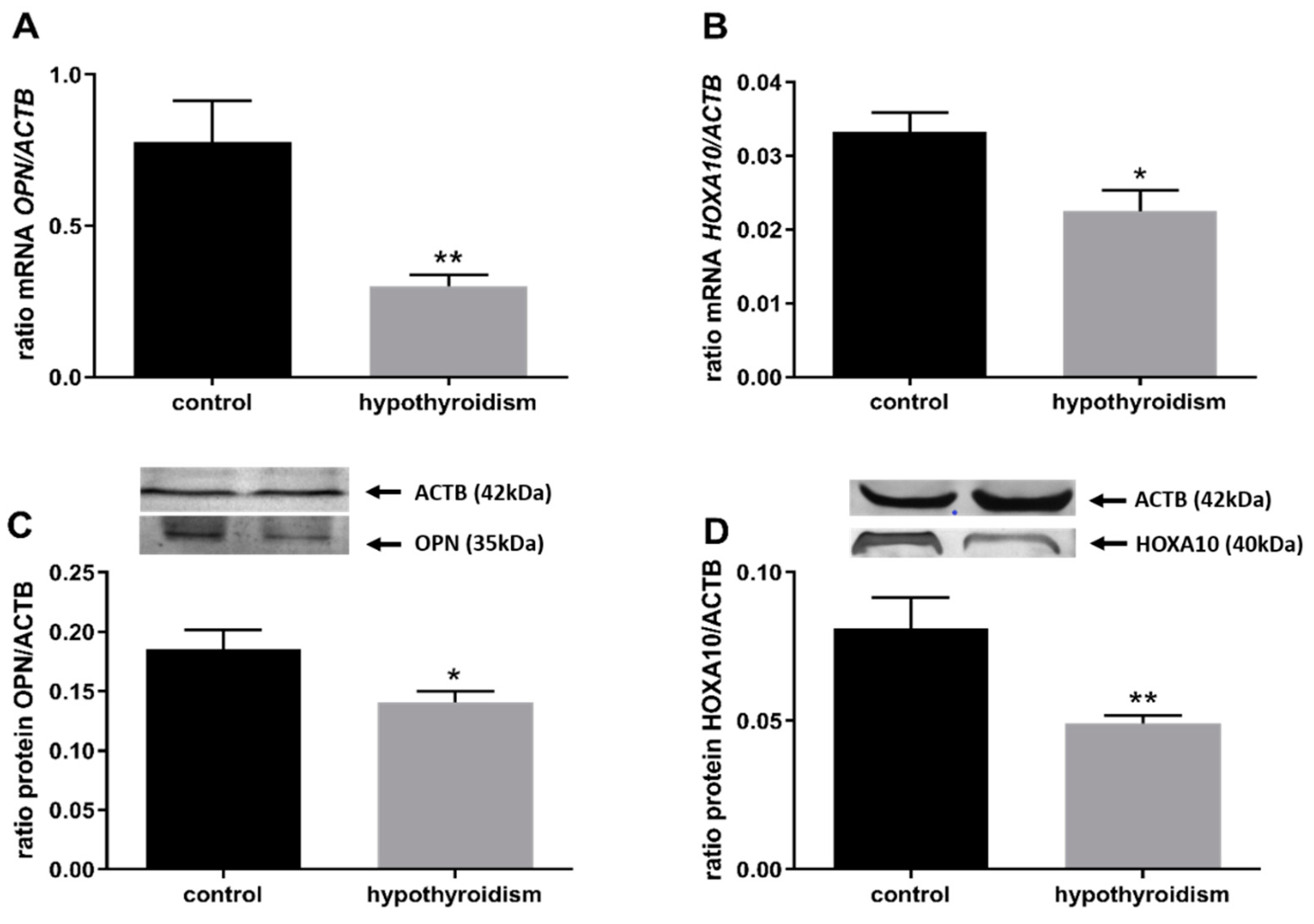
3.2. Uterine Receptivity
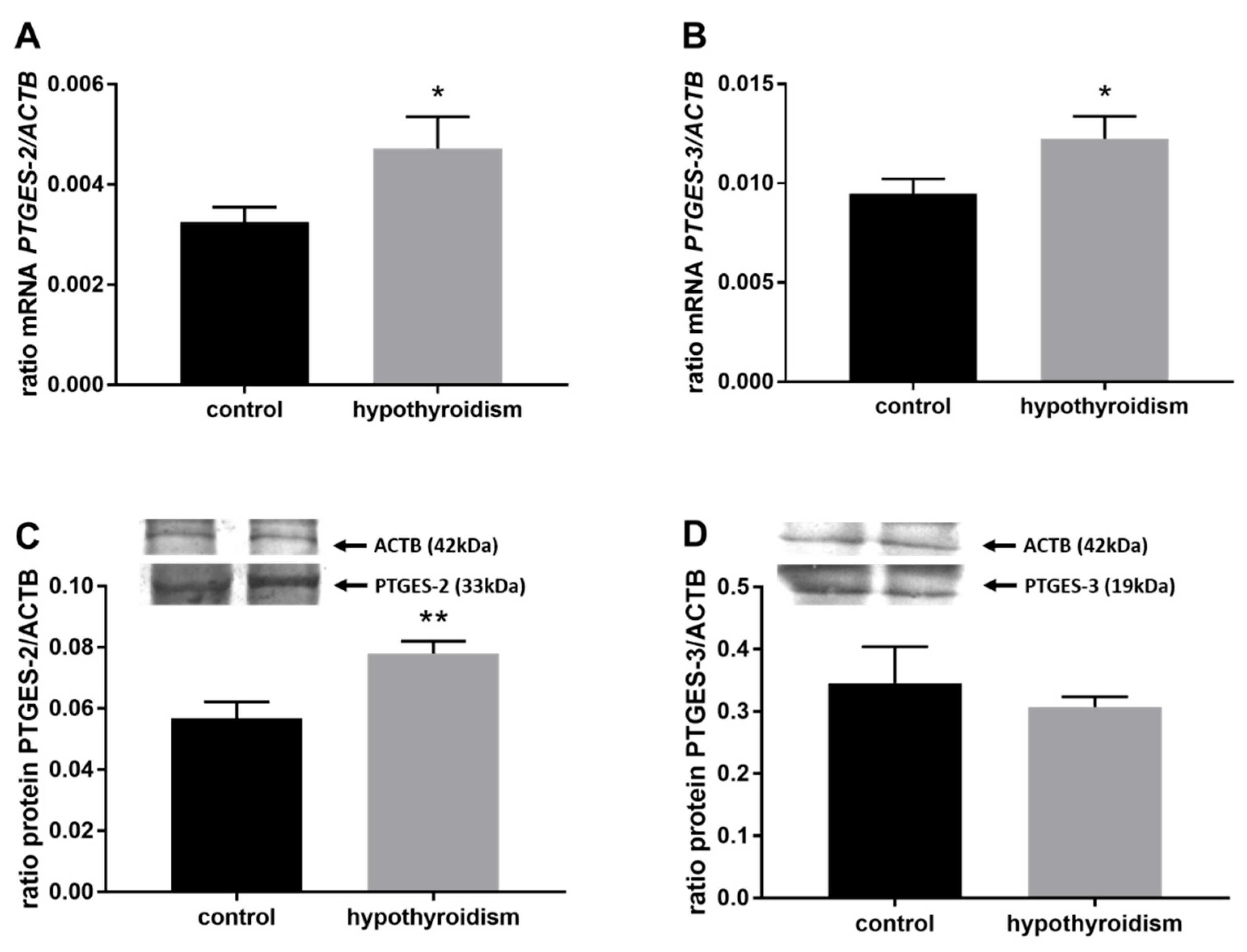
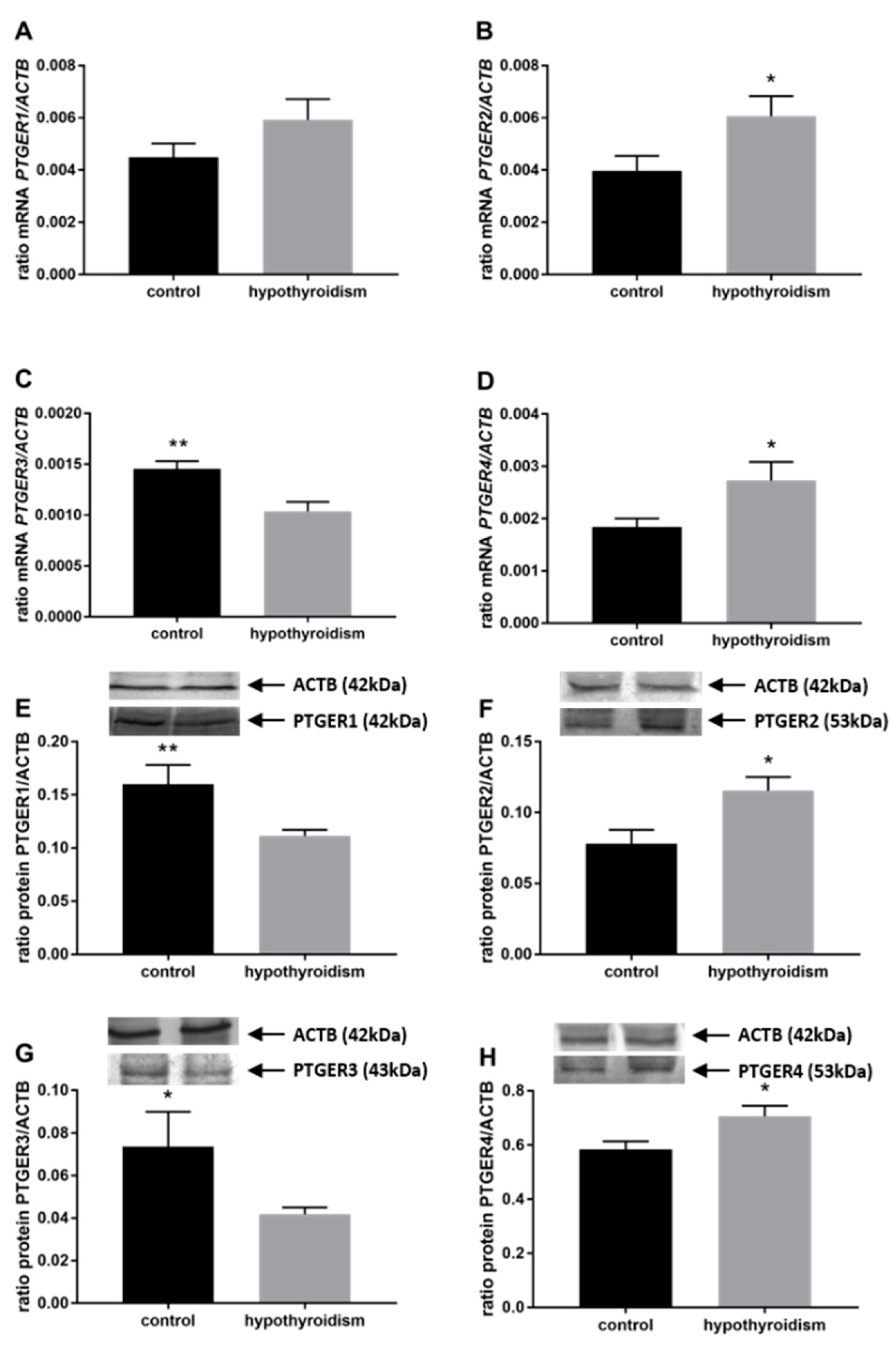
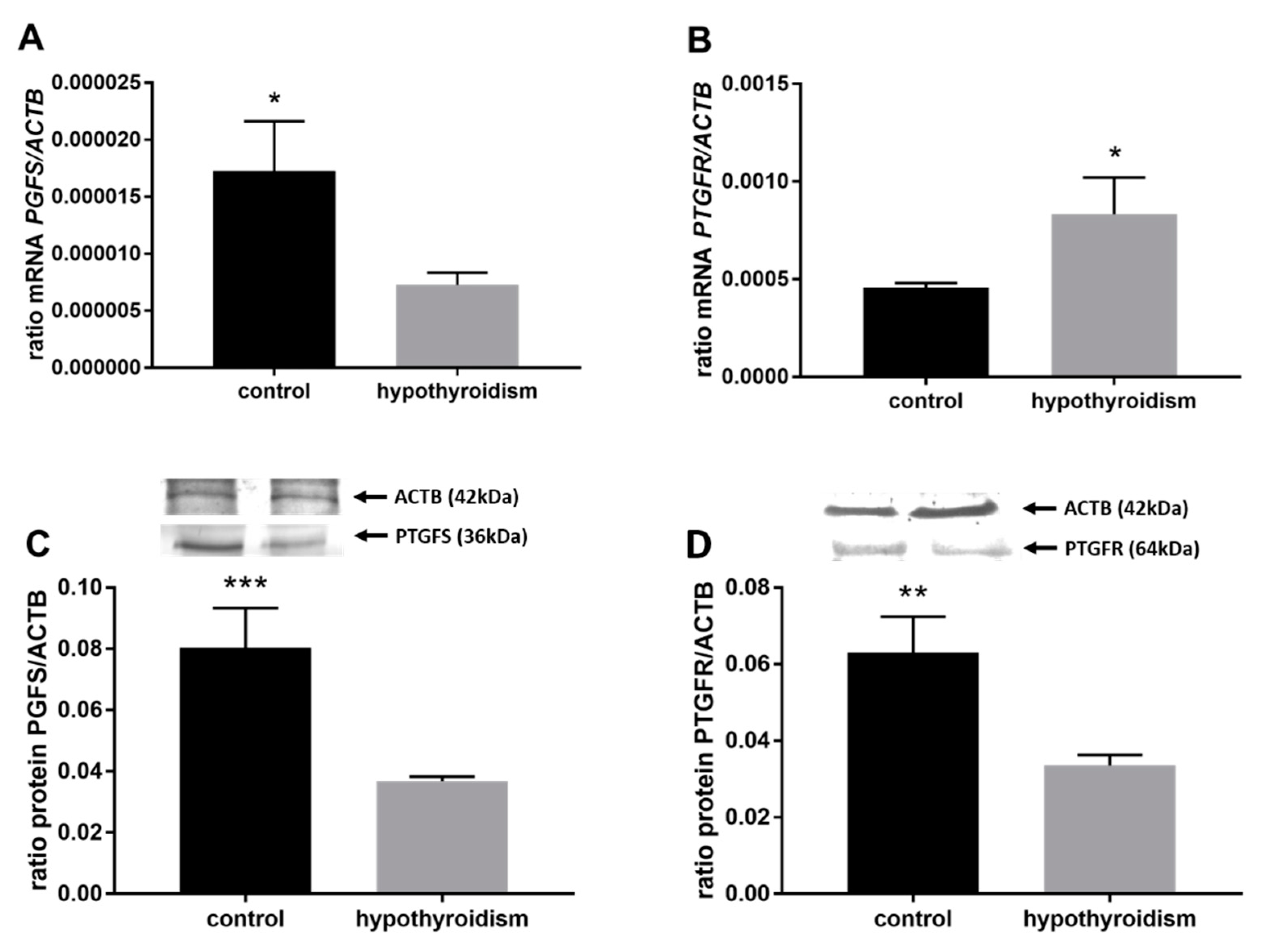
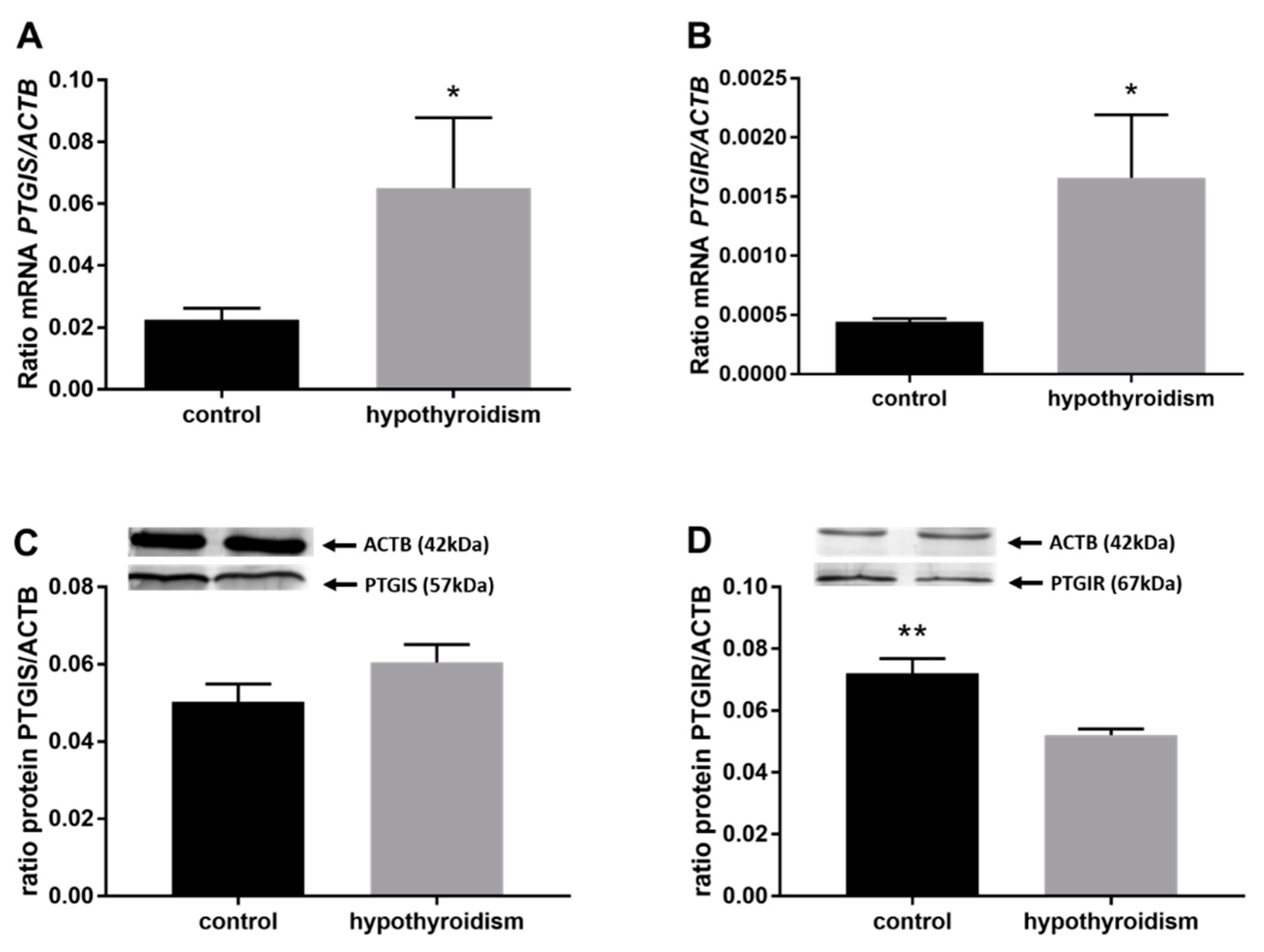
3.3. Prostaglandin Signaling in the Uterine Tissue
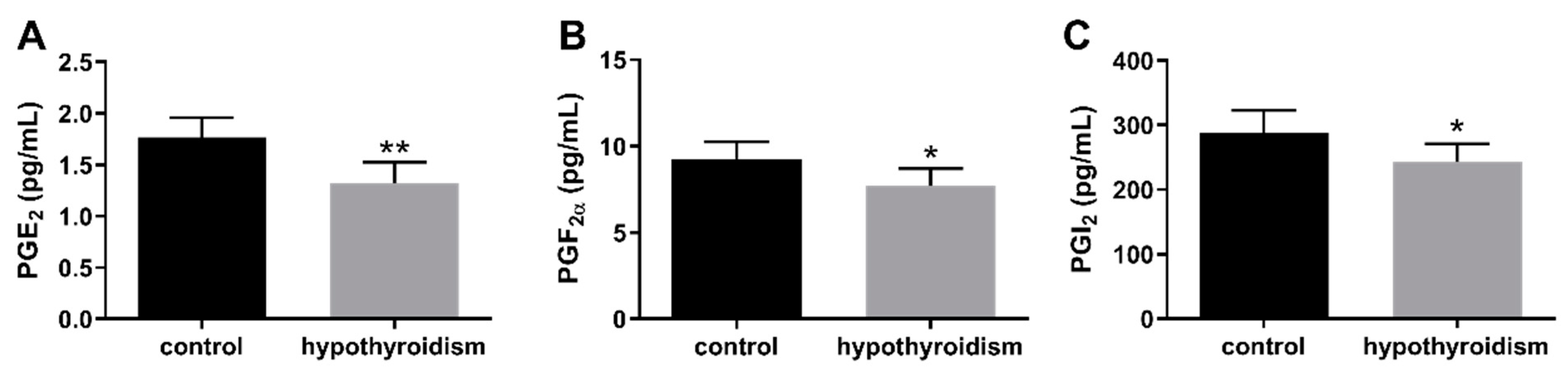
3.4. The Concentrations of Prostaglandins in the Uterine Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jannini, E.A.; Ulisse, S.; D’Armiento, M. Thyroid hormone and male gonadal function. Endocr. Rev. 1995, 16, 443–459. [Google Scholar] [PubMed]
- Metz, L.D.; Seidler, F.J.; McCook, E.C.; Slotkin, T.A. Cardiac alpha-adrenergic receptor expression is regulated by thyroid hormone during a critical developmental period. J. Mol. Cell Cardiol. 1996, 28, 1033–1044. [Google Scholar] [CrossRef]
- Krassas, G.E. Thyroid disease and female reproduction. Fertil. Steril. 2000, 74, 1063–1070. [Google Scholar] [CrossRef]
- Choksi, N.Y.; Jahnke, G.D.; St Hilaire, C.; Shelby, M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth. Defects Res. B Dev. Reprod. Toxicol. 2003, 68, 479–491. [Google Scholar] [CrossRef]
- Kong, L.; Wei, Q.; Fedail, J.S.; Shi, F.; Nagaoka, K.; Watanabe, G. Effects of thyroid hormones on the antioxidative status in the uterus of young adult rats. J. Reprod. Dev. 2015, 61, 219–227. [Google Scholar] [CrossRef]
- Thomas, R.; Reid, R.L. Thyroid disease and reproductive dysfunction. J. Obstet. Gynaecol. 1987, 70, 789–798. [Google Scholar]
- Parija, S.C.; Raviprakash, V.; Telang, A.G.; Varshney, V.P.; Mishra, S.K. Influence of hypothyroid state on 45Ca2+ influx and sensitivity of rat uterus to nifedipine and diltiazem. Eur. J. Pharmacol. 2001, 421, 207–213. [Google Scholar]
- Inuwa, I.; Williams, M.A. Morphometric study on the uterine horn and thyroid gland in hypothyroid, and thyroxine-treated hypothyroid rats. J. Anat. 1996, 188, 383–393. [Google Scholar]
- Doufas, A.G.; Mastorakos, G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann. N. Y. Acad. Sci. 2000, 900, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Reh, A.; Chaudhry, S.; Mendelsohn, F.; Im, S.; Rolnitzky, L.; Amarosa, A.; Levitz, M.; Srinivasa, S.; Krey, L.; Berkeley, A.S.; et al. Effect of autoimmune thyroid disease in older euthyroid infertile woman during the first 35 days of an IVF cycle. Fertil. Steril. 2011, 95, 1178–1181. [Google Scholar] [CrossRef]
- Dittrich, R.; Beckmann, M.W.; Oppelt, P.G.; Hoffmann, I.; Lotz, L.; Kuwert, T.; Mueller, A. Thyroid hormone receptors and reproduction. J. Reprod. Immunol. 2011, 90, 58–66. [Google Scholar] [CrossRef]
- Turnbull, L.W.; Lesny, P.; Killick, S.R. Assessment of uterine receptivity prior to embryo transfer: A review of currently available imaging modalities. Hum. Reprod. Update. 1995, 1, 505–514. [Google Scholar] [CrossRef]
- Shahzad, H.; Giribabu, N.; Karim, K.; Kassim, N.; Muniandy, S.; Kumar, K.E.; Salleh, N. Quercetin interferes with the fluid volume and receptivity development of the uterus in rats during the peri-implantation period. Reprod. Toxicol. 2017, 71, 42–54. [Google Scholar] [CrossRef]
- Yoshinaga, K. Uterine receptivity for blastocyst implantation. Ann. N. Y. Acad. Sci. 1988, 541, 423–443. [Google Scholar] [CrossRef]
- Murphy, C.R. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004, 14, 259–267. [Google Scholar] [CrossRef]
- Okada, A.; Ohta, Y.; Inoue, S.; Hiroi, H.; Muramatsu, M.; Iguchi, T. Expression of estrogen, progesterone and androgen receptors in the oviduct of developing, cyclic and preimplantation rats. J. Mol. Endocrinol. 2003, 30, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Psychoyos, A. Uterine receptivity for nidation. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Satokata, I.; Benson, G.; Maas, R. Sexually dimorphic sterility phenotypes in Hoxa-10 deficient mice. Nature 1995, 374, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Avici, A.; Olive, D.; Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Investig. 1998, 101, 1379–1384. [Google Scholar] [CrossRef]
- Bagot, C.N.; Kliman, H.J.; Taylor, H.S. Maternal HOXA10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev. Dyn. 2001, 222, 538–544. [Google Scholar] [CrossRef]
- Apparao, K.B.; Murray, M.J.; Fritz, M.A.; Meyer, W.R.; Chamberes, A.F.; Truong, P.R.; Lessey, B.A. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during menstrual cycle but regulated differentially. J. Clin. Endocrinol. Metab. 2001, 86, 4991–5000. [Google Scholar]
- Casals, G.; Ordi, J.; Creus, M.; Fa’bregues, F.; Carmona, F.; Casamitjana, R.; Balasch, J. Osteopontin and avb3 integrin as markers of endometrial receptivity: The effect of different hormone therapies. Reprod. Biomed. Online 2010, 21, 349–359. [Google Scholar] [CrossRef]
- Loftin, C.D.; Tiano, H.F.; Langenbach, R. Phenotypes of the COX-deficient mice indicte physiological and pathological roles for COX-1 and COX-2. Prostaglandins Lipid Mediat. 2002, 68, 177–185. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L. Prostaglandin endoperoxide synthases- 1 and -2. Adv. Immunol. 1996, 62, 167–215. [Google Scholar]
- Morawska, E.; Kaczmarek, M.M.; Blitek, A. Regulation of prostacyclin synthase expression and prostacyclin content in the pig endometrium. Theriogenology 2012, 78, 2071–2086. [Google Scholar] [CrossRef]
- Helliwell, R.J.; Adams, L.F.; Mitchell, M.D. Prostaglandin synthases: Recent developments and a novel hypothesis. Prostaglandins Leukot Essent. Fatt. Acids 2004, 70, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Akahoshi, T.; Jiang, S.; Namai, R.; Kitasato, H.; Endo, H.; Kameya, T.; Kondo, H. Induction of neutrophil death resembling neither apoptosis nor necrosis by ONO-AE-248, a selective agonist for PGE2 receptor subtype 3. J. Leukoc. Biol. 2000, 68, 187–193. [Google Scholar] [PubMed]
- Jabbour, H.N.; Sales, K.J. Prostaglandin receptor signaling and function in human endometrial pathology. Trends Endocrinol. Metab. 2004, 15, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Blesson, C.S.; Büttner, E.; Masironi, B.; Sahlin, L. Prostaglandin receptors EP and FP are regulated by estradiol and progesterone in the uterus of ovariectomized rats. Reprod. Biol. Endocrinol. 2012, 10, 3. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Zaragoza, D.B.; Mitchell, B.F.; Robertson, S.; Olson, D.M. Prostaglandin F2alpha and its receptor as activators of human decidua. Semin. Reprod. Med. 2007, 25, 60–68. [Google Scholar] [CrossRef]
- Narumiya, S.; FitzGerald, G.A. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Investig. 2001, 108, 25–30. [Google Scholar] [CrossRef]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Yang, Z.M.; Das, S.K.; Wang, J.; Sugimoto, Y.; Ichikawa, A.; Dey, S.K. Potential sites of prostaglandin action in the periimplantation mouse uterus: Differentia expression and regulation of prostaglandin receptor genes. Biol. Reprod. 1997, 56, 368–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grigsby, P.L.; Sooranna, S.R.; Adu-Amankwa, B.; Pitzer, B.; Brockman, D.E.; Johnson, M.R.; Myatt, L. Regional expression of prostaglandin E2 and F2alpha receptors in hyman myometrium, amnion and choriodecidua with advancing gestation and labor. Biol. Reprod. 2006, 75, 297–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catalano, R.D.; Wilson, M.R.; Boddy, S.C.; Jabbour, H.N. Comprehensive expression analysis of prostanoid enzymes and receptors in the human endometrium across the menstrual cycle. Mol. Hum. Reprod. 2011, 17, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kraeling, R.R.; Rampacek, G.B.; Fiorello, N.A. Inhibition of pregnancy with indomethacin in mature gilts and prepubertal gilts induced to ovulate. Biol. Reprod. 1985, 32, 105–110. [Google Scholar] [CrossRef]
- Davis, D.L.; Blair, R.M. Studies of uterine secretion and products of primary cultures of endometrial cells in pigs. J. Reprod. Fertil. Supply 1993, 48, 143–155. [Google Scholar] [CrossRef]
- Lim, H.; Gupta, R.A.; Ma, W.G.; Parija, B.C.; Moller, D.E.; Morrow, J.D.; Dubois, R.D.; Trzaskos, J.M.; Dey, S.K. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes. Dev. 1999, 13, 1561–1574. [Google Scholar] [CrossRef]
- Cammas, L.; Reinaud, P.; Bordas, N.; Dubois, O.; Germain, G.; Charpigny, G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction 2006, 131, 917–927. [Google Scholar] [CrossRef]
- Kengni, J.H.; St-Louis, I.; Parent, S.; Leblanc, V.; Shooner, C.; Asselin, E. Regulation of prostaglandin D synthase and prostacyclin synthase in endometrium of cyclic, pregnant and pseudopregnant rats and their regulation by sex steroids. J. Endocrinol. 2007, 195, 301–311. [Google Scholar] [CrossRef]
- Gillio-Meina, C.; Phang, S.H.; Mather, J.P.; Knight, T.G. Expression patterns and role of prostaglandin-endoperoxide synthases, prostaglandin E synthases, prostacyclin synthases, prostacyclin synthase, prostacyclin receptor, peroxisome proliferator-activated receptor delta and retinoid x receptor alpha in rat endometrium during artificially-induced decidualization. Reproduction 2009, 137, 537–552. [Google Scholar] [PubMed]
- Ulbrich, S.E.; Schulke, K.; Groebner, A.E.; Reichenbach, H.D.; Angioni, C.; Geisslinger, G.; Meyer, H.H.D. Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction 2009, 138, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Pakrasi, P.L.; Jain, A.K. Evaluation of cyclooxygenase 2 derived endogenous prostacyclin in mouse preimplantation embryo development in vitro. Life Sci. 2007, 80, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Pakrasi, P.; Jain, A.K. Cyclooxygenase-2-derived endogenous prostacyclin reduces apoptosis and enhances embryo viability in mouse. Prostaglandins Leukot. Essent. Fatty Acids 2008, 79, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Wun, W.S.; Goldsby, J.S.; Wun, I.C.; Falconi, S.M.; Wu, K.K. Prostacyclin enhances embryo hatching but not sperm motility. Hum. Reprod. 2003, 18, 2582–2589. [Google Scholar] [CrossRef][Green Version]
- Huang, J.C.; Goldsby, J.S.; Wun, W.S. Prostacyclin enhances the implantation and life birth potentials of mouse embryos. Hum. Reprod. 2004, 19, 1856–1860. [Google Scholar] [CrossRef]
- Salleh, N. Diverse roles of prostaglandins in blastocyst implantation. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Shahnazi, M.; Niuri, M.; Mohaddes, G.; Latifi, Z.; Fattahi, A.; Mohammadi, M. Prostaglandin E pathway in uterine tissue during window of preimplantation in female mice mated with intact and seminal vesicle-excited male. Reprod. Sci. 2017, 1, 1–9. [Google Scholar]
- Wacławik, A. Novel insights into the mechanism of pregnancy establishment: Regulation of prostaglandin synthesis and signaling in pig. Reproduction 2011, 142, 389–399. [Google Scholar] [CrossRef]
- Jena, S.; Bhanja, S. Hypothyroidism alters antioxidant defence system in rat brainstem during postnatal development and adulthood. Neurol. Sci. 2014, 35, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Persani, L. Clinical review: Central hypothyroidism: Pathogenic, diagnostic, and therapeutic challenges. J. Clin. Endocrinol. Metab. 2012, 97, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Biondi, B.; Cooper, D.S. Thyroid hormone therapy for hypothyroidism. Endocrine 2019, 66, 18–26. [Google Scholar] [CrossRef]
- Gruters, A.; Krude, H. Detection and treatment of congenital hypothyroidism. Nat. Rev. Endocrinol. 2012, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Colicchia M Campagnolo, L.; Baldini, E.; Ulisse, S.; Valensise, H.; Moretti, C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum. Reprod. Update. 2014, 20, 884–904. [Google Scholar] [CrossRef]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid function and human reproductive health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef]
- Tohei, A. Studies on the functional relationship between thyroid, adrenal and gonadal hormones. J. Reprod. Dev. 2004, 50, 9–20. [Google Scholar] [CrossRef]
- Hapon, M.B.; Motta, A.B.; Ezquer, M.; Bonafede, M.; Jahn, A.G. Hypothyroidism prolongs corpus luteum function in the pregnant rat. Reproduction 2007, 133, 197–205. [Google Scholar] [CrossRef]
- Zanatta, A.; Rocha, A.M.; Carvalho, F.M.; Pereira, R.M.; Taylor, H.S.; Motta, E.L.; Baracat, E.C.; Serafini, P.C. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: A review. J. Assist. Reprod. Genet. 2010, 27, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.S.; Lin, X.; Itskovich, V.V.; Aguinaldo, J.G.; Chaplin, W.F.; Denhardt, D.T.; Fayad, Z.A. Prenatal detection of embryo resorption in osteopontin-deficient mice using serial noninvasive magnetic resonance microscopy. Pediatr. Res. 2004, 55, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Von Wolff, M.; Bohlmann, M.K.; Fiedler, C.; Ursel, S.; Strowitzki, T. Osteopontin is up-regulated in human decidual stromal cells. Fertil. Steril. 2004, 81, 741–748. [Google Scholar] [CrossRef]
- Qi, Q.R.; Xie, Q.Z.; Liu, X.L.; Zhou, Y. Osteopontin is expressed in the mouse uterus during early pregnancy and promotes mouse blastocyst attachment and invasion in vitro. PLoS ONE 2014, 9, e104955. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk JE Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef]
- St-Louis, I.; Singh, M.; Brasseur, K.; Leblanc, V.; Parent, S.; Asselin, E. Expression of COX-1 and COX-2 in the endometrium of cyclic, pregnant and in a model of pseudopregnant rats and their regulation by sex steroids. Reprod. Biol. Endocrinol. 2010, 24, 103. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhaaran, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/ antipyretic drugs: Cloning, structure and expression. Proc. Natl. Acad. Sci. USA 2002, 90, 13926–13931. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M. Novel contraceptive targets to inhibit ovulation: The prostaglandin E2 pathway. Hum. Reprod. Update. 2015, 21, 652–670. [Google Scholar] [CrossRef]

| Gene Symbol | GeneBank Accession No. | Forward Primer Reverse Primer | Primer Size (bp) |
|---|---|---|---|
| PTGS2 | NM_017232 | 5′-AAAGGCCTCCATTGACCAGA-3′ 5′-TCGATGTCATGGTAGAGGGC-3′ | 20 20 |
| PTGES2 | NM_001107832.1 | 5′-AAAGGAAGCCAGGACGGAGGA-3′ 5′-CCTCGGCAGGTGTTCGGT-3′ | 21 18 |
| PTGES3 | M_001130989.1 | 5′-GCTGCCGGAGAGGAGTCG-3′ 5′-AGGCTGCATGGTGAACGGG-3′ | 18 19 |
| PTGER1 | M_001278475.1 | 5′-GCTCCCTGCCTTTCACAATCT-3′ 5′-TCTCAGGACTGGTGGTCTAAGGA-3′ | 21 23 |
| PTGER2 | NM_031088.1 | 5′-GAAAGGACTTCTATGGCGGAGG-3′ 5′-AAGCAAAGATTGTGAAAGGCAGG-3′ | 22 24 |
| PTGER3 | NM_012704.1 | 5′-CGCAGATGGGAAAGGAGAAGGA-3′ 5′-AGGTTGTTCATCATCTGGCAGAACT-3′ | 22 25 |
| PTGER4 | NM_032076.3 | 5′-CATTCCCGCTCGTGGTGCGA-3′ 5′TCTGCTGATGGTCTTTCACCACAC-3′ | 19 23 |
| PGFS | NM_138510.1 | 5′-GGTATCTCTGAAGCCAGGGGA-3′ 5′-TTGGACACCCCGATGGACTTG-3′ | 21 21 |
| PTGFR | NM_013115.1 | 5′-CCCTTTCTGGTGACGATGGC-3′ 5′-TCCGTAGCAGAATGTAGACCCA-3′ | 20 22 |
| PTGIS | NM_031557.2 | 5′-GGGCCTCCTGACTTCCTGTTG-3′ 5′-AGCTTTTCCTGCTCTCGGTGT-3′ | 21 21 |
| PTGIR | NM_001077644.1 | 5′-TCCCTGCCTCTCACGATCAG-3′ 5′-AAAACGGAAGGCGTGGAGGT-3′ | 20 20 |
| OPN | AB001382.1 | 5′-TGAAAGTGGCTGAGTTTGGC-3′ 5′-TCGTCGTCATCATCGTCCAT-3′ | 20 20 |
| HOXA10 | NM_001129878.1 | 5′-CATTCAGGCCCCATCTCAGA-3′ 5′-TTCAGCCCCTCATAGCCAAA-3′ | 20 20 |
| ACTB | KJ696744.1 | 5′-CCACACCCGCCACCAGTTCG-3′ 5′-CTAGGGCGGCCCACGATGGA-3′ | 20 20 |
| Protein | Catalog N° | Host | Dilution | Predicted Molecular Weight (kDa) |
|---|---|---|---|---|
| PTGS-2 | Santa Cruz, sc-7951 | Rabbit | 1:100 | 85 |
| PTGES-2 | Cayman, 160145 | Rabbit | 1:200 | 33 |
| PTGES-3 | Abcam, ab92503 | Rabbit | 1:10,000 | 19 |
| PTGER1 | Abcam, ab183073 | Rabbit | 1:3000 | 42 |
| PTGER2 | Abcam, ab16151 | Mouse | 1:200 | 53 |
| PTGER3 | Abcam, ab16152 | Mouse | 1:100 | 43 |
| PTGER4 | Santa Cruz, sc-20677 | Rabbit | 1:100 | 53 |
| PGFS | Abcam, ab84327 | Rabbit | 1:500 | 36 |
| PTGFR | Cayman, 101802 | Rabbit | 1:200 | 64 |
| PTGIS | Abcam, ab23668 | Rabbit | 1:250 | 57 |
| PTGIR | Cayman, 10005518 | Rabbit | 1:200 | 67 |
| OPN | Abcam ab8448 | Rabbit | 1:1000 | 66 |
| HOXA10 | Abcam ab23392 | Rabbit | 1:1000 | 42 |
| ACTB | SigmaAldrich A2228 | Mouse | 1:4000 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk-Zieba, I.; Staszkiewicz-Chodor, J.; Boruszewska, D.; Lukaszuk, K.; Jaworska, J.; Woclawek-Potocka, I. Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling. Animals 2021, 11, 2636. https://doi.org/10.3390/ani11092636
Kowalczyk-Zieba I, Staszkiewicz-Chodor J, Boruszewska D, Lukaszuk K, Jaworska J, Woclawek-Potocka I. Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling. Animals. 2021; 11(9):2636. https://doi.org/10.3390/ani11092636
Chicago/Turabian StyleKowalczyk-Zieba, Ilona, Joanna Staszkiewicz-Chodor, Dorota Boruszewska, Krzysztof Lukaszuk, Joanna Jaworska, and Izabela Woclawek-Potocka. 2021. "Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling" Animals 11, no. 9: 2636. https://doi.org/10.3390/ani11092636
APA StyleKowalczyk-Zieba, I., Staszkiewicz-Chodor, J., Boruszewska, D., Lukaszuk, K., Jaworska, J., & Woclawek-Potocka, I. (2021). Hypothyroidism Affects Uterine Function via the Modulation of Prostaglandin Signaling. Animals, 11(9), 2636. https://doi.org/10.3390/ani11092636






